LECTURE 7 4 ACIDBASE TITRATIONS What are indicators

LECTURE 7. 4 – ACID/BASE TITRATIONS

What are indicators?

I. Acid/Base Indicators • Indicators – Some compounds change color depending on p. H. • Therefore, these compounds tell us the general p. H of our solution based their color

How are indicators used in titrations?

I. Titrations • Often times, we have solutions of unknown concentrations • Titration – The controlled addition of solution of known concentration/volume to a solution of known volume, but unknown concentration.
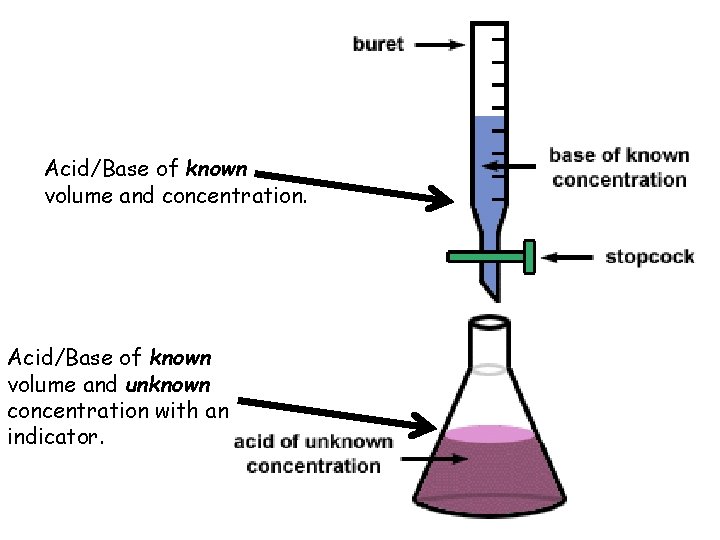
Acid/Base of known volume and concentration. Acid/Base of known volume and unknown concentration with an indicator.
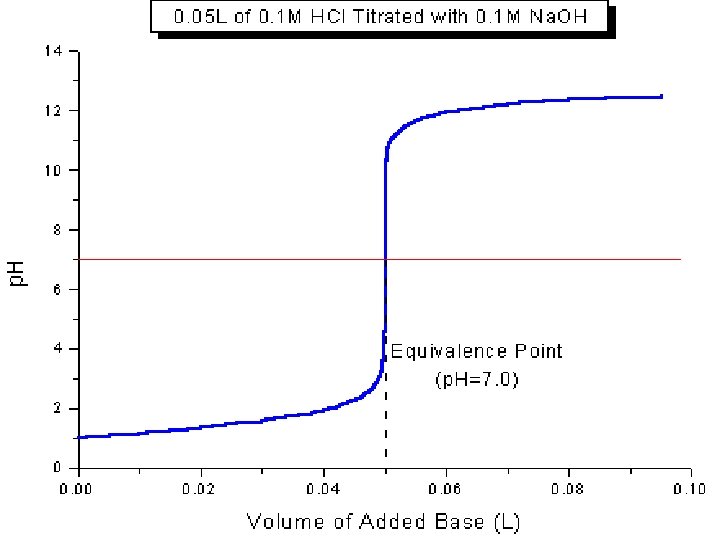

II. Equivalence Point • Equivalence Point – The point at which the two solutions used in a titration are present in equal amounts. • We use indicators to spot this point • For this class, always at p. H =7 • Moles of acid = moles of base at this point
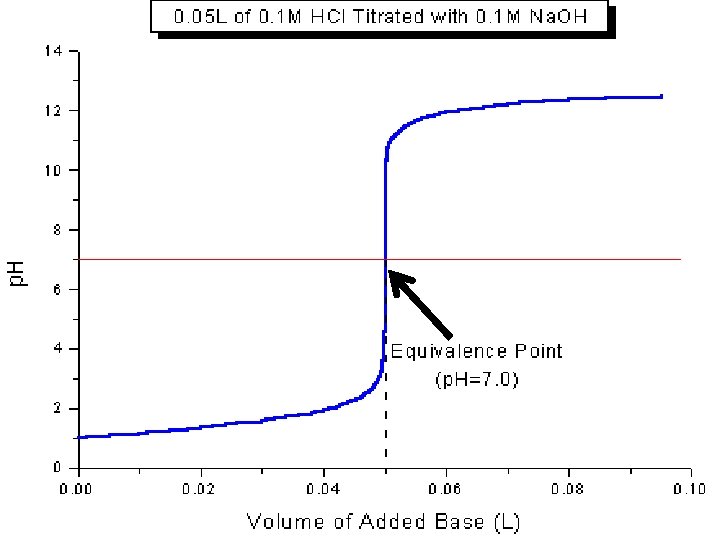

How are titrations used to calculate concentrations?
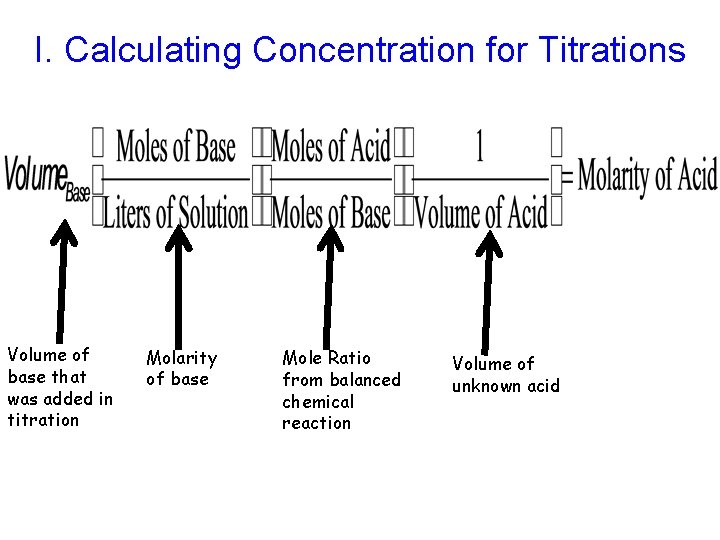
I. Calculating Concentration for Titrations Volume of base that was added in titration Molarity of base Mole Ratio from balanced chemical reaction Volume of unknown acid
Class Example • If it takes 0. 540 L of 0. 1 M Na. OH to neutralize 0. 125 L of an HCl solution, what is the concentration of the HCl?
Class Example • If it takes 0. 140 L of 0. 051 M HNO 3 to neutralize 0. 225 L of an Li. OH solution, what is the concentration of the HNO 3?

Table Talk • It takes 2. 540 L of 1. 1 M HBr to neutralize 1. 25 L of an KOH solution, what is the concentration of the HBr?
- Slides: 14