Lecture 7 Orogeny Continental Dynamics and Regional Metamorphism












- Slides: 33

Lecture 7: Orogeny, Continental Dynamics, and Regional Metamorphism Questions: • What is the general age and tectonic structure of continents? • Why are the mobile belts on continental margins so wide, when oceanic plate boundaries are so narrow? What is this telling us about the rheology of continental lithosphere? • What is the relationship between orogenic events and regional metamorphism, and what can you learn by studying metamorphic rocks? Tools: • Continuum and fracture mechanics • Metamorphic petrology and thermodynamics (again)

Lecture 7: Continents and Orogeny • There is a general large-scale structure of continents: – Old stable cores surrounded by younger deformed belts 2

Continents and Orogeny • Stable continental regions, undeformed since precambrian time, are called cratons (particularly if Archean in age). Where precambrian crystalline (i. e. , igneous and metamorphic) rocks are exposed, that part of the craton is called a shield (example: Canadian shield). • Where the craton is covered by a relatively flat-lying undeformed sequence of paleozoic and later sediments, it is called a platform. Parts of platforms may experience prolonged subsidence and accumulate thick sedimentary basins. In between basins there may be regions (arches or domes) that have long stood relatively high and accumulated little sediment. 3

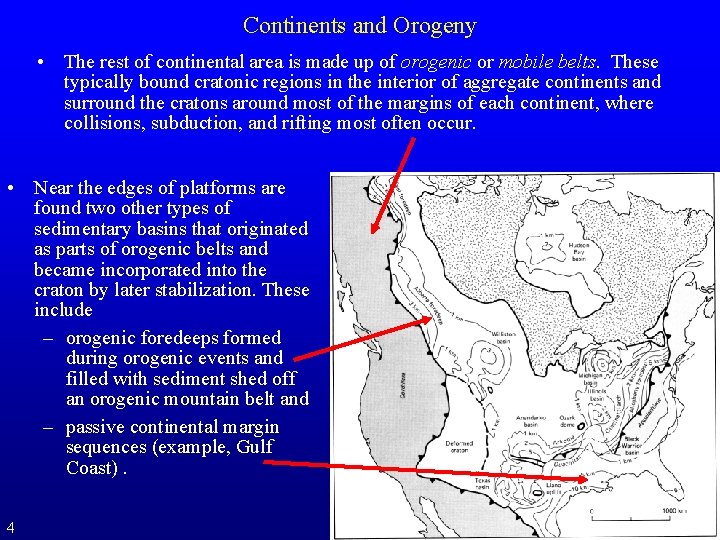
Continents and Orogeny • The rest of continental area is made up of orogenic or mobile belts. These typically bound cratonic regions in the interior of aggregate continents and surround the cratons around most of the margins of each continent, where collisions, subduction, and rifting most often occur. • Near the edges of platforms are found two other types of sedimentary basins that originated as parts of orogenic belts and became incorporated into the craton by later stabilization. These include – orogenic foredeeps formed during orogenic events and filled with sediment shed off an orogenic mountain belt and – passive continental margin sequences (example, Gulf Coast). 4

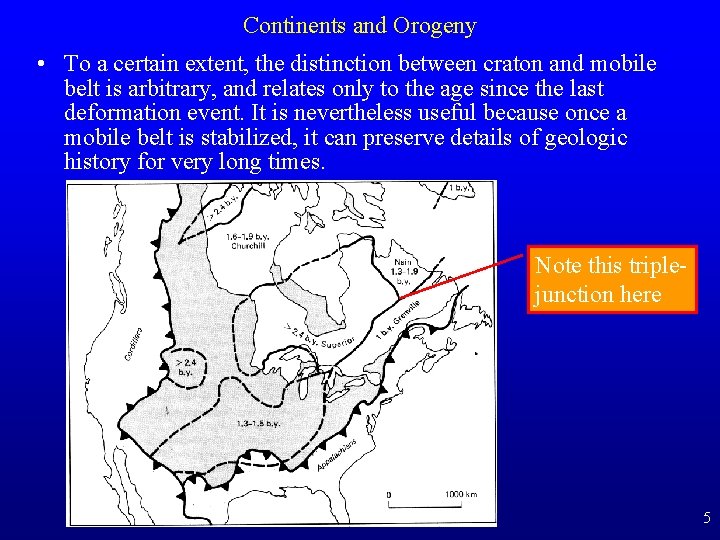
Continents and Orogeny • To a certain extent, the distinction between craton and mobile belt is arbitrary, and relates only to the age since the last deformation event. It is nevertheless useful because once a mobile belt is stabilized, it can preserve details of geologic history for very long times. Note this triplejunction here 5

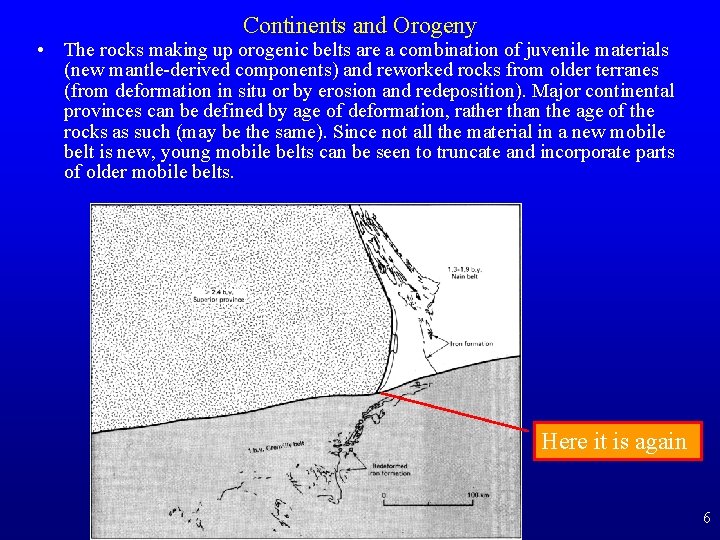
Continents and Orogeny • The rocks making up orogenic belts are a combination of juvenile materials (new mantle-derived components) and reworked rocks from older terranes (from deformation in situ or by erosion and redeposition). Major continental provinces can be defined by age of deformation, rather than the age of the rocks as such (may be the same). Since not all the material in a new mobile belt is new, young mobile belts can be seen to truncate and incorporate parts of older mobile belts. Here it is again 6

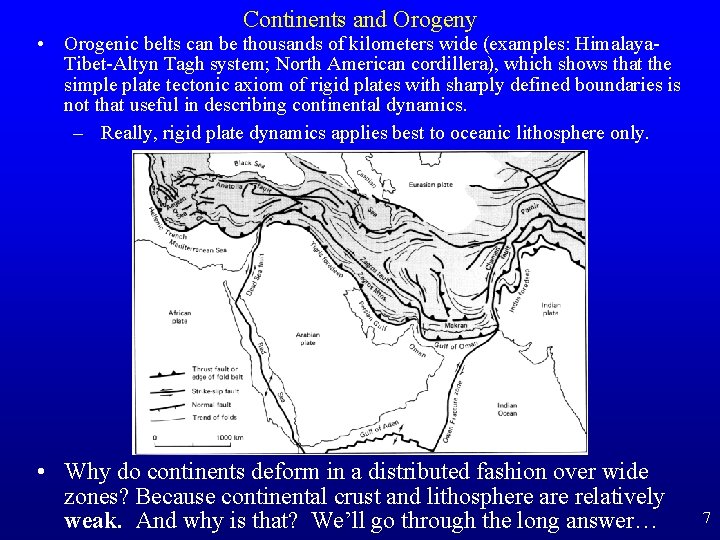
Continents and Orogeny • Orogenic belts can be thousands of kilometers wide (examples: Himalaya. Tibet-Altyn Tagh system; North American cordillera), which shows that the simple plate tectonic axiom of rigid plates with sharply defined boundaries is not that useful in describing continental dynamics. – Really, rigid plate dynamics applies best to oceanic lithosphere only. • Why do continents deform in a distributed fashion over wide zones? Because continental crust and lithosphere are relatively weak. And why is that? We’ll go through the long answer… 7

Rheology at Plate Scale • It is possible to find clear examples where obviously weak mechanical properties of crust contribute directly to distributed deformation, as in this picture of the Zagros fold-and-thrust belt, which is full of salt (the dark spots are where the salt layers have risen as buoyant, effectively fluid blobs called diapirs or salt domes (the image is 175 km across). • Broadly speaking, we can understand the difference between continents and oceans in this regard by considering the strength of granitic (quartz-dominated) and ultramafic (olivine-dominated) rock as functions of pressure and temperature… • This requires us to go into continuum mechanics, which describes how materials deform (strain) in response to applied forces (stress). 8

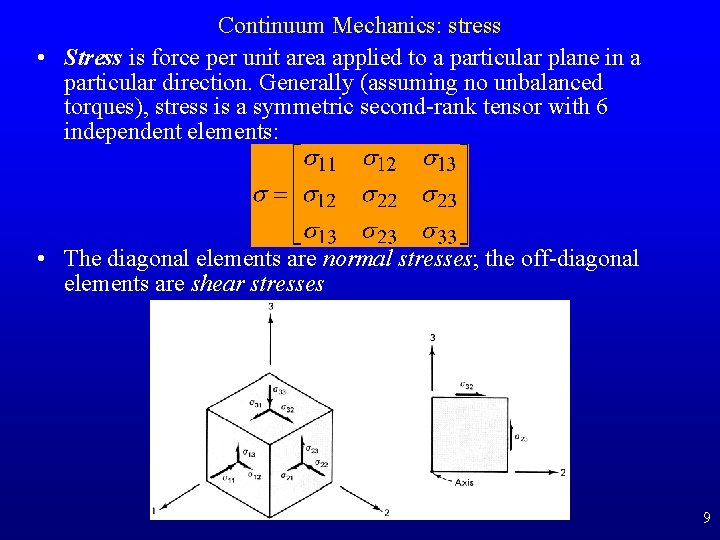
Continuum Mechanics: stress • Stress is force per unit area applied to a particular plane in a particular direction. Generally (assuming no unbalanced torques), stress is a symmetric second-rank tensor with 6 independent elements: • The diagonal elements are normal stresses; the off-diagonal elements are shear stresses 9

Continuum Mechanics: stress • We can always find a coordinate system in which the stress tensor is diagonal, which defines the stress ellipsoid, whose axes are the principal stresses s 1, s 2, s 3. – By convention, s 1 is the maximum compressive (positive) stress, s 2 is the intermediate stress, and s 3 is the minimum compressive or maximum tensile (negative) stress. • The trace of the stress tensor is independent of coordinate system and is three times the mean stress: – sm = (s 11+s 22+s 33)/3 = (s 1+s 2+s 3)/3. – IF AND ONLY IF the three principal stresses are equal and the shear stresses are all zero, we have a hydrostatic state of stress and the mean stress equals the pressure. – The stress tensor minus the diagonal mean stress tensor is the deviatoric stress tensor. Differential stress, s 1 -s 3, however, is a scalar. 10

Continuum Mechanics: strain • Strain, on the other hand, is the change in shape and size of a body during deformation. We exclude rigid-body translation and rotation from strain; only change in shape and change in size count 11

Continuum Mechanics: strain • Strain is always expressed in dimensionless terms. – So a change in length L of a line can be expressed by e = DL/L. – A change in volume V is expressed as DV/V. – A shear strain can be expressed by the perpendicular displacement of the end of a line over its length g = D/L or by an angular strain tan y = D/L. • In general, strain, like stress, is a second-rank tensor (e) with six independent elements – (in this case the antisymmetric component of deformation went into rotation, rather than the force balance argument for stress). – It can also be expressed by a principal strain ellipse in a suitable coordinate system and be decomposed into volumetric strain and shear strain. • The strain rate, or strain per unit time, is usually expressed. 12

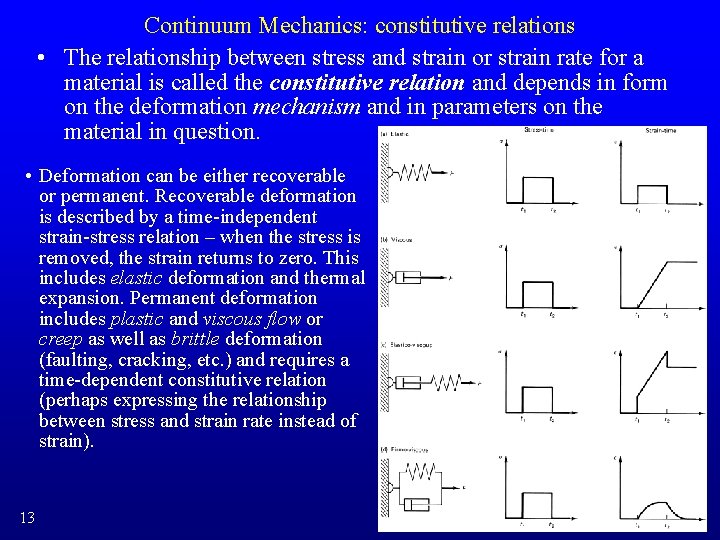
Continuum Mechanics: constitutive relations • The relationship between stress and strain or strain rate for a material is called the constitutive relation and depends in form on the deformation mechanism and in parameters on the material in question. • Deformation can be either recoverable or permanent. Recoverable deformation is described by a time-independent strain-stress relation – when the stress is removed, the strain returns to zero. This includes elastic deformation and thermal expansion. Permanent deformation includes plastic and viscous flow or creep as well as brittle deformation (faulting, cracking, etc. ) and requires a time-dependent constitutive relation (perhaps expressing the relationship between stress and strain rate instead of strain). 13

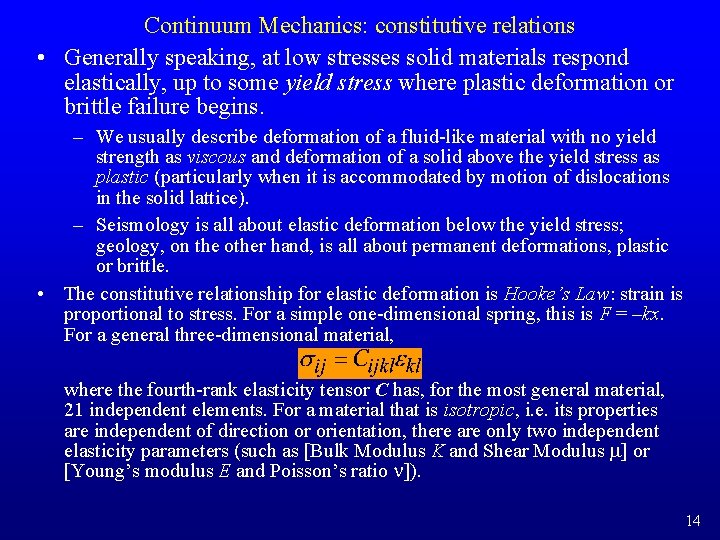
Continuum Mechanics: constitutive relations • Generally speaking, at low stresses solid materials respond elastically, up to some yield stress where plastic deformation or brittle failure begins. – We usually describe deformation of a fluid-like material with no yield strength as viscous and deformation of a solid above the yield stress as plastic (particularly when it is accommodated by motion of dislocations in the solid lattice). – Seismology is all about elastic deformation below the yield stress; geology, on the other hand, is all about permanent deformations, plastic or brittle. • The constitutive relationship for elastic deformation is Hooke’s Law: strain is proportional to stress. For a simple one-dimensional spring, this is F = –kx. For a general three-dimensional material, where the fourth-rank elasticity tensor C has, for the most general material, 21 independent elements. For a material that is isotropic, i. e. its properties are independent of direction or orientation, there are only two independent elasticity parameters (such as [Bulk Modulus K and Shear Modulus m] or [Young’s modulus E and Poisson’s ratio n]). 14

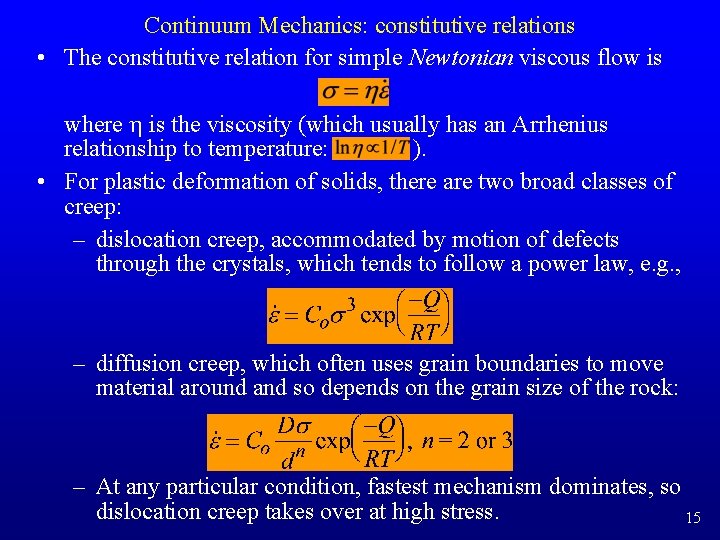
Continuum Mechanics: constitutive relations • The constitutive relation for simple Newtonian viscous flow is where h is the viscosity (which usually has an Arrhenius relationship to temperature: ). • For plastic deformation of solids, there are two broad classes of creep: – dislocation creep, accommodated by motion of defects through the crystals, which tends to follow a power law, e. g. , – diffusion creep, which often uses grain boundaries to move material around and so depends on the grain size of the rock: – At any particular condition, fastest mechanism dominates, so dislocation creep takes over at high stress. 15

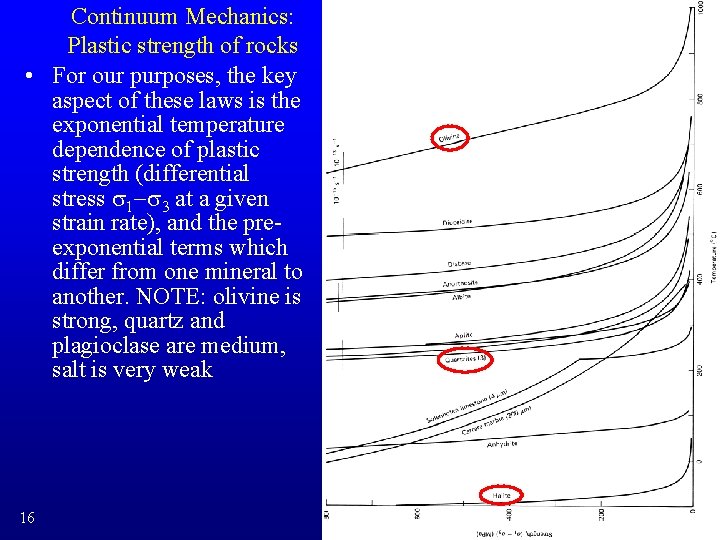
Continuum Mechanics: Plastic strength of rocks • For our purposes, the key aspect of these laws is the exponential temperature dependence of plastic strength (differential stress s 1 -s 3 at a given strain rate), and the preexponential terms which differ from one mineral to another. NOTE: olivine is strong, quartz and plagioclase are medium, salt is very weak 16

Continuum Mechanics: Brittle Failure • To complete a first-order understanding of the strength of crust and lithosphere, we need to venture into brittle rheology and fracture mechanics (briefly). • Whereas plastic flow is strongly temperature dependent (weaker at high T), brittle deformation is strongly pressure dependent (stronger at high P), since (1) most crack modes effectively require an increase in volume and (2) sliding is resisted by friction, which is proportional to normal stress. • Preview: since P and T increase together along a geotherm, any rock will be weaker with regard to brittle deformation at the surface of the earth and weaker with regard to plastic flow at large depth; the boundary between these regimes is called the brittle-plastic or brittle-ductile transition. Whichever mode is weaker controls the strength of the rock under given conditions. Please note: Brittle-ductile transition is NOT the same as lithosphere-asthenosphere boundary! 17

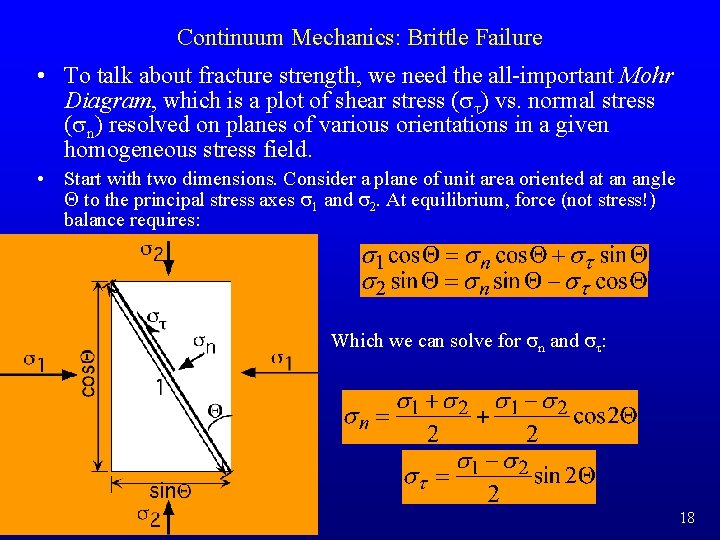
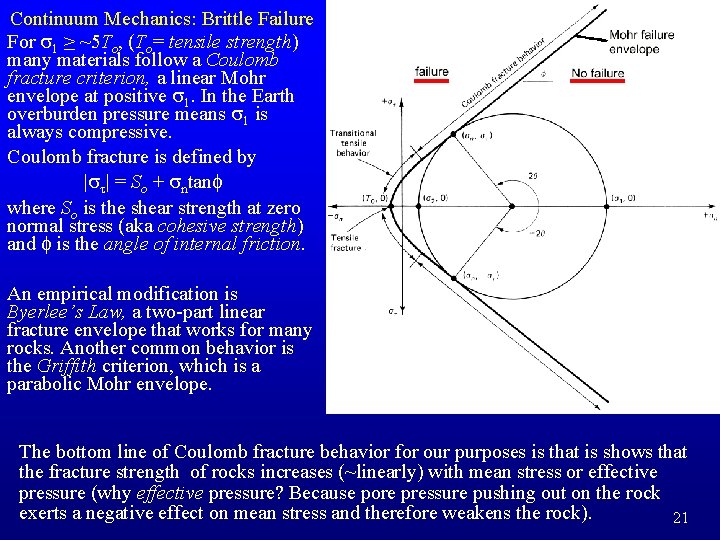
Continuum Mechanics: Brittle Failure • To talk about fracture strength, we need the all-important Mohr Diagram, which is a plot of shear stress (st) vs. normal stress (sn) resolved on planes of various orientations in a given homogeneous stress field. • Start with two dimensions. Consider a plane of unit area oriented at an angle Q to the principal stress axes s 1 and s 2. At equilibrium, force (not stress!) balance requires: Which we can solve for sn and st: 18

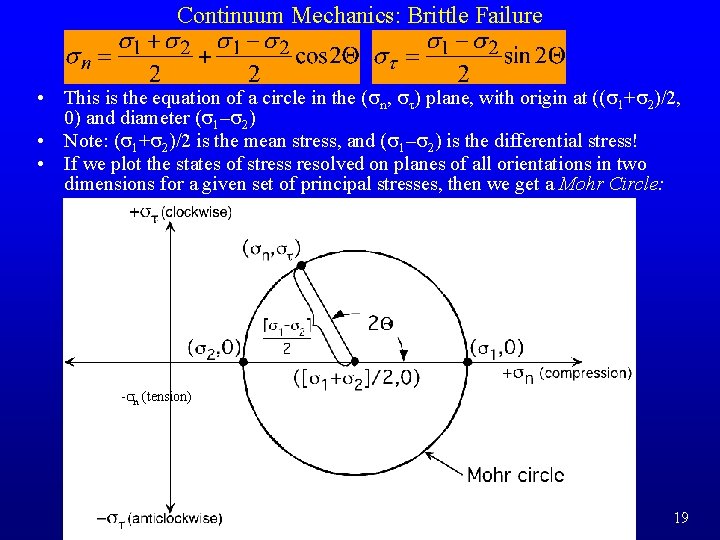
Continuum Mechanics: Brittle Failure • This is the equation of a circle in the (sn, st) plane, with origin at ((s 1+s 2)/2, 0) and diameter (s 1–s 2) • Note: (s 1+s 2)/2 is the mean stress, and (s 1–s 2) is the differential stress! • If we plot the states of stress resolved on planes of all orientations in two dimensions for a given set of principal stresses, then we get a Mohr Circle: -sn (tension) 19

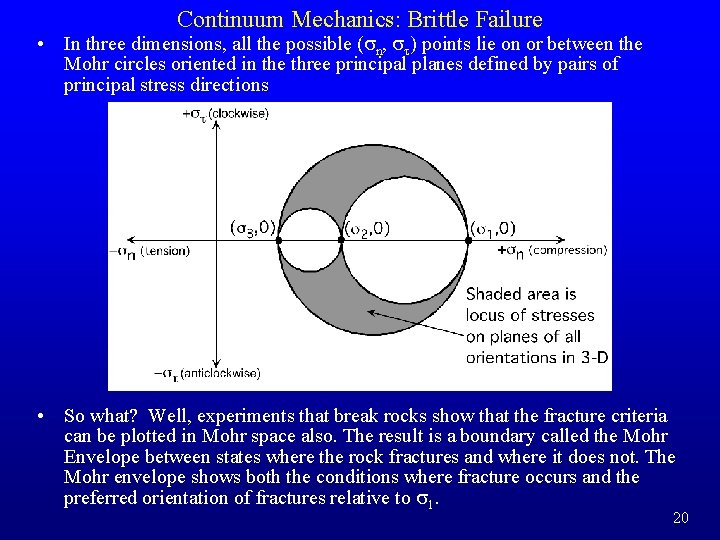
Continuum Mechanics: Brittle Failure • In three dimensions, all the possible (sn, st) points lie on or between the Mohr circles oriented in the three principal planes defined by pairs of principal stress directions • So what? Well, experiments that break rocks show that the fracture criteria can be plotted in Mohr space also. The result is a boundary called the Mohr Envelope between states where the rock fractures and where it does not. The Mohr envelope shows both the conditions where fracture occurs and the preferred orientation of fractures relative to s 1. 20

Continuum Mechanics: Brittle Failure For s 1 ≥ ~5 To, (To= tensile strength) many materials follow a Coulomb fracture criterion, a linear Mohr envelope at positive s 1. In the Earth overburden pressure means s 1 is always compressive. Coulomb fracture is defined by |st| = So + sntanf where So is the shear strength at zero normal stress (aka cohesive strength) and f is the angle of internal friction. An empirical modification is Byerlee’s Law, a two-part linear fracture envelope that works for many rocks. Another common behavior is the Griffith criterion, which is a parabolic Mohr envelope. The bottom line of Coulomb fracture behavior for our purposes is that is shows that the fracture strength of rocks increases (~linearly) with mean stress or effective pressure (why effective pressure? Because pore pressure pushing out on the rock exerts a negative effect on mean stress and therefore weakens the rock). 21

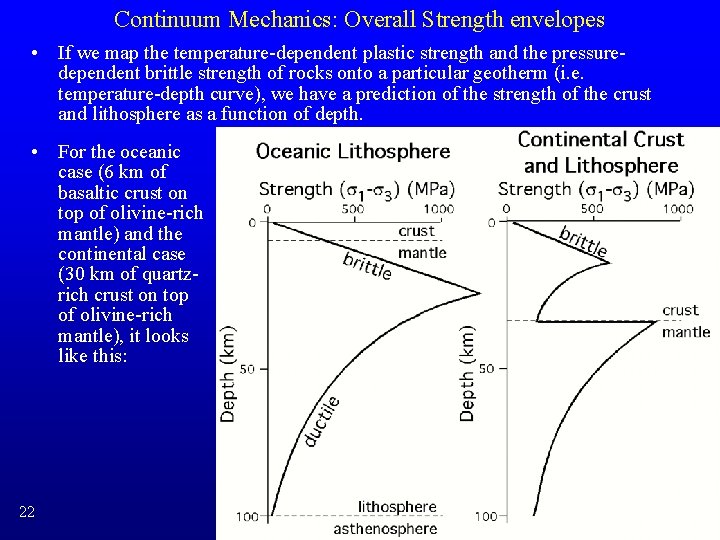
Continuum Mechanics: Overall Strength envelopes • If we map the temperature-dependent plastic strength and the pressuredependent brittle strength of rocks onto a particular geotherm (i. e. temperature-depth curve), we have a prediction of the strength of the crust and lithosphere as a function of depth. • For the oceanic case (6 km of basaltic crust on top of olivine-rich mantle) and the continental case (30 km of quartzrich crust on top of olivine-rich mantle), it looks like this: 22

Continuum Mechanics: Conclusion • So, why are oceanic plates rigid but continents undergo distributed deformation? • Because continental crust is thick and quartz has a weak plastic strength. Although thermal gradient in continents is lower, and at large depth the lithosphere is colder and stronger, what really matters is that we do not encounter olivine, which is strong in plastic deformation, until larger depth and therefore much higher temperature under continents. • We can also understand how strain concentration to plate boundaries works: – Mid-ocean ridges are weak because adiabatic rise of asthenosphere brings the hot, weak plastic domain almost to the surface; the brittle layer is only ~2 km thick – Subduction zones may be weak because high fluid pressures lower the mean stress across their faults and promote brittle behavior to large depths. 23

Regional Metamorphism • One major consequence of continental deformation is regional metamorphism. – Orogenic events drive vertical motions and departures from stable conductive geothermal gradients. Shallow crust is deeply buried under nonhydrostatic stress and undergoes coupled chemical reaction and ductile deformation. The same event at later stages may uplift deep crust into mountain ranges where erosion can unroof it for geologists to view. – Generally, in map view the surface will expose rocks of a variety of metamorphic grades (i. e. , peak P and T), either because of differential uplift or because igneous activity heated rocks close to the core of the orogeny. The sequence of metamorphic grades exposed across a terrain is called the metamorphic field gradient and is characteristic of the type of orogeny. • we have already seen the blueschist path of low-T, high-P metamorphism leading to eclogite facies, associated with the forearc of subduction zones. • In the arc itself, the dominant process is heating by large scale igneous activity, and we see a relatively high-T path leading to granulite facies. • In collisional mountain belts, burial is dominant and what results is an intermediate P-T path called the Barrovian sequence 24

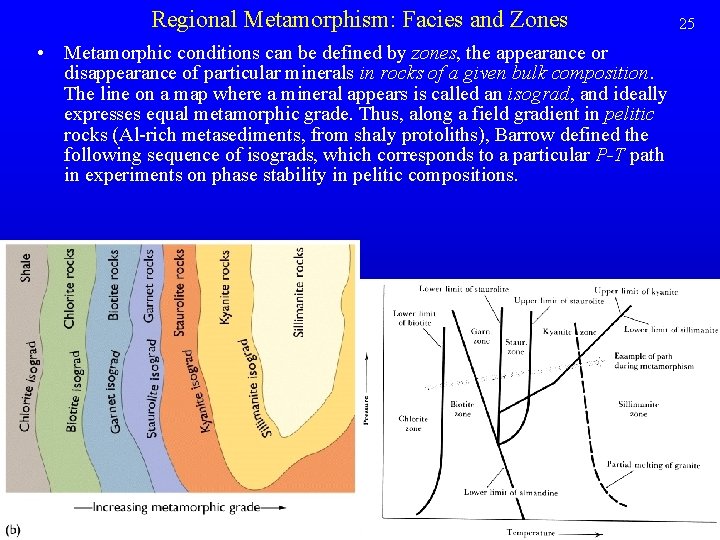
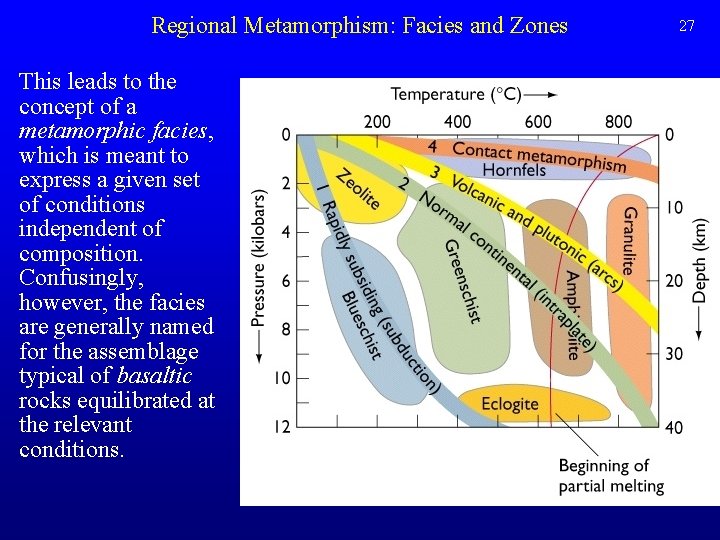
Regional Metamorphism: Facies and Zones • Metamorphic conditions can be defined by zones, the appearance or disappearance of particular minerals in rocks of a given bulk composition. The line on a map where a mineral appears is called an isograd, and ideally expresses equal metamorphic grade. Thus, along a field gradient in pelitic rocks (Al-rich metasediments, from shaly protoliths), Barrow defined the following sequence of isograds, which corresponds to a particular P-T path in experiments on phase stability in pelitic compositions. 25

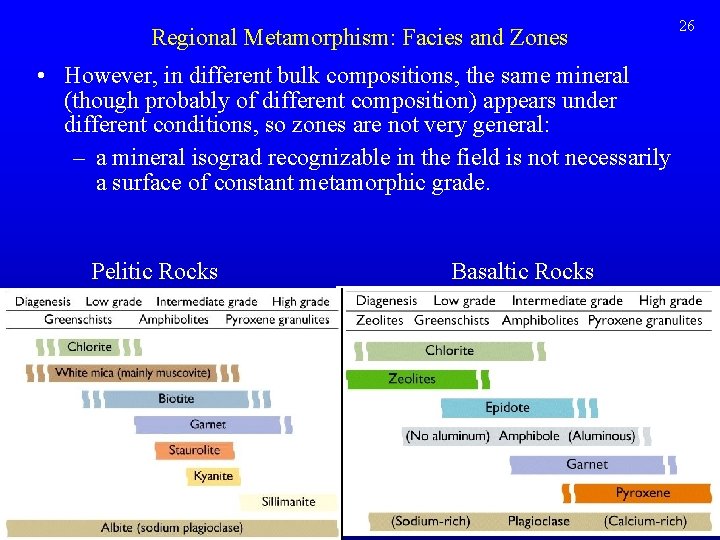
Regional Metamorphism: Facies and Zones • However, in different bulk compositions, the same mineral (though probably of different composition) appears under different conditions, so zones are not very general: – a mineral isograd recognizable in the field is not necessarily a surface of constant metamorphic grade. Pelitic Rocks Basaltic Rocks 26

Regional Metamorphism: Facies and Zones This leads to the concept of a metamorphic facies, which is meant to express a given set of conditions independent of composition. Confusingly, however, the facies are generally named for the assemblage typical of basaltic rocks equilibrated at the relevant conditions. 27

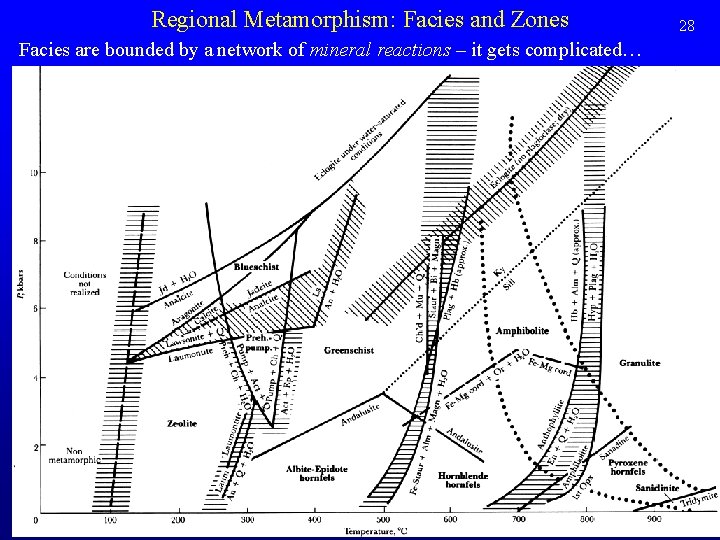
Regional Metamorphism: Facies and Zones Facies are bounded by a network of mineral reactions – it gets complicated… 28

Mineral reactions and geothermobarometry • Some mineral reactions precisely indicate particular P-T conditions, especially those involving pure phases. – Thus: the andalusite-kyanite-sillimanite triple point and univariant reactions are based on the stable structures of the pure aluminosilicate (Al 2 Si. O 5) phases. No other constituents dissolve in these minerals, so nothing except kinetics affects the reactions. • Most reactions involve phases of variable composition and hence it is necessary to measure phase compositions and use thermodynamic reasoning to interpret the results in terms of P and T. – A metamorphic assemblage can be bracketed into a given region of P-T space using the mineral reactions that bound the stability of the observed assemblage. Continuous mineral reactions involving solutions are used to quantify T or P. – A reaction that is very T-sensitive and relatively P-insensitive makes a good geothermometer. A reaction that is P- sensitive and relatively Tinsensitive makes a good geobarometer. A combination of (at least) two such reactions yields a thermobarometer, an estimate of T and P. 29

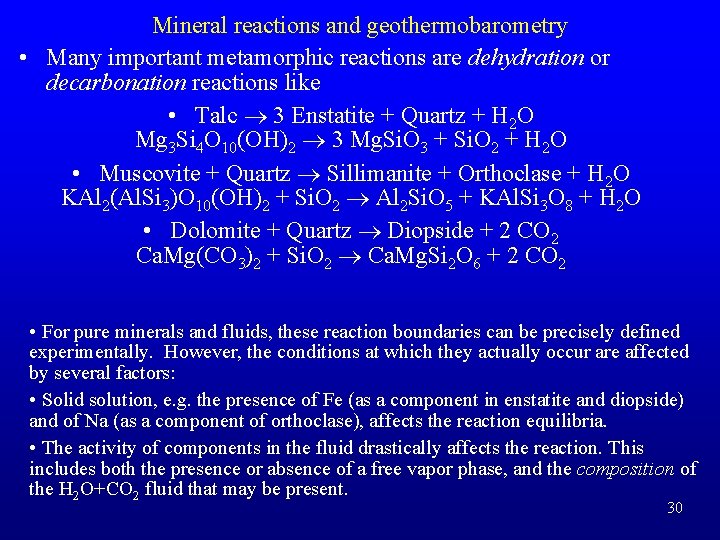
Mineral reactions and geothermobarometry • Many important metamorphic reactions are dehydration or decarbonation reactions like • Talc ® 3 Enstatite + Quartz + H 2 O Mg 3 Si 4 O 10(OH)2 ® 3 Mg. Si. O 3 + Si. O 2 + H 2 O • Muscovite + Quartz ® Sillimanite + Orthoclase + H 2 O KAl 2(Al. Si 3)O 10(OH)2 + Si. O 2 ® Al 2 Si. O 5 + KAl. Si 3 O 8 + H 2 O • Dolomite + Quartz ® Diopside + 2 CO 2 Ca. Mg(CO 3)2 + Si. O 2 ® Ca. Mg. Si 2 O 6 + 2 CO 2 • For pure minerals and fluids, these reaction boundaries can be precisely defined experimentally. However, the conditions at which they actually occur are affected by several factors: • Solid solution, e. g. the presence of Fe (as a component in enstatite and diopside) and of Na (as a component of orthoclase), affects the reaction equilibria. • The activity of components in the fluid drastically affects the reaction. This includes both the presence or absence of a free vapor phase, and the composition of the H 2 O+CO 2 fluid that may be present. 30

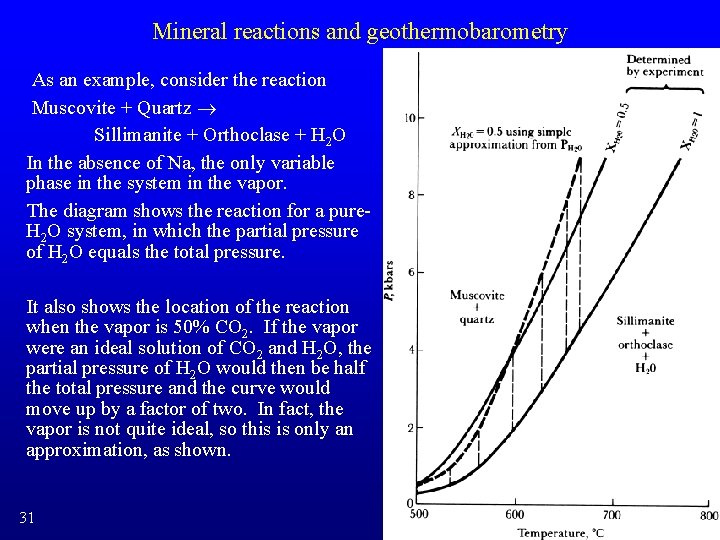
Mineral reactions and geothermobarometry As an example, consider the reaction Muscovite + Quartz ® Sillimanite + Orthoclase + H 2 O In the absence of Na, the only variable phase in the system in the vapor. The diagram shows the reaction for a pure. H 2 O system, in which the partial pressure of H 2 O equals the total pressure. It also shows the location of the reaction when the vapor is 50% CO 2. If the vapor were an ideal solution of CO 2 and H 2 O, the partial pressure of H 2 O would then be half the total pressure and the curve would move up by a factor of two. In fact, the vapor is not quite ideal, so this is only an approximation, as shown. 31

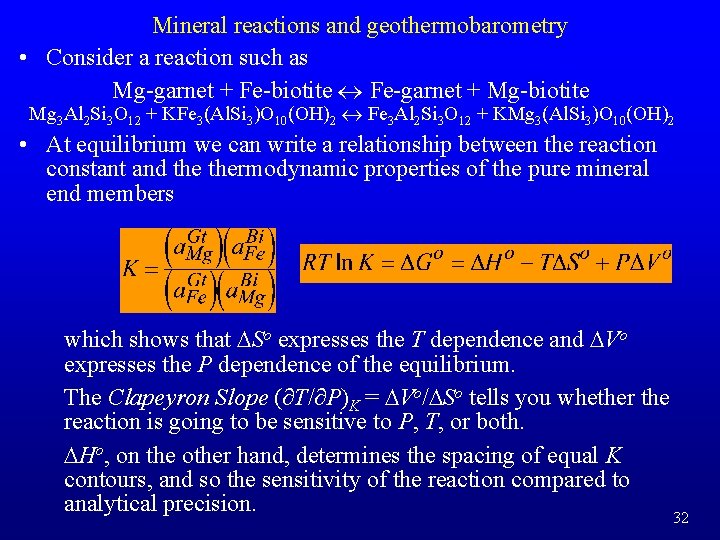
Mineral reactions and geothermobarometry • Consider a reaction such as Mg-garnet + Fe-biotite « Fe-garnet + Mg-biotite Mg 3 Al 2 Si 3 O 12 + KFe 3(Al. Si 3)O 10(OH)2 « Fe 3 Al 2 Si 3 O 12 + KMg 3(Al. Si 3)O 10(OH)2 • At equilibrium we can write a relationship between the reaction constant and thermodynamic properties of the pure mineral end members which shows that DSo expresses the T dependence and DVo expresses the P dependence of the equilibrium. The Clapeyron Slope (∂T/∂P)K = DVo/DSo tells you whether the reaction is going to be sensitive to P, T, or both. DHo, on the other hand, determines the spacing of equal K contours, and so the sensitivity of the reaction compared to analytical precision. 32

Mineral reactions and geothermobarometry • For garnet-biotite Fe-Mg exchange, – DVo should be small, since Fe and Mg fit in the same sites with little volume strain of the lattice. – DSo should be relatively big because of Fe-Mg ordering phenomena. – Indeed, the calibrated geothermometer equation in this case is 3 RTln. K = – 12454 cal + (4. 662 cal/K)T – (0. 057 cal/bar)P So a measured K of 0. 222, for example • If at 5 kbar, implies T = 661 °C • If at 10 kbar, implies T = 682 °C Relative to typical T uncertainty of ± 50 °C quoted for most geothermometers, this is indeed insensitive to pressure. • The opposite case could be, e. g. , Ca exchange between garnet and plagioclase, which has a big volume change due to coupled Ca-Al subsitution and so is a good barometer. 33