Lecture 7 IC V Nb Ta Group 14

Lecture 7 (IC) V, Nb, Ta. Group 14 (Part 1)
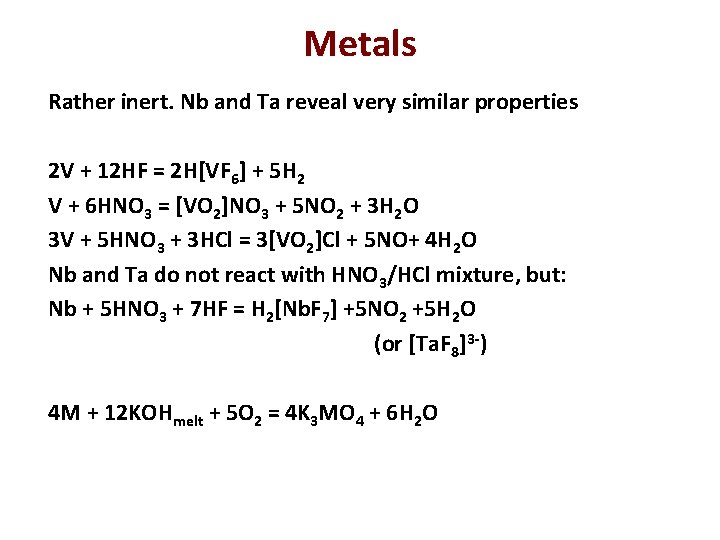
Metals Rather inert. Nb and Ta reveal very similar properties 2 V + 12 HF = 2 H[VF 6] + 5 H 2 V + 6 HNO 3 = [VO 2]NO 3 + 5 NO 2 + 3 H 2 O 3 V + 5 HNO 3 + 3 HCl = 3[VO 2]Cl + 5 NO+ 4 H 2 O Nb and Ta do not react with HNO 3/HCl mixture, but: Nb + 5 HNO 3 + 7 HF = H 2[Nb. F 7] +5 NO 2 +5 H 2 O (or [Ta. F 8]3 -) 4 M + 12 KOHmelt + 5 O 2 = 4 K 3 MO 4 + 6 H 2 O
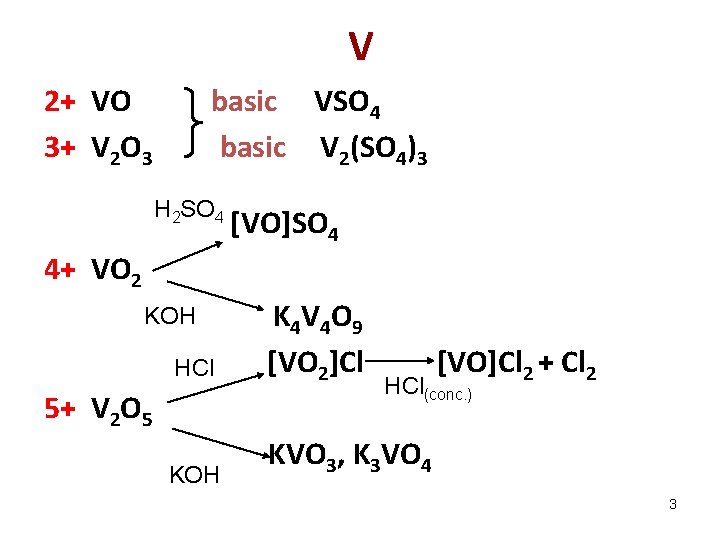
V 2+ VO 3+ V 2 O 3 basic VSO 4 basic V 2(SO 4)3 H 2 SO 4 [VO]SO 4 4+ VO 2 HCl K 4 V 4 O 9 [VO 2]Cl KOH KVO 3, K 3 VO 4 KOH 5+ V 2 O 5 [VO]Cl 2 + Cl 2 HCl(conc. ) 3
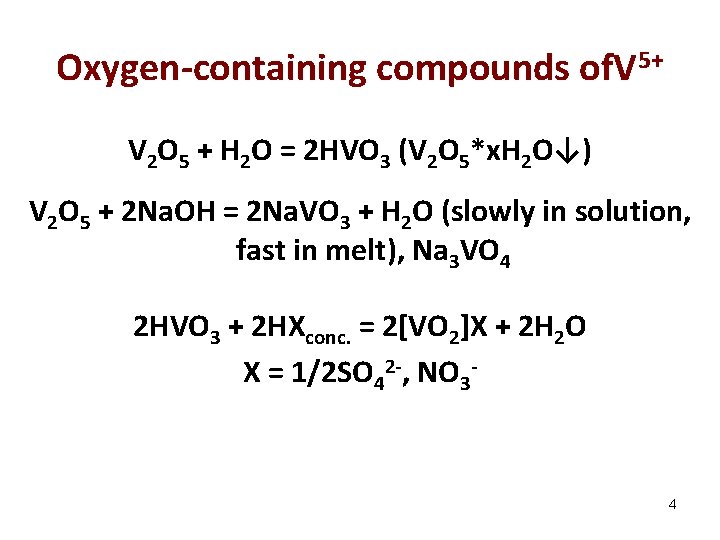
Oxygen-containing compounds of. V 5+ V 2 O 5 + H 2 O = 2 HVO 3 (V 2 O 5*x. H 2 O↓) V 2 O 5 + 2 Na. OH = 2 Na. VO 3 + H 2 O (slowly in solution, fast in melt), Na 3 VO 4 2 HVO 3 + 2 HXconc. = 2[VO 2]X + 2 H 2 O X = 1/2 SO 42 -, NO 3 - 4
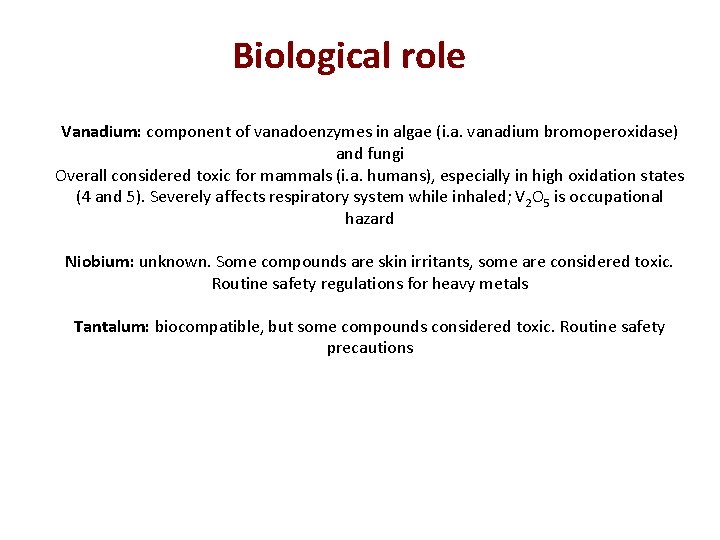
Biological role Vanadium: component of vanadoenzymes in algae (i. a. vanadium bromoperoxidase) and fungi Overall considered toxic for mammals (i. a. humans), especially in high oxidation states (4 and 5). Severely affects respiratory system while inhaled; V 2 O 5 is occupational hazard Niobium: unknown. Some compounds are skin irritants, some are considered toxic. Routine safety regulations for heavy metals Tantalum: biocompatible, but some compounds considered toxic. Routine safety precautions
Occurence C – 11 th (CO 2, Ca. CO 3, coal, crude oil and gas, living creatures) Si – 2 nd (Si. O 2 (sand), silicates = soil, rocks, ores Ge – 54 th, Cu 3 Ge. S 4 (germanite) Sn – 27 th, Sn. O 2 (cassiterite) Pb – 60 th, Pb. S (halenite), Pb. SO 4, Pb. CO 3 etc 6
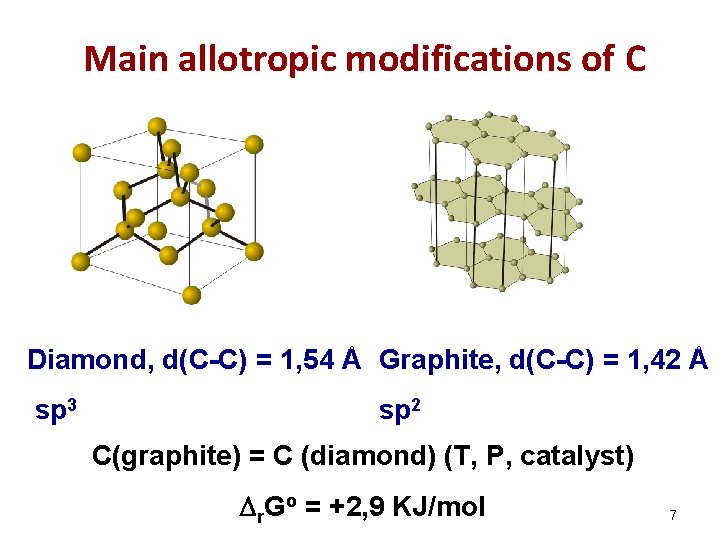
Main allotropic modifications of С Diamond, d(С-С) = 1, 54 Å Graphite, d(С-С) = 1, 42 Å sp 3 sp 2 С(graphite) = С (diamond) (T, P, catalyst) r. Go = +2, 9 KJ/mol 7

Carbides General formula: (metal)x. Cy, C has a negative ox. state Ionic: with aluminum, alkali and alkali earth metals Metal-like – d- and f-element. Solid, conductive, very hard Covalent – B 4 C and Si. С (has diamond-type structure, extremely hard) Ionic carbides can be methanides (Be 2 C, Al 4 C 3) and acetylenides (MIIC 2, MI 2 C 2) 2 Al 2 O 3 + 9 C = Al 4 C 3 + 6 CO (high Т) Ca. O + 3 C = Ca. C 2 + CO (high Т) Al 4 C 3 + 12 H 2 O = 4 Al(OH)3 + 3 CH 4 Ca. C 2 + 2 H 2 O = Ca(OH)2 + C 2 H 2 8
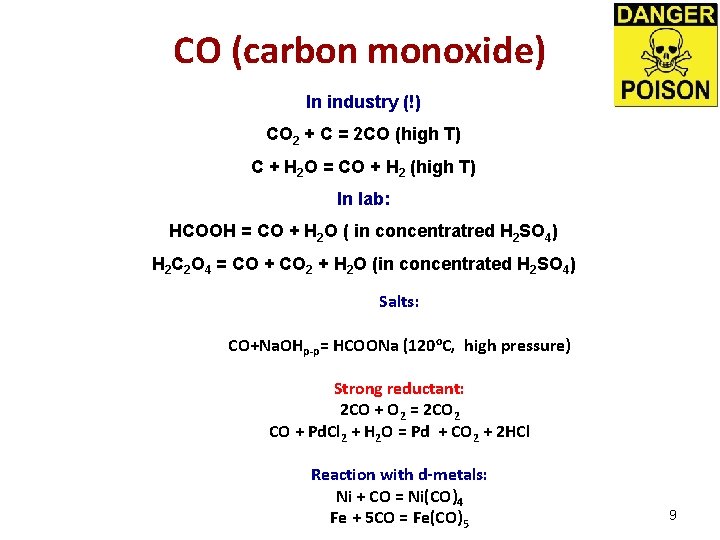
СО (carbon monoxide) In industry (!) CO 2 + C = 2 CO (high Т) С + H 2 O = CO + H 2 (high Т) In lab: HCOOH = СO + H 2 O ( in concentratred H 2 SO 4) H 2 C 2 O 4 = СO + CO 2 + H 2 O (in concentrated H 2 SO 4) Salts: CO+Na. OHр-р= HCOONa (120 o. C, high pressure) Strong reductant: 2 CO + O 2 = 2 CO 2 СO + Pd. Cl 2 + H 2 O = Pd + CO 2 + 2 HCl Reaction with d-metals: Ni + CO = Ni(CO)4 Fe + 5 CO = Fe(CO)5 9
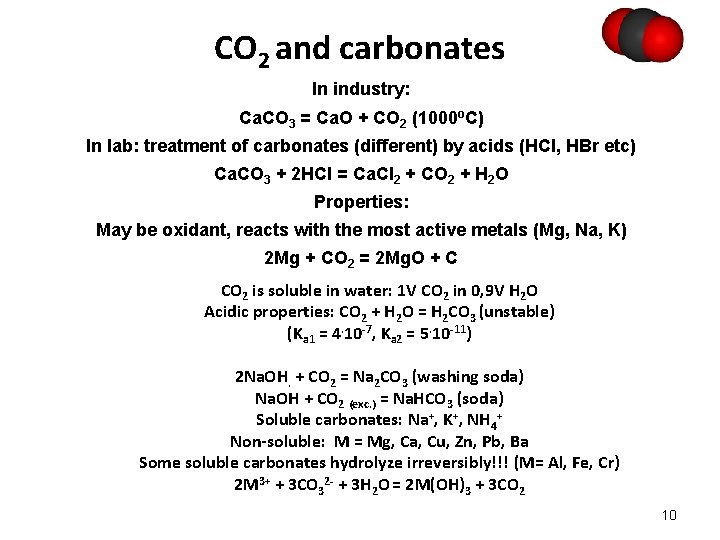
СO 2 and carbonates In industry: Сa. CO 3 = Ca. O + CO 2 (1000 o. C) In lab: treatment of carbonates (different) by acids (HCl, HBr etc) Ca. CO 3 + 2 HCl = Ca. Cl 2 + CO 2 + H 2 O Properties: May be oxidant, reacts with the most active metals (Mg, Na, K) 2 Mg + CO 2 = 2 Mg. O + C CO 2 is soluble in water: 1 V CO 2 in 0, 9 V H 2 O Acidic properties: СO 2 + H 2 O = H 2 CO 3 (unstable) (Ka 1 = 4. 10 -7, Ka 2 = 5. 10 -11) 2 Na. OH. + CO 2 = Na 2 CO 3 (washing soda) Na. OH + CO 2 (exc. ) = Na. HCO 3 (soda) Soluble carbonates: Na+, K+, NH 4+ Non-soluble: M = Mg, Ca, Cu, Zn, Pb, Ba Some soluble carbonates hydrolyze irreversibly!!! (M= Al, Fe, Cr) 2 M 3+ + 3 CO 32 - + 3 H 2 O = 2 M(OH)3 + 3 CO 2 10
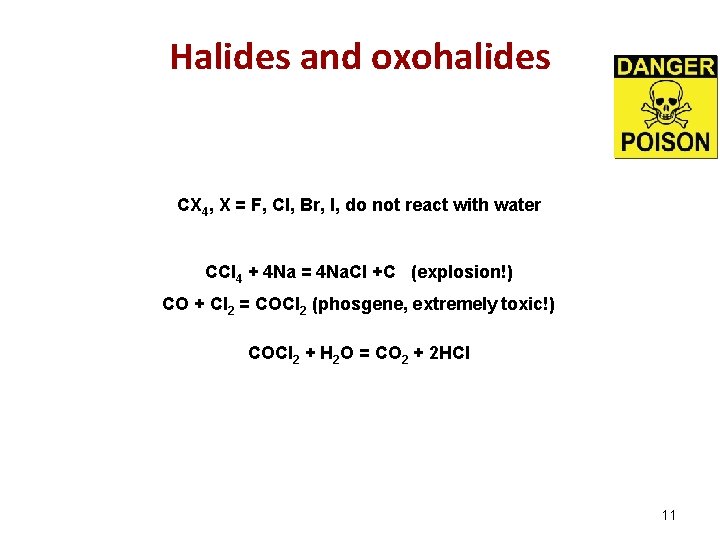
Halides and oxohalides CX 4, X = F, Cl, Br, I, do not react with water СCl 4 + 4 Na = 4 Na. Cl +C (explosion!) СO + Cl 2 = COCl 2 (phosgene, extremely toxic!) COCl 2 + H 2 O = CO 2 + 2 HCl 11

S-containing compounds CS 2 carbon disulfide. Industrial and lab use: solvent Flammable, poison!!! C + 2 S = CS 2 ( in industry, high T ) CS 2 + K 2 S = K 2 CS 3 thiocarbonate K 2 CS 3 + 2 HCl = 2 KCl + H 2 CS 3 Thiocarbonic acid Ka 1 = 2. 10 -3, Ka 2 = 7. 10 -9 H 2 CS 3 = H 2 S + CS 2 (slowly, when heated) 12
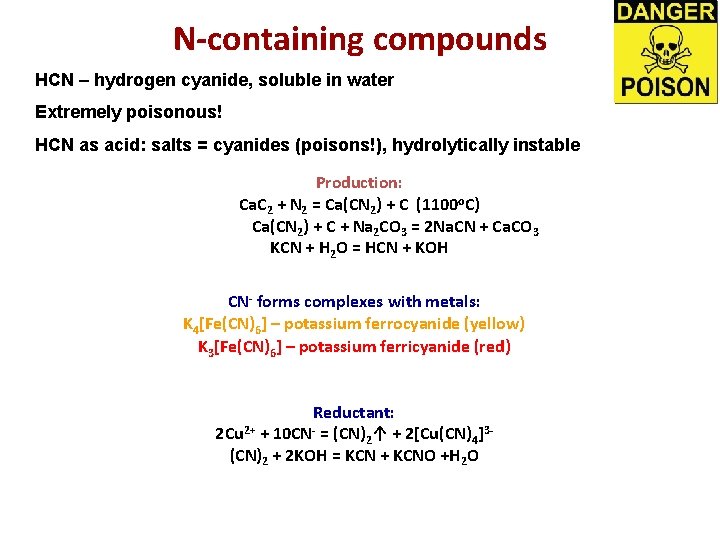
N-containing compounds HCN – hydrogen cyanide, soluble in water Extremely poisonous! HCN as acid: salts = cyanides (poisons!), hydrolytically instable Production: Ca. C 2 + N 2 = Ca(CN 2) + C (1100 o. C) Ca(CN 2) + C + Na 2 CO 3 = 2 Na. CN + Ca. CO 3 KCN + H 2 O = HCN + KOH CN- forms complexes with metals: K 4[Fe(CN)6] – potassium ferrocyanide (yellow) K 3[Fe(CN)6] – potassium ferricyanide (red) Reductant: 2 Cu 2+ + 10 CN- = (CN)2↑ + 2[Cu(CN)4]3(CN)2 + 2 KOH = KCN + KCNO +H 2 O
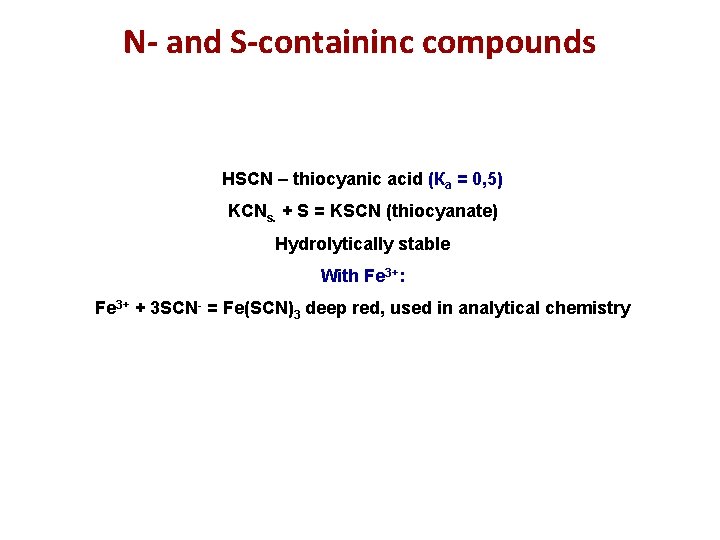
N- and S-containinc compounds HSCN – thiocyanic acid (Ка = 0, 5) KCNs. + S = KSCN (thiocyanate) Hydrolytically stable With Fe 3+: Fe 3+ + 3 SCN- = Fe(SCN)3 deep red, used in analytical chemistry
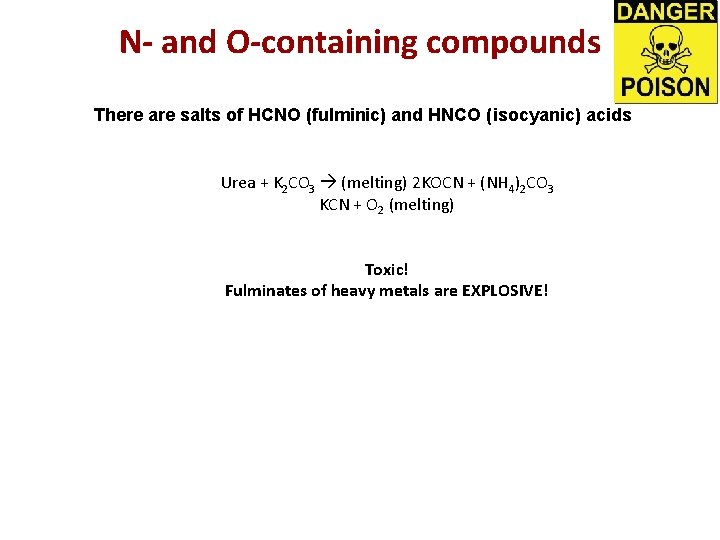
N- and O-containing compounds There are salts of HCNO (fulminic) and HNCO (isocyanic) acids Urea + K 2 CO 3 (melting) 2 KOCN + (NH 4)2 CO 3 KCN + O 2 (melting) Toxic! Fulminates of heavy metals are EXPLOSIVE!
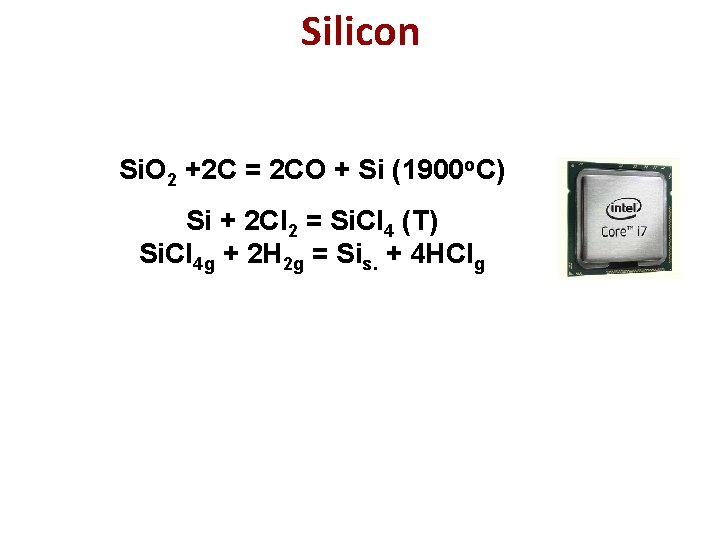
Silicon Si. O 2 +2 C = 2 CO + Si (1900 o. C) Si + 2 Cl 2 = Si. Cl 4 (Т) Si. Cl 4 g + 2 H 2 g = Sis. + 4 HClg
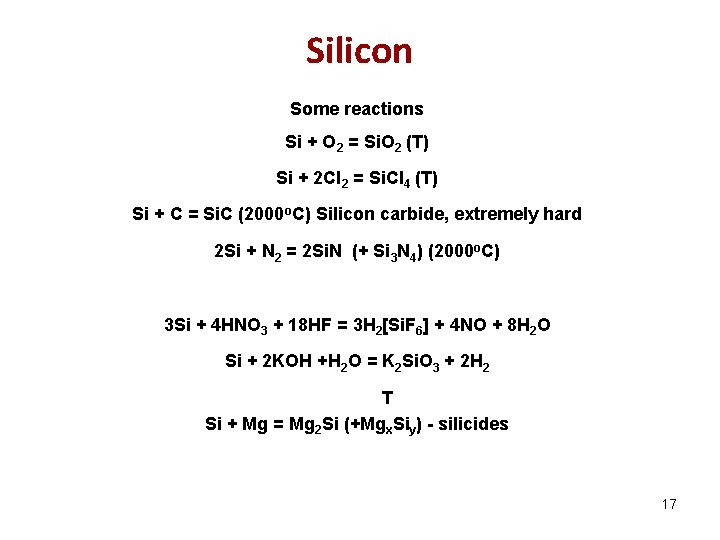
Silicon Some reactions Si + O 2 = Si. O 2 (Т) Si + 2 Cl 2 = Si. Cl 4 (Т) Si + C = Si. C (2000 o. C) Silicon carbide, extremely hard 2 Si + N 2 = 2 Si. N (+ Si 3 N 4) (2000 o. C) 3 Si + 4 HNO 3 + 18 HF = 3 H 2[Si. F 6] + 4 NO + 8 H 2 O Si + 2 KOH +H 2 O = K 2 Si. O 3 + 2 H 2 T Si + Mg = Mg 2 Si (+Mgx. Siy) - silicides 17
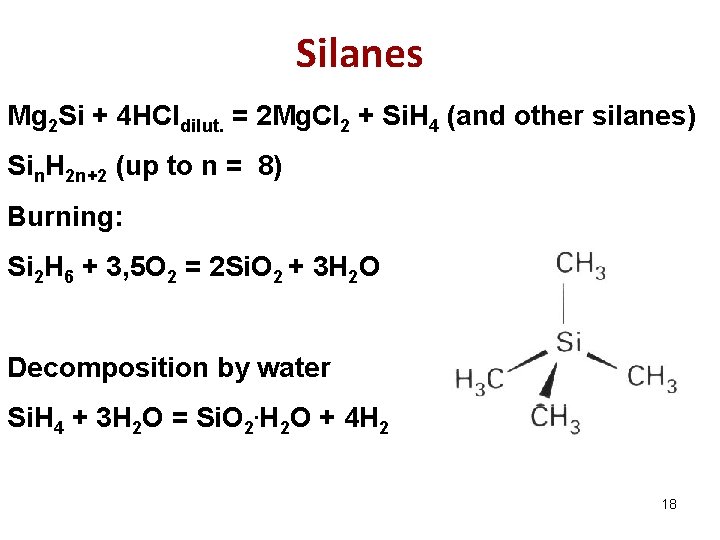
Silanes Mg 2 Si + 4 HCldilut. = 2 Mg. Cl 2 + Si. H 4 (and other silanes) Sin. H 2 n+2 (up to n = 8) Burning: Si 2 H 6 + 3, 5 O 2 = 2 Si. O 2 + 3 H 2 O Decomposition by water Si. H 4 + 3 H 2 O = Si. O 2. H 2 O + 4 H 2 18
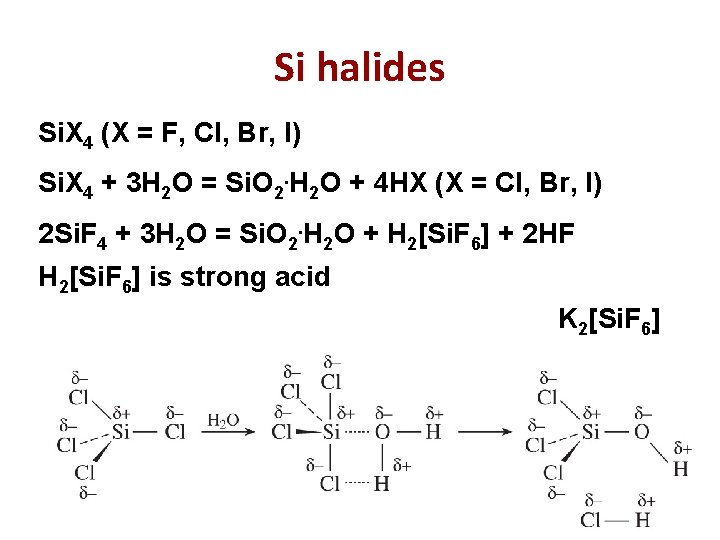
Si halides Si. X 4 (X = F, Cl, Br, I) Si. X 4 + 3 H 2 O = Si. O 2. H 2 O + 4 HX (X = Cl, Br, I) 2 Si. F 4 + 3 H 2 O = Si. O 2. H 2 O + H 2[Si. F 6] + 2 HF H 2[Si. F 6] is strong acid K 2[Si. F 6] 19

Si. O 2 1) Non-soluble in water and most of acids, except of HF: 2) Si. O 2 + 6 HF = H 2[Si. F 6] + 2 H 2 O 3) Si. O 2 + 2 Na. OHs. = Na 2 Si. O 3 + H 2 O (melting) Silicic acids: meta H 2 Si. O 3, orto H 4 Si. O 4 Non-soluble in water! Na 2 Si. O 3 + HCl x. Si. O 2. y. H 2 O + Na. Cl (гель) Salts (soluble): K 2 Si. O 3, Na 2 Si. O 3 (“liquid glass”). Non-soluble – all natural silicates 20
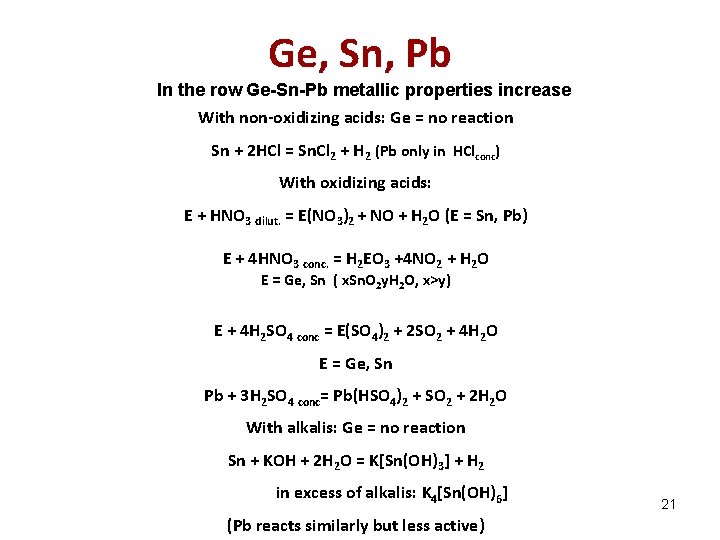
Ge, Sn, Pb In the row Ge-Sn-Pb metallic properties increase With non-oxidizing acids: Ge = no reaction Sn + 2 HCl = Sn. Cl 2 + H 2 (Pb only in HClconc) With oxidizing acids: E + HNO 3 dilut. = E(NO 3)2 + NO + H 2 O (E = Sn, Pb) E + 4 HNO 3 conc. = H 2 EO 3 +4 NO 2 + H 2 O E = Ge, Sn ( x. Sn. O 2 y. H 2 O, x>y) E + 4 H 2 SO 4 conc = E(SO 4)2 + 2 SO 2 + 4 H 2 O E = Ge, Sn Pb + 3 H 2 SO 4 conc= Pb(HSO 4)2 + SO 2 + 2 H 2 O With alkalis: Ge = no reaction Sn + KOH + 2 H 2 O = K[Sn(OH)3] + H 2 in excess of alkalis: K 4[Sn(OH)6] (Pb reacts similarly but less active) 21
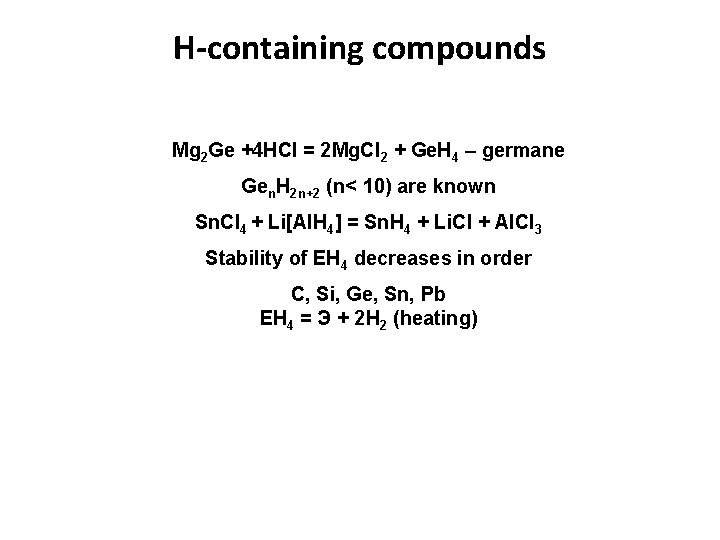
H-containing compounds Mg 2 Ge +4 HCl = 2 Mg. Cl 2 + Ge. H 4 – germane Gen. H 2 n+2 (n< 10) are known Sn. Cl 4 + Li[Al. H 4] = Sn. H 4 + Li. Cl + Al. Cl 3 Stability of EН 4 decreases in order C, Si, Ge, Sn, Pb EН 4 = Э + 2 Н 2 (heating)
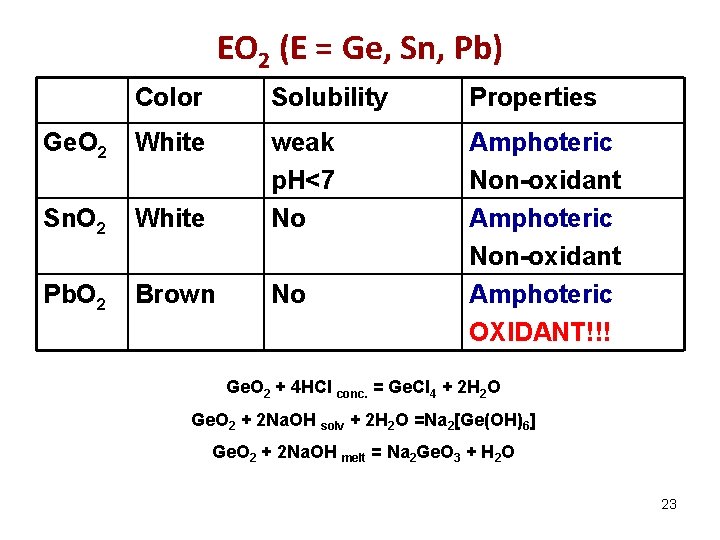
EО 2 (E = Ge, Sn, Pb) Color Solubility Properties Ge. O 2 White Sn. O 2 White weak р. Н<7 No Pb. O 2 Brown No Amphoteric Non-oxidant Amphoteric OXIDANT!!! Ge. O 2 + 4 HCl conc. = Ge. Cl 4 + 2 H 2 O Ge. O 2 + 2 Na. OH solv + 2 H 2 O =Na 2[Ge(OH)6] Ge. O 2 + 2 Na. OH melt = Na 2 Ge. O 3 + H 2 O 23
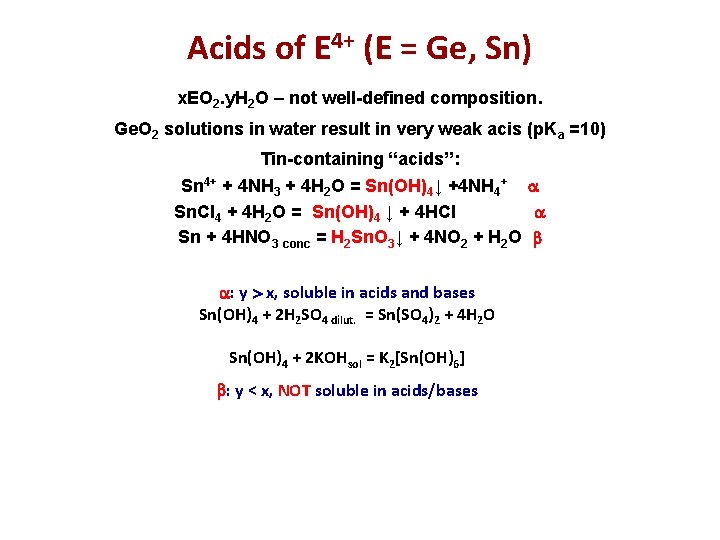
Acids of E 4+ (E = Ge, Sn) x. EО 2. y. H 2 O – not well-defined composition. Ge. O 2 solutions in water result in very weak acis (p. Ka =10) Tin-containing “acids”: Sn 4+ + 4 NH 3 + 4 H 2 O = Sn(OH)4↓ +4 NH 4+ Sn. Cl 4 + 4 H 2 O = Sn(OH)4 ↓ + 4 HCl Sn + 4 HNO 3 conc = H 2 Sn. O 3↓ + 4 NO 2 + H 2 O : y x, soluble in acids and bases Sn(OH)4 + 2 H 2 SO 4 dilut. = Sn(SO 4)2 + 4 H 2 O Sn(OH)4 + 2 KOHsol = K 2[Sn(OH)6] : y < x, NOT soluble in acids/bases
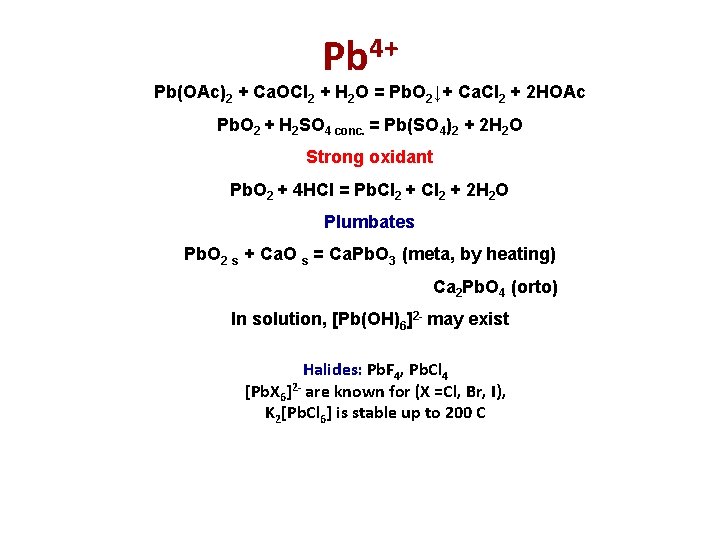
Pb 4+ Pb(OAc)2 + Ca. OCl 2 + H 2 O = Pb. O 2↓+ Ca. Cl 2 + 2 HOAc Pb. O 2 + H 2 SO 4 conc. = Pb(SO 4)2 + 2 H 2 O Strong oxidant Pb. O 2 + 4 HCl = Pb. Cl 2 + 2 H 2 O Plumbates Pb. O 2 s + Ca. O s = Ca. Pb. O 3 (meta, by heating) Ca 2 Pb. O 4 (orto) In solution, [Pb(OH)6]2 - may exist Halides: Pb. F 4, Pb. Cl 4 [Pb. X 6]2 - are known for (X =Cl, Br, I), K 2[Pb. Cl 6] is stable up to 200 C
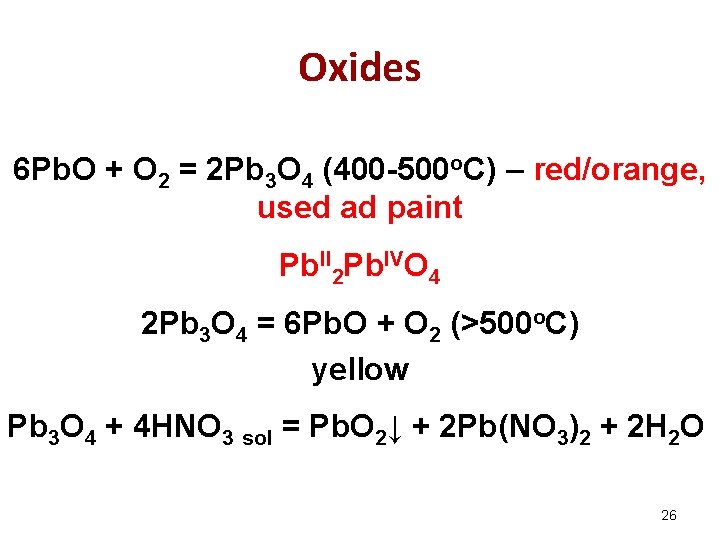
Oxides 6 Pb. O + O 2 = 2 Pb 3 O 4 (400 -500 o. C) – red/orange, used ad paint Pb. II 2 Pb. IVO 4 2 Pb 3 O 4 = 6 Pb. O + O 2 (>500 o. C) yellow Pb 3 O 4 + 4 HNO 3 sol = Pb. O 2↓ + 2 Pb(NO 3)2 + 2 H 2 O 26
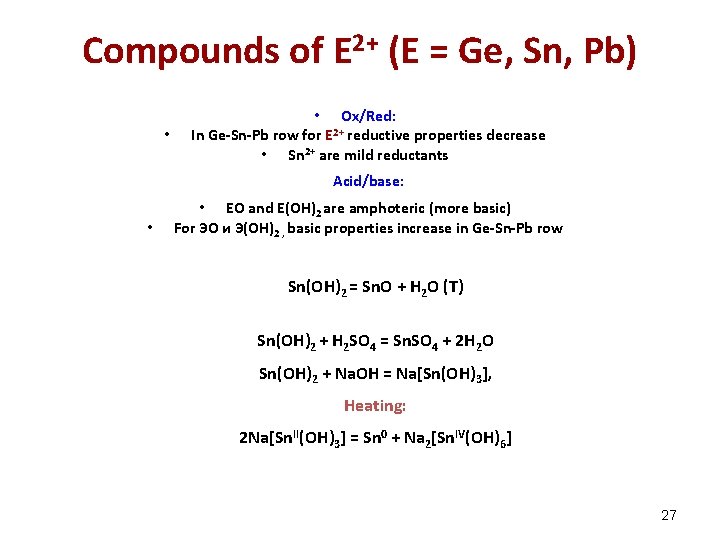
Compounds of E 2+ (E = Ge, Sn, Pb) • • Ox/Red: In Ge-Sn-Pb row for E 2+ reductive properties decrease • Sn 2+ are mild reductants Acid/base: • • EО and E(ОН)2 are amphoteric (more basic) For ЭО и Э(ОН)2 , basic properties increase in Ge-Sn-Pb row Sn(OH)2 = Sn. O + H 2 O (Т) Sn(OH)2 + H 2 SO 4 = Sn. SO 4 + 2 H 2 O Sn(OH)2 + Na. OH = Na[Sn(OH)3], Heating: 2 Na[Sn. II(OH)3] = Sn 0 + Na 2[Sn. IV(OH)6] 27
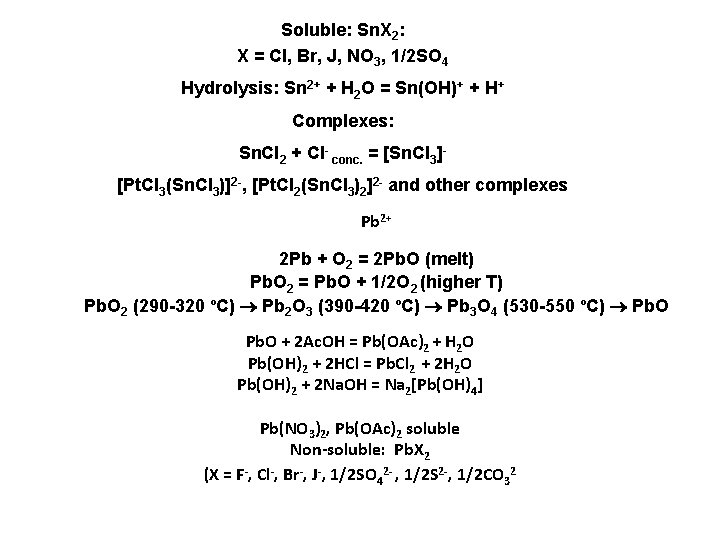
Soluble: Sn. X 2: X = Cl, Br, J, NO 3, 1/2 SO 4 Hydrolysis: Sn 2+ + H 2 O = Sn(OH)+ + H+ Complexes: Sn. Cl 2 + Cl- conc. = [Sn. Cl 3][Pt. Cl 3(Sn. Cl 3)]2 -, [Pt. Cl 2(Sn. Cl 3)2]2 - and other complexes Pb 2+ 2 Pb + O 2 = 2 Pb. O (melt) Pb. O 2 = Pb. O + 1/2 O 2 (higher Т) Pb. O 2 (290 -320 ºС) Pb 2 O 3 (390 -420 ºС) Pb 3 O 4 (530 -550 ºС) Рb. O Pb. O + 2 Ac. OH = Pb(OAc)2 + H 2 O Pb(OH)2 + 2 HCl = Pb. Cl 2 + 2 H 2 O Pb(OH)2 + 2 Na. OH = Na 2[Pb(OH)4] Pb(NO 3)2, Pb(OAc)2 soluble Non-soluble: Pb. X 2 (X = F-, Cl-, Br-, J-, 1/2 SO 42 - , 1/2 S 2 -, 1/2 СО 32
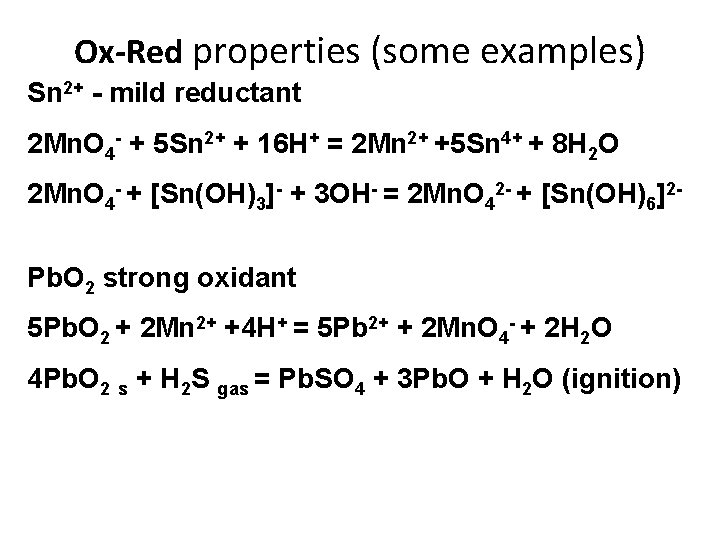
Ox-Red properties (some examples) Sn 2+ - mild reductant 2 Mn. O 4 - + 5 Sn 2+ + 16 H+ = 2 Mn 2+ +5 Sn 4+ + 8 H 2 O 2 Mn. O 4 - + [Sn(OH)3]- + 3 OH- = 2 Mn. O 42 - + [Sn(OH)6]2 Pb. O 2 strong oxidant 5 Pb. O 2 + 2 Mn 2+ +4 H+ = 5 Pb 2+ + 2 Mn. O 4 - + 2 H 2 O 4 Pb. O 2 s + H 2 S gas = Pb. SO 4 + 3 Pb. O + H 2 O (ignition)

Sulfides and thio salts Ge. S, Sn. S, Pb. S do not react with H 2 S But!!! EIIS + Na 2 S 2 = Na 2 EIVS 3 (Э = Ge, Sn) Ge. S 2, Sn. S 2: Sn. S 2 + Na 2 S = Na 2 Sn. S 3 thiostannate Na 2 Sn. S 3 + 2 HCl = Sn. S 2↓ + H 2 S + 2 Na. Cl
- Slides: 30