LECTURE 7 QUANTUM PHYSICS III Instructor ShihChieh Hsu








- Slides: 41

LECTURE 7 QUANTUM PHYSICS III Instructor: Shih-Chieh Hsu

Virtual Visit on Sep 10 2 9: 30 am Sep 10 (Thu) B 305 � � UW Research Dr. Associate Sam Meehan and UW Physics Graduate Nikola Whallon from CERN ATLAS Control Room ATLAS virtual visit Vidyo – 9213753 http: //vidyoportal. cern. ch/flex. html? roomdirect. html&key=P 5 b. Tn 2 wuu gkb 10: 50 am Sep 10 (Thu) PAA A 112 � LHC Master class – search for Higgs from real data

ATLAS Virtual Visit PAB B 305 3 9: 20 am PAB B 305

4 Old Quantum Theory

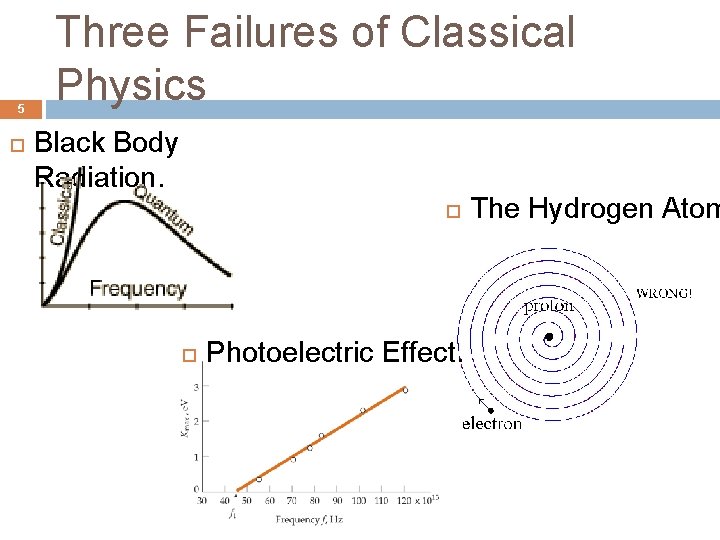
5 Three Failures of Classical Physics Black Body Radiation. Photoelectric Effect. The Hydrogen Atom

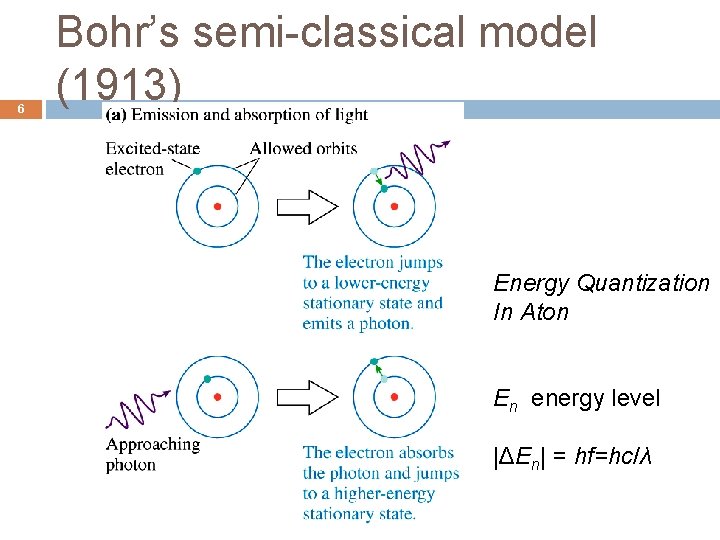
6 Bohr’s semi-classical model (1913) Energy Quantization In Aton En energy level |ΔEn| = hf=hc/λ

7 New Quantum Theory

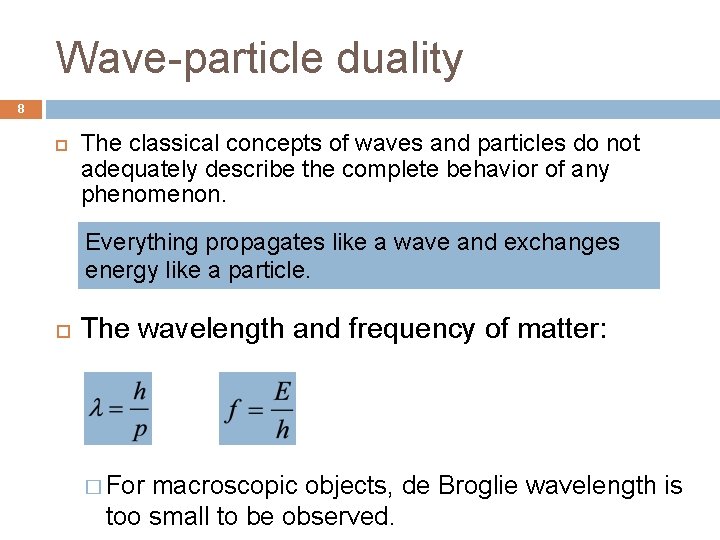
Wave-particle duality 8 The classical concepts of waves and particles do not adequately describe the complete behavior of any phenomenon. Everything propagates like a wave and exchanges energy like a particle. The wavelength and frequency of matter: � For macroscopic objects, de Broglie wavelength is too small to be observed.

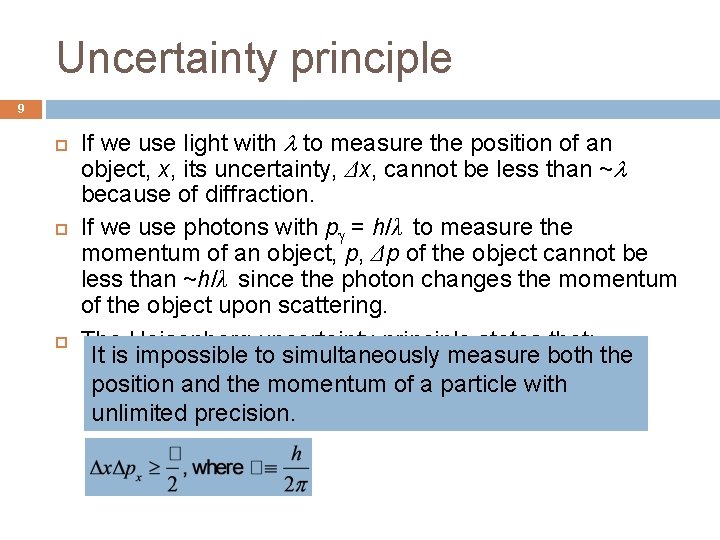
Uncertainty principle 9 If we use light with to measure the position of an object, x, its uncertainty, Δx, cannot be less than ~ because of diffraction. If we use photons with p = h/λ to measure the momentum of an object, p, Δp of the object cannot be less than ~h/λ since the photon changes the momentum of the object upon scattering. The Heisenberg uncertainty principle states that: It is impossible to simultaneously measure both the position and the momentum of a particle with unlimited precision.

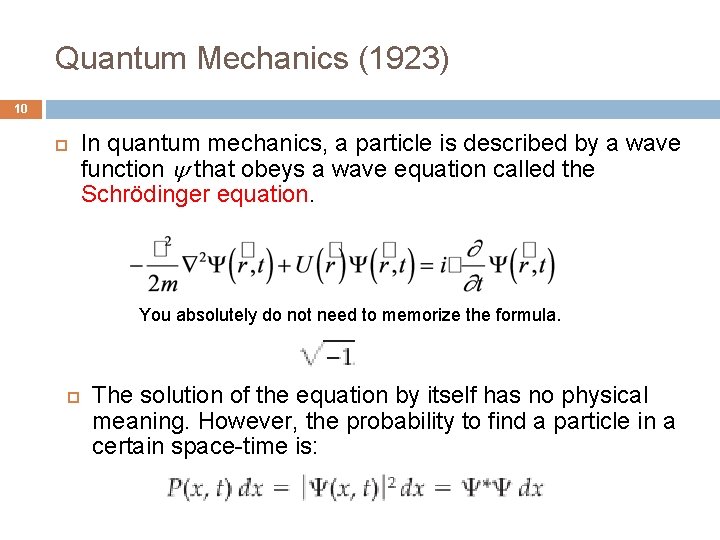
Quantum Mechanics (1923) 10 In quantum mechanics, a particle is described by a wave function that obeys a wave equation called the Schrödinger equation. You absolutely do not need to memorize the formula. The solution of the equation by itself has no physical meaning. However, the probability to find a particle in a certain space-time is:

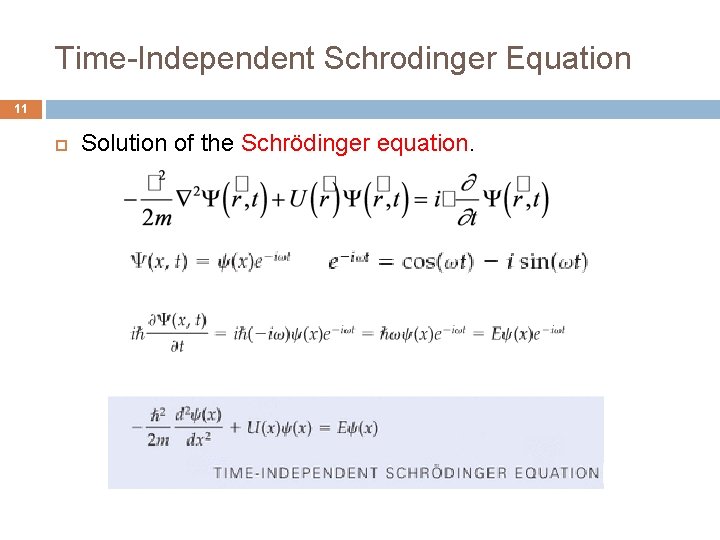
Time-Independent Schrodinger Equation 11 Solution of the Schrödinger equation.

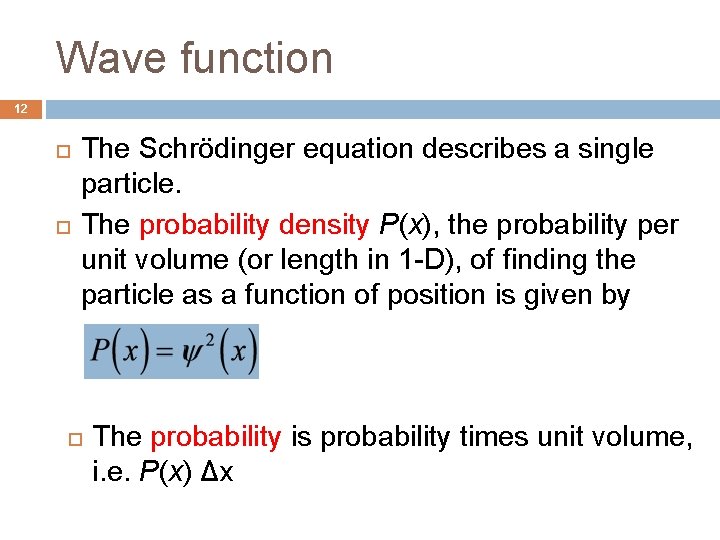
Wave function 12 The Schrödinger equation describes a single particle. The probability density P(x), the probability per unit volume (or length in 1 -D), of finding the particle as a function of position is given by The probability is probability times unit volume, i. e. P(x) Δx

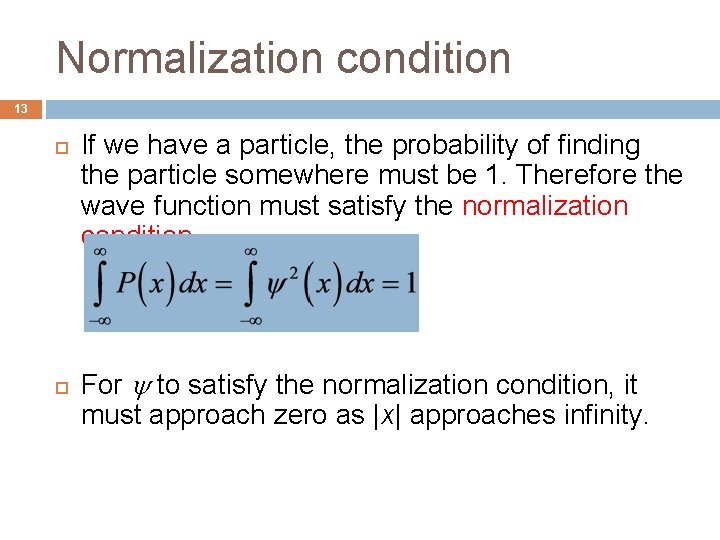
Normalization condition 13 If we have a particle, the probability of finding the particle somewhere must be 1. Therefore the wave function must satisfy the normalization condition. For to satisfy the normalization condition, it must approach zero as |x| approaches infinity.

Copenhagen Interpretation 14 1927, Bohr, Heisenberg, Pauli had converged to a consensus based on Bohr's concept of complementarity, which states that a physical phenomenon may manifest itself in two different ‘complementary' ways depending on the experiment set up to investigate it. Thus light, for example, could appear sometimes as a wave and sometimes as a particle. Although mutually exclusive, both pictures were necessary to obtain a full description of the phenomenon.

Schrödinger’s Cat 15 1935, Austrian physicist Erwin Schrödinger proposed this thought experiment, often described as a paradox, to illustrate what he saw as the problem of the Copenhagen interpretation of quantum mechanics applied to everyday objects.

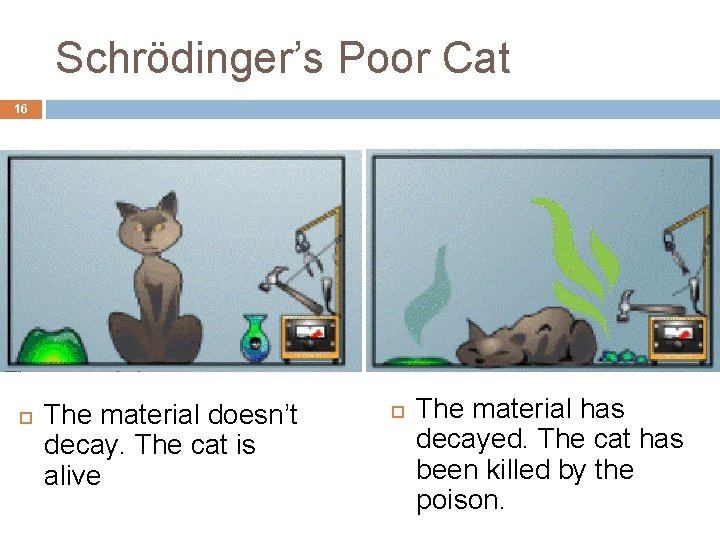
Schrödinger’s Poor Cat 16 The material doesn’t decay. The cat is alive The material has decayed. The cat has been killed by the poison.

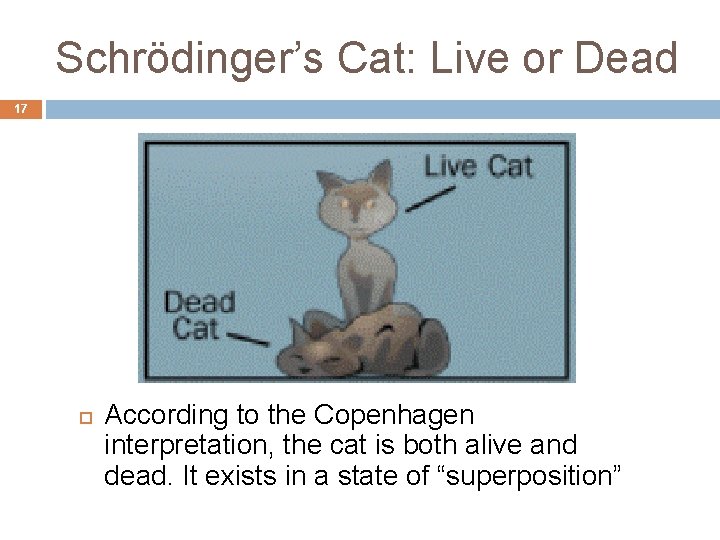
Schrödinger’s Cat: Live or Dead 17 According to the Copenhagen interpretation, the cat is both alive and dead. It exists in a state of “superposition”

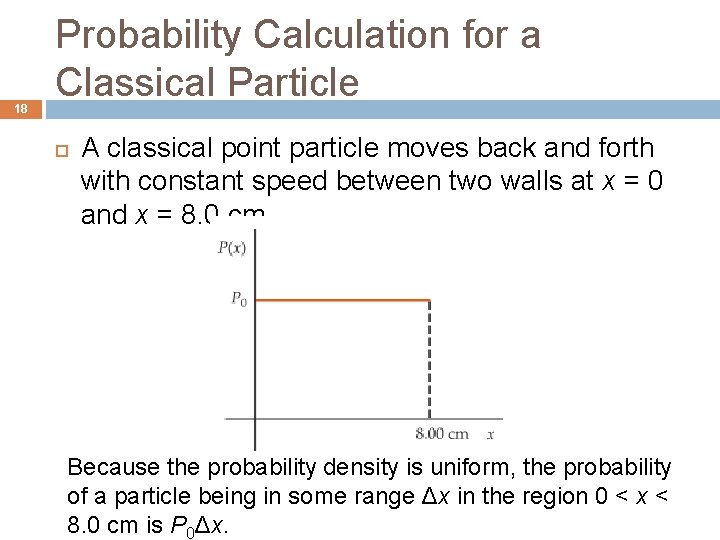
18 Probability Calculation for a Classical Particle A classical point particle moves back and forth with constant speed between two walls at x = 0 and x = 8. 0 cm. Because the probability density is uniform, the probability of a particle being in some range Δx in the region 0 < x < 8. 0 cm is P 0Δx.

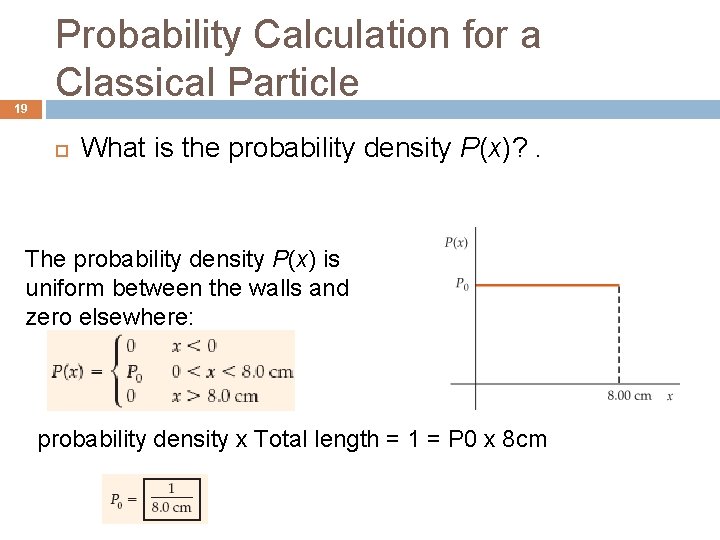
19 Probability Calculation for a Classical Particle What is the probability density P(x)? . The probability density P(x) is uniform between the walls and zero elsewhere: probability density x Total length = 1 = P 0 x 8 cm

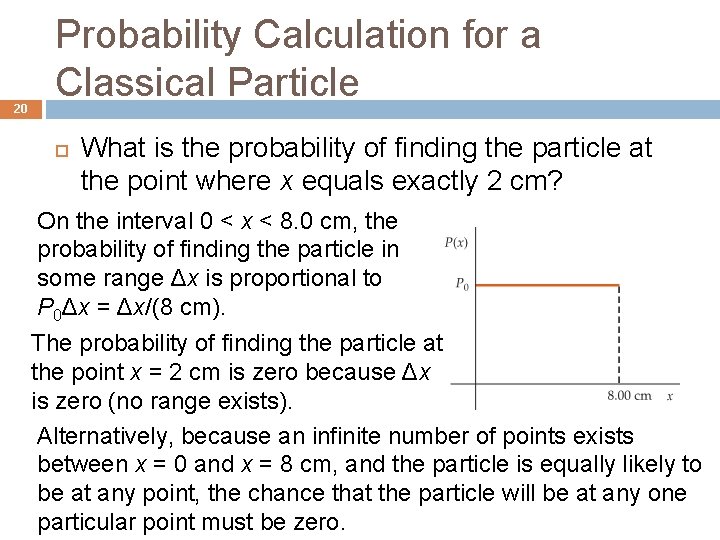
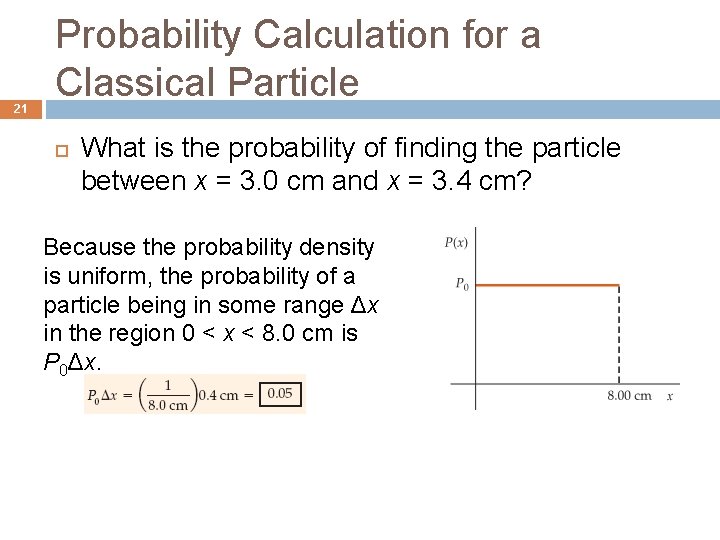
20 Probability Calculation for a Classical Particle What is the probability of finding the particle at the point where x equals exactly 2 cm? On the interval 0 < x < 8. 0 cm, the probability of finding the particle in some range Δx is proportional to P 0Δx = Δx/(8 cm). The probability of finding the particle at the point x = 2 cm is zero because Δx is zero (no range exists). Alternatively, because an infinite number of points exists between x = 0 and x = 8 cm, and the particle is equally likely to be at any point, the chance that the particle will be at any one particular point must be zero.

21 Probability Calculation for a Classical Particle What is the probability of finding the particle between x = 3. 0 cm and x = 3. 4 cm? Because the probability density is uniform, the probability of a particle being in some range Δx in the region 0 < x < 8. 0 cm is P 0Δx.

A particle in a box 22 Consider a particle of mass m confined to a onedimensional box of length L. � Classical Mechanics: The particle with any values of energy and momentum bounces back and forth between the walls of the box. � Quantum Mechanics: The particle is described by a wave function , and 2 describes the probability of finding the particle in some region.

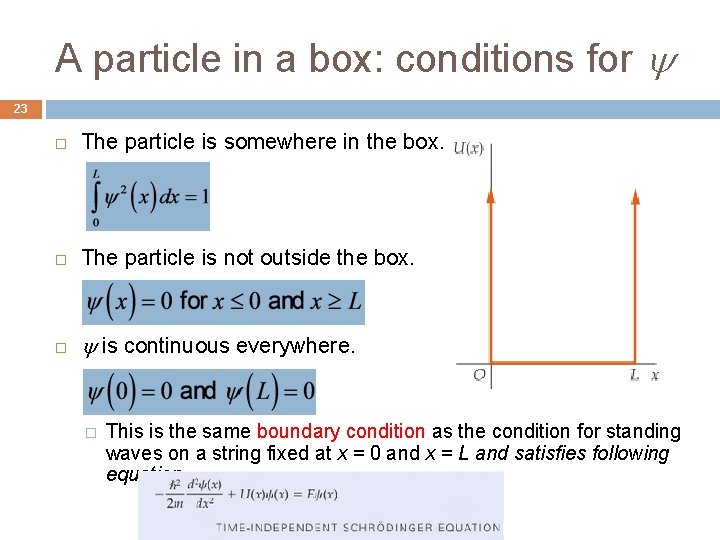
A particle in a box: conditions for 23 The particle is somewhere in the box. The particle is not outside the box. is continuous everywhere. � This is the same boundary condition as the condition for standing waves on a string fixed at x = 0 and x = L and satisfies following equation

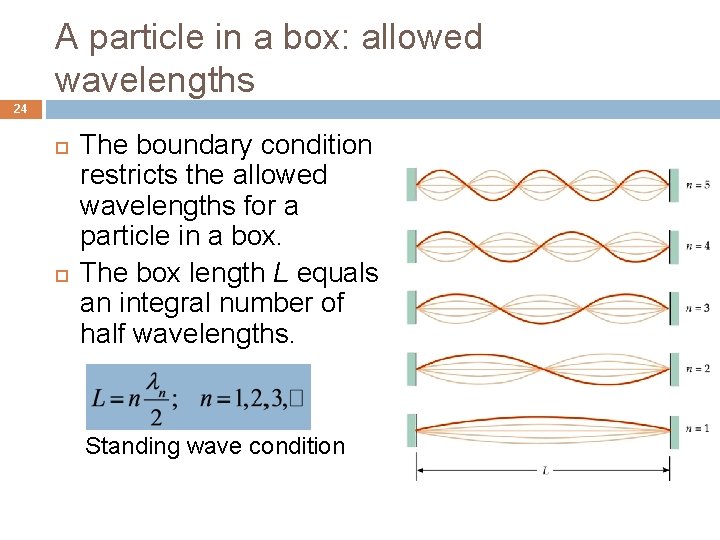
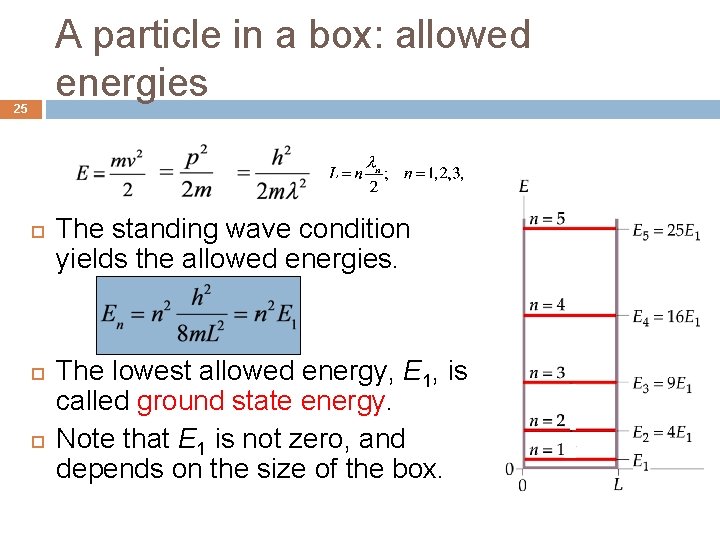
A particle in a box: allowed wavelengths 24 The boundary condition restricts the allowed wavelengths for a particle in a box. The box length L equals an integral number of half wavelengths. Standing wave condition

A particle in a box: allowed energies 25 The standing wave condition yields the allowed energies. The lowest allowed energy, E 1, is called ground state energy. Note that E 1 is not zero, and depends on the size of the box.

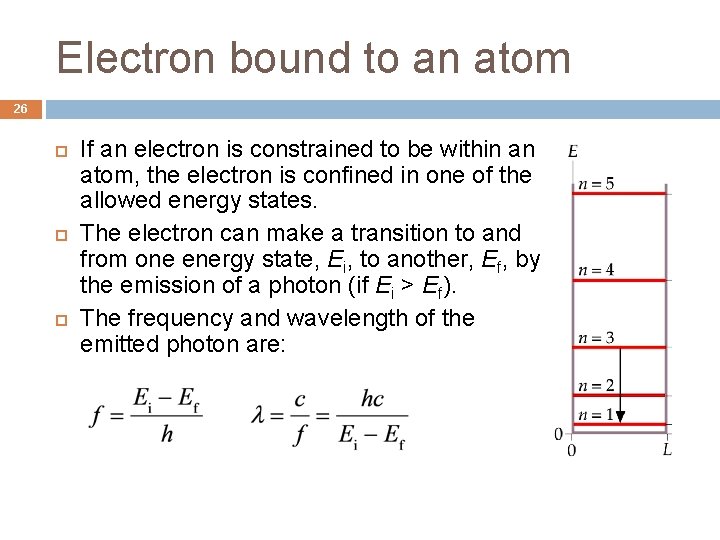
Electron bound to an atom 26 If an electron is constrained to be within an atom, the electron is confined in one of the allowed energy states. The electron can make a transition to and from one energy state, Ei, to another, Ef, by the emission of a photon (if Ei > Ef). The frequency and wavelength of the emitted photon are:

Quantum number 27 The number “n” is called a quantum number. It characterizes n for a particular state and for the energy of that state, En. For a particle in a 1 -D box, a quantum number arises from the boundary condition on : (0) = 0 and (L) = 0. For a particle in a 3 -D box, three quantum numbers arise, one associated with a boundary condition in each dimension.

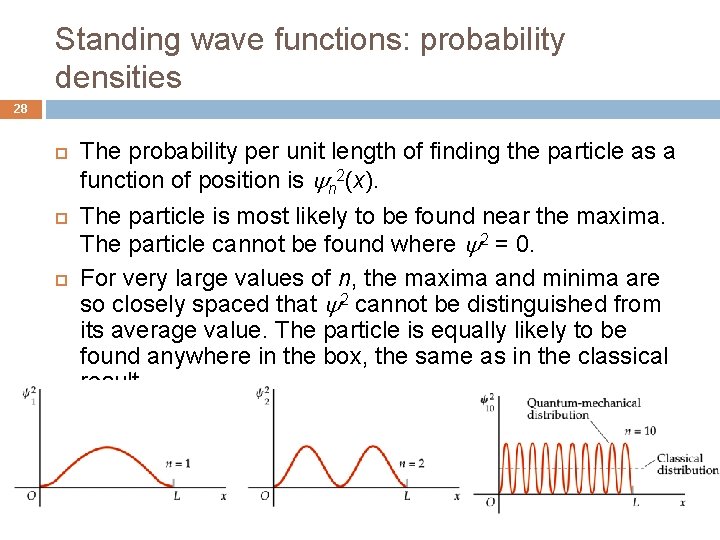
Standing wave functions: probability densities 28 The probability per unit length of finding the particle as a function of position is n 2(x). The particle is most likely to be found near the maxima. The particle cannot be found where 2 = 0. For very large values of n, the maxima and minima are so closely spaced that 2 cannot be distinguished from its average value. The particle is equally likely to be found anywhere in the box, the same as in the classical result.

Large quantum number 29 The fractional energy difference of adjacent states becomes very small as the quantum number increases. For a very large n, energy quantization is not important. Bohr’s correspondence principle states: In the limit of very large quantum numbers, the classical calculation and the quantum calculation must yield the same results.

Clicker Question 19 -2 There are three 1 -D boxes, B 1, B 2, and B 3, with length L, 2 L, and 3 L, respectively. Each box contains an electron in the state for n = 10. Rank the boxes according the number of maxima for the probability density of the electron, greatest first. � � B 1, B 2, B 3, B 2, B 1 B 2, B 3, B 1 They are all tie.

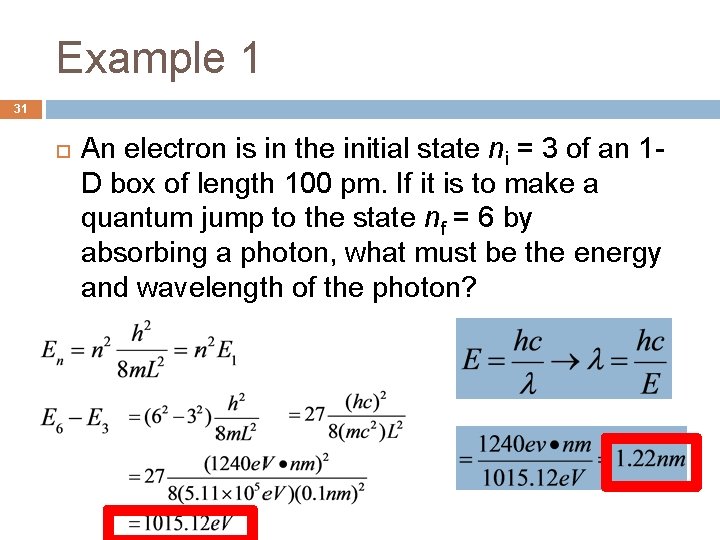
Example 1 31 An electron is in the initial state ni = 3 of an 1 D box of length 100 pm. If it is to make a quantum jump to the state nf = 6 by absorbing a photon, what must be the energy and wavelength of the photon?

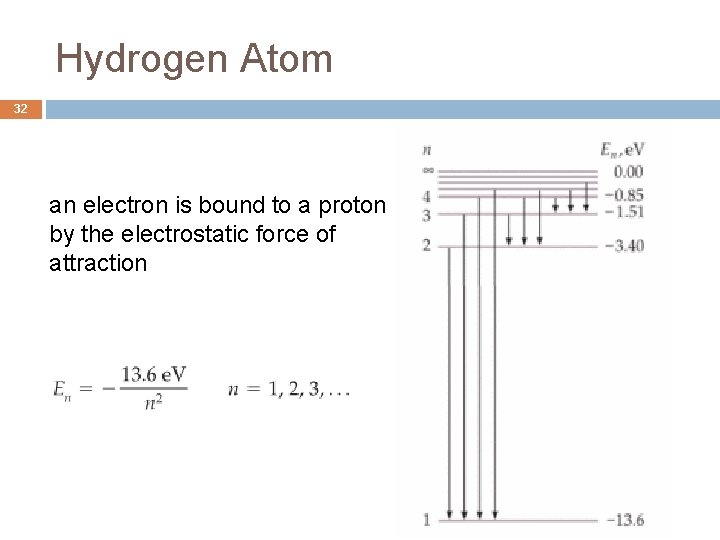
Hydrogen Atom 32 an electron is bound to a proton by the electrostatic force of attraction

Timeline 33

34 Backup

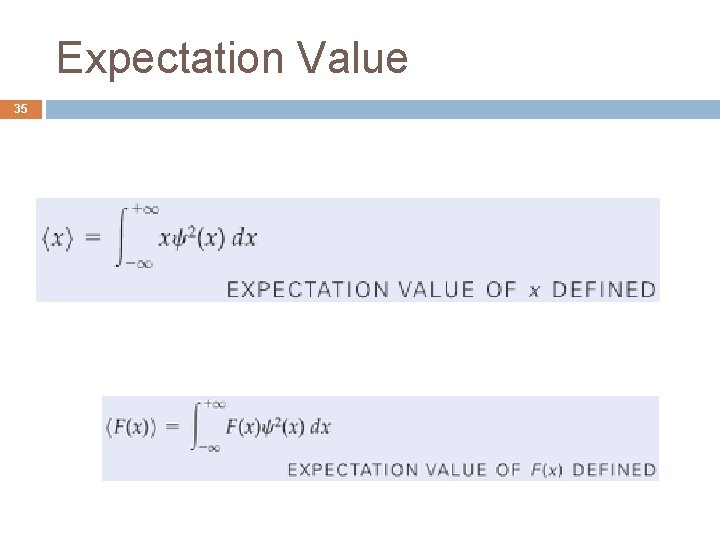
Expectation Value 35

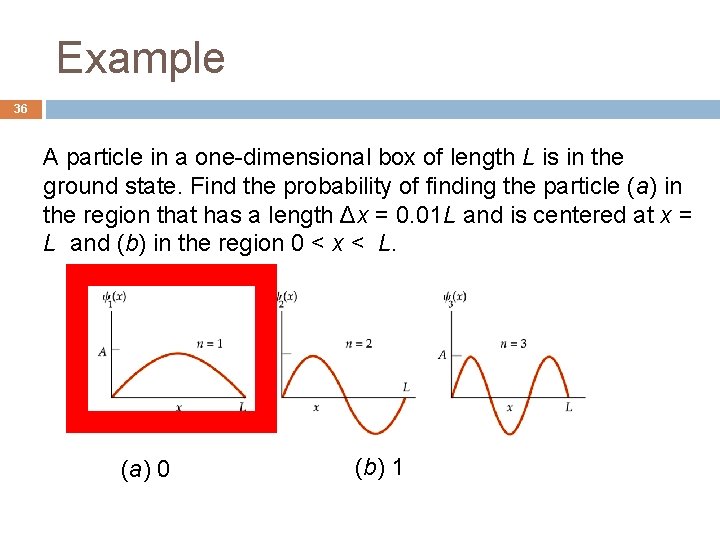
Example 36 A particle in a one-dimensional box of length L is in the ground state. Find the probability of finding the particle (a) in the region that has a length Δx = 0. 01 L and is centered at x = L and (b) in the region 0 < x < L. (a) 0 (b) 1

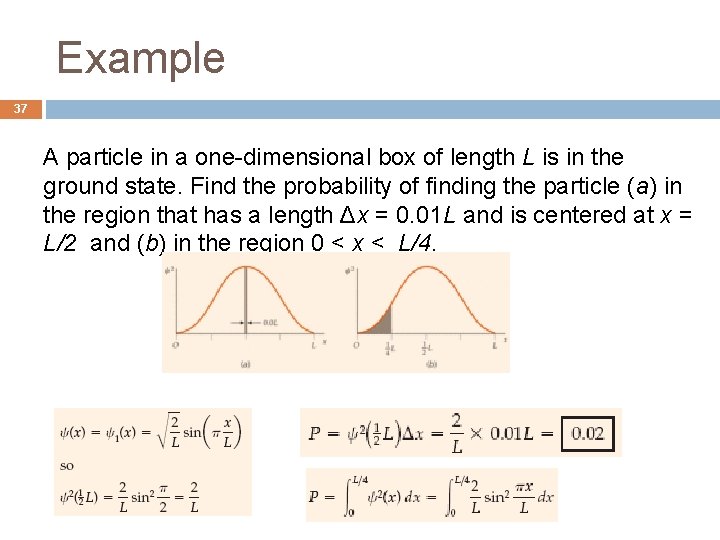
Example 37 A particle in a one-dimensional box of length L is in the ground state. Find the probability of finding the particle (a) in the region that has a length Δx = 0. 01 L and is centered at x = L/2 and (b) in the region 0 < x < L/4.

Example 3 38 The photons in a monochromatic beam are scattered by electrons. The wavelength of the photons that are scattered at an angle of 135° with the direction of the incident photon beam is 2. 3 percent more than the wavelength of the incident photons. a) b) What is the wavelength of the incident photons? What is the kinetic energy of the electron?

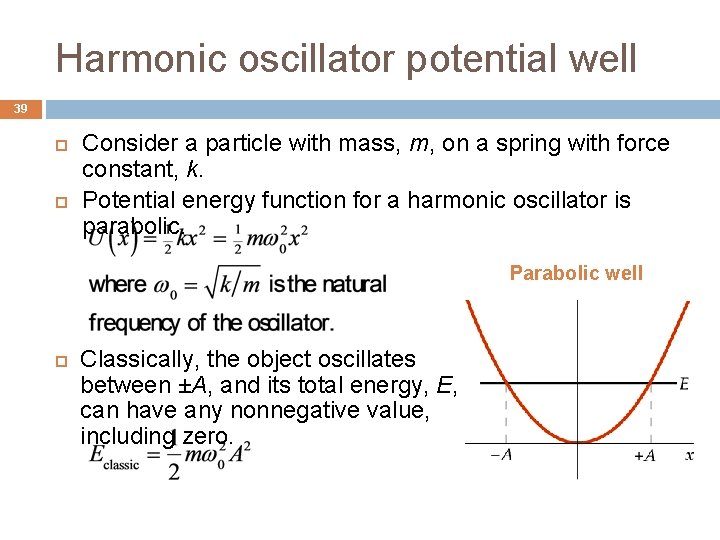
Harmonic oscillator potential well 39 Consider a particle with mass, m, on a spring with force constant, k. Potential energy function for a harmonic oscillator is parabolic. Parabolic well Classically, the object oscillates between ±A, and its total energy, E, can have any nonnegative value, including zero.

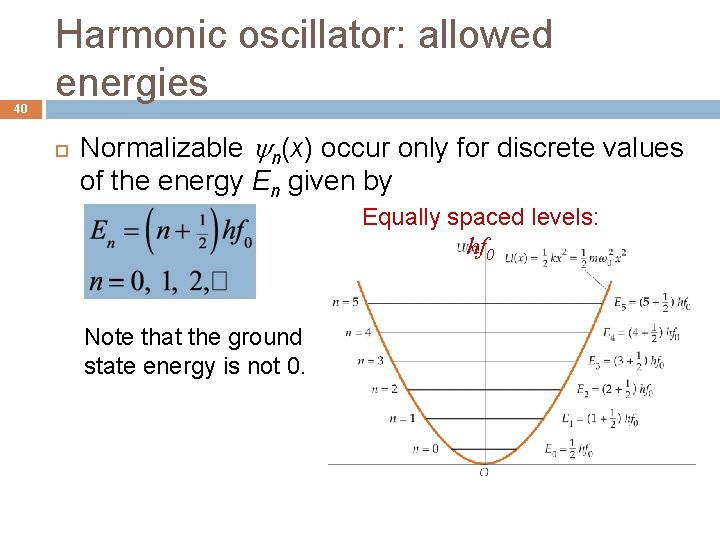
40 Harmonic oscillator: allowed energies Normalizable n(x) occur only for discrete values of the energy En given by Equally spaced levels: hf 0 Note that the ground state energy is not 0.

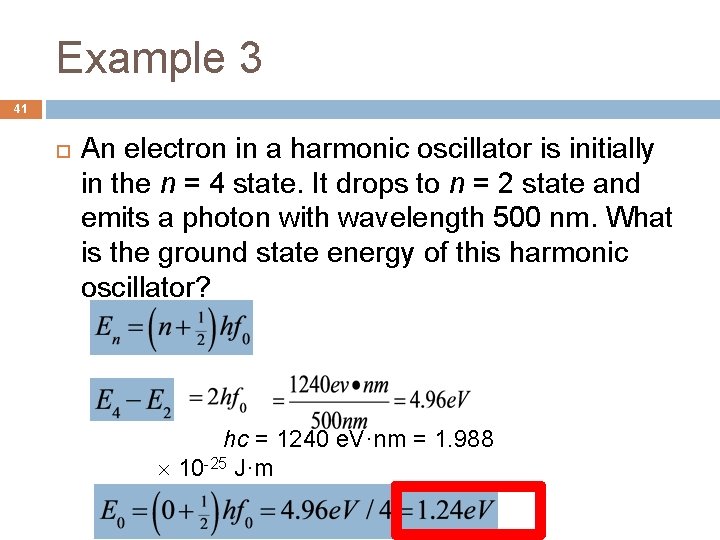
Example 3 41 An electron in a harmonic oscillator is initially in the n = 4 state. It drops to n = 2 state and emits a photon with wavelength 500 nm. What is the ground state energy of this harmonic oscillator? hc = 1240 e. V·nm = 1. 988 10 -25 J·m