Lecture 6 The Periodic Table and Naming Simple

Lecture #6 The Periodic Table and Naming Simple Compounds Chemistry 142 A James B. Callis, Instructor Winter Quarter, 2006
Classification of the Elements • Most of the elements are metals - metallic luster, ability to conduct electricity and heat, and malleability. • The remaining elements are classified as nonmetals - no luster, poor conductors of electricity and heat and brittleness.


Sub-Classes of Metals • Alkali metals - (lithium, sodium, potassium, rubidium and cesium) - soft, low melting points, react with water to liberate hydrogen, form 1: 1 compounds with chlorine. • Alkaline earths - (beryllium, magnesium, calcium, strontium, barium and radium) - react in a 1: 2 ratio with chlorine. • Transition metals - (e. g. iron, copper, silver, gold, tungsten and cobalt) - structural metals • Metalloids - (antimony, arsenic, boron, silicon and tellurium) - intermediate between metals and nonmetals.

Non-Metals • Chalcogens - (oxygen, sulfur, selenium and tellurium) - form 1: 1 compounds with alkaline-earths, but 2: 1 compounds with alkali-metals. • Halogens - (fluorine, chlorine, bromine and iodine) - highly reactive and form 1: 1 compounds with alkali-metals. • Noble gases - (helium, neon, argon, krypton, radon) - virtually inert to chemical reactions.
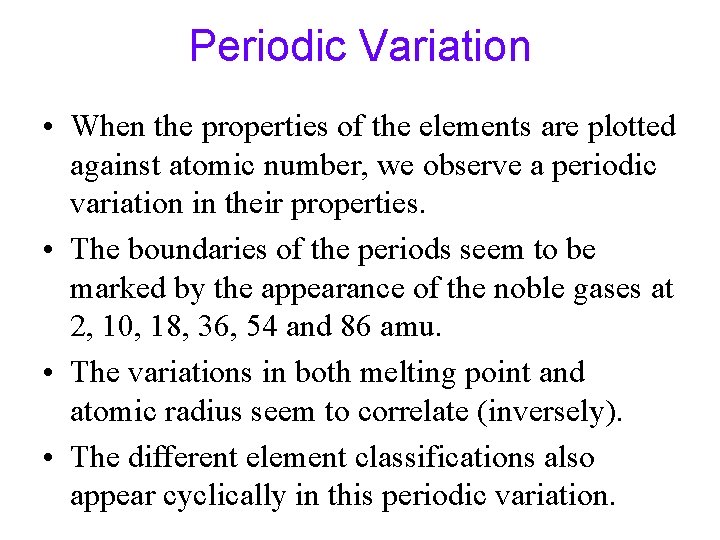
Periodic Variation • When the properties of the elements are plotted against atomic number, we observe a periodic variation in their properties. • The boundaries of the periods seem to be marked by the appearance of the noble gases at 2, 10, 18, 36, 54 and 86 amu. • The variations in both melting point and atomic radius seem to correlate (inversely). • The different element classifications also appear cyclically in this periodic variation.
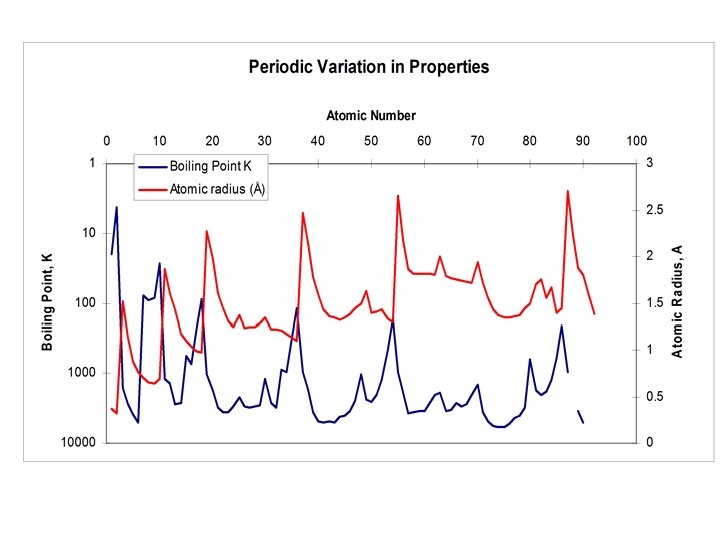
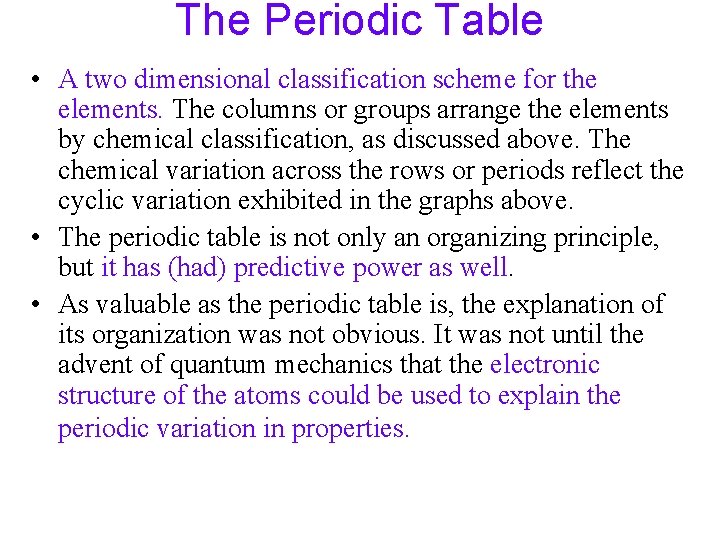
The Periodic Table • A two dimensional classification scheme for the elements. The columns or groups arrange the elements by chemical classification, as discussed above. The chemical variation across the rows or periods reflect the cyclic variation exhibited in the graphs above. • The periodic table is not only an organizing principle, but it has (had) predictive power as well. • As valuable as the periodic table is, the explanation of its organization was not obvious. It was not until the advent of quantum mechanics that the electronic structure of the atoms could be used to explain the periodic variation in properties.

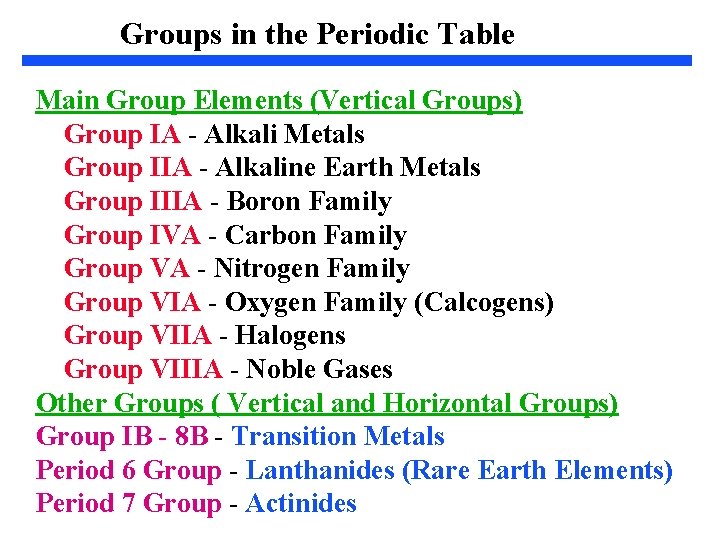
Groups in the Periodic Table Main Group Elements (Vertical Groups) Group IA - Alkali Metals Group IIA - Alkaline Earth Metals Group IIIA - Boron Family Group IVA - Carbon Family Group VA - Nitrogen Family Group VIA - Oxygen Family (Calcogens) Group VIIA - Halogens Group VIIIA - Noble Gases Other Groups ( Vertical and Horizontal Groups) Group IB - 8 B - Transition Metals Period 6 Group - Lanthanides (Rare Earth Elements) Period 7 Group - Actinides
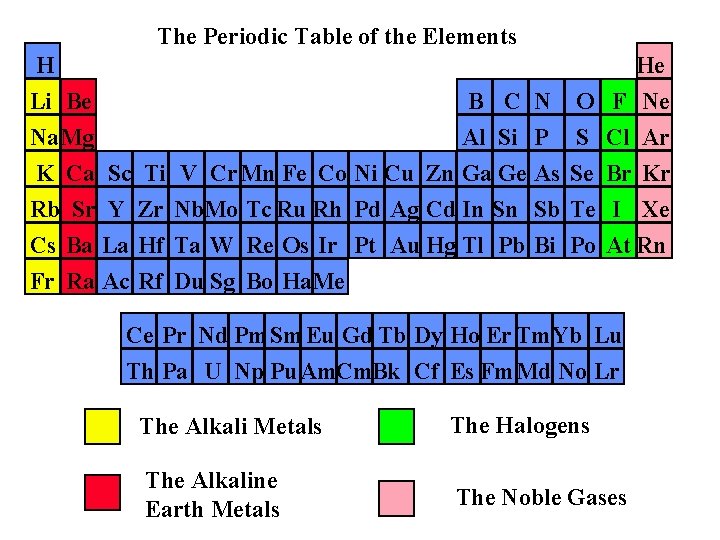
The Periodic Table of the Elements H Li Be Na. Mg B C N Al Si P K Ca Sc Ti Rb Sr Y Zr Cs Ba La Hf Fr Ra Ac Rf He O F Ne S Cl Ar V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Nb. Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Du Sg Bo Ha. Me Ce Pr Nd Pm. Sm Eu Gd Tb Dy Ho Er Tm. Yb Lu Th Pa U Np Pu. Am. Cm. Bk Cf Es Fm. Md No Lr The Alkali Metals The Halogens The Alkaline Earth Metals The Noble Gases
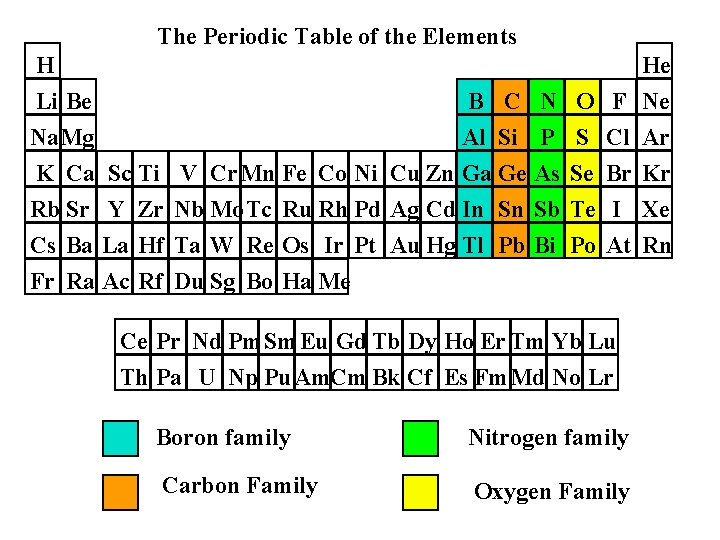
The Periodic Table of the Elements H Li Be Na. Mg He B C N O F Ne Al Si P S Cl Ar K Ca Sc Ti Rb Sr Y Zr Cs Ba La Hf Fr Ra Ac Rf V Cr. Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Du Sg Bo Ha Me Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu. Am. Cm Bk Cf Es Fm. Md No Lr Boron family Nitrogen family Carbon Family Oxygen Family
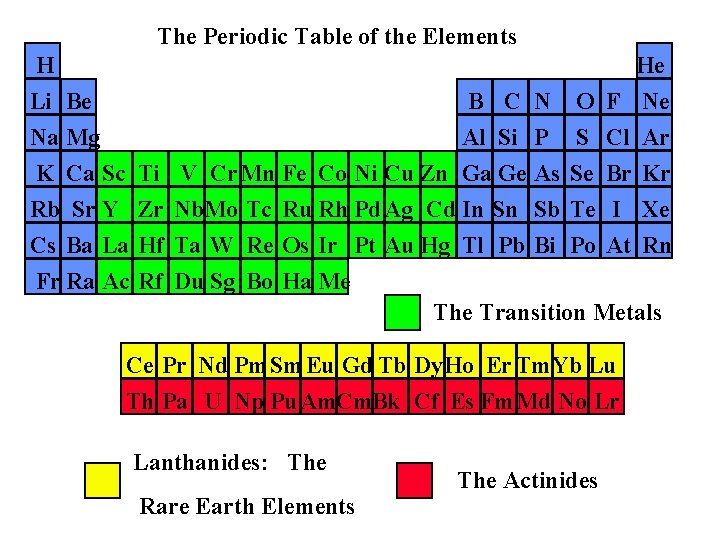
The Periodic Table of the Elements H Li Be Na Mg B C N Al Si P K Ca Sc Rb Sr Y Cs Ba La Fr Ra Ac Ti Zr Hf Rf He O F Ne S Cl Ar V Cr. Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Nb. Mo Tc Ru Rh Pd. Ag Cd In Sn Sb Te I Xe Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Du Sg Bo Ha Me The Transition Metals Ce Pr Nd Pm. Sm Eu Gd Tb Dy. Ho Er Tm. Yb Lu Th Pa U Np Pu. Am. Cm. Bk Cf Es Fm. Md No Lr Lanthanides: The Rare Earth Elements The Actinides

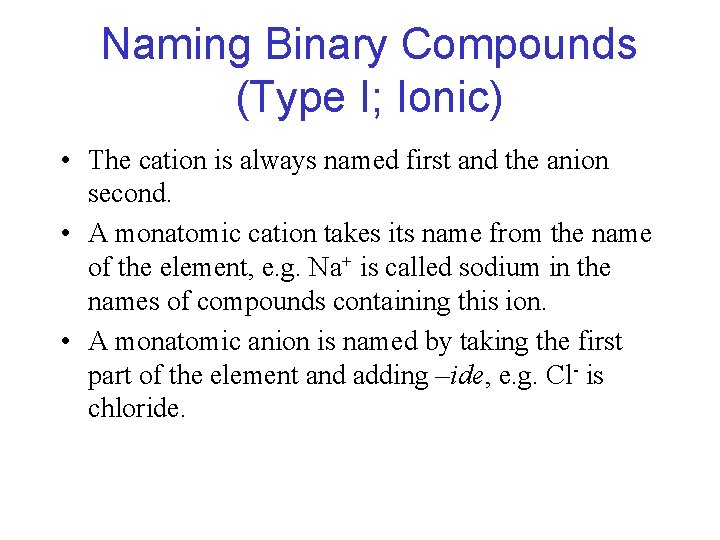
Naming Binary Compounds (Type I; Ionic) • The cation is always named first and the anion second. • A monatomic cation takes its name from the name of the element, e. g. Na+ is called sodium in the names of compounds containing this ion. • A monatomic anion is named by taking the first part of the element and adding –ide, e. g. Cl- is chloride.

Type I
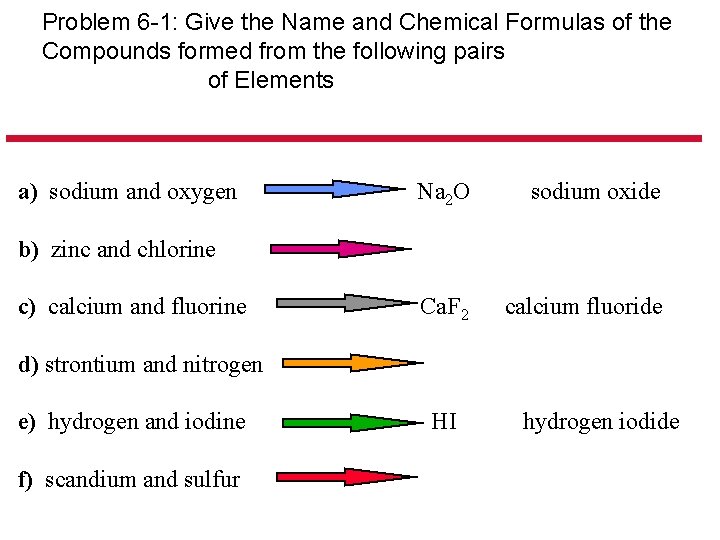
Problem 6 -1: Give the Name and Chemical Formulas of the Compounds formed from the following pairs of Elements a) sodium and oxygen Na 2 O sodium oxide Ca. F 2 calcium fluoride b) zinc and chlorine c) calcium and fluorine d) strontium and nitrogen e) hydrogen and iodine f) scandium and sulfur HI hydrogen iodide
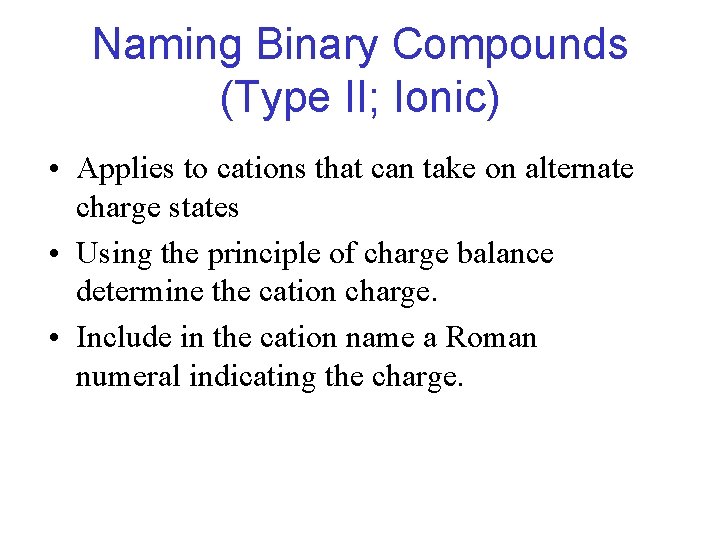
Naming Binary Compounds (Type II; Ionic) • Applies to cations that can take on alternate charge states • Using the principle of charge balance determine the cation charge. • Include in the cation name a Roman numeral indicating the charge.

Type II
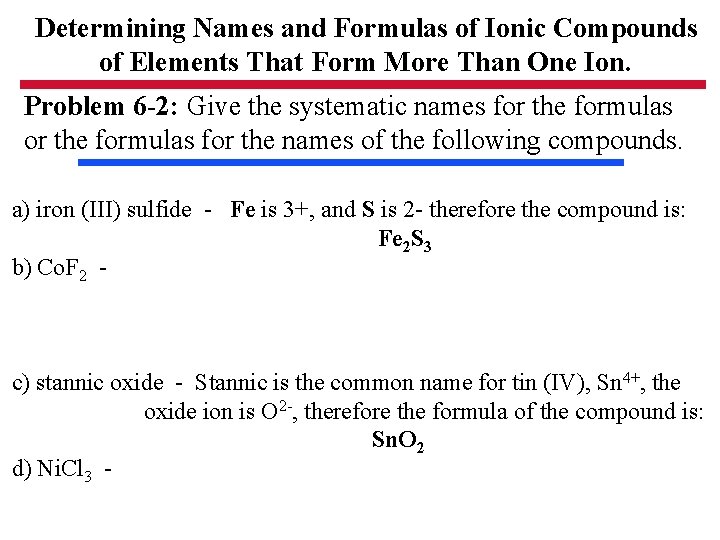
Determining Names and Formulas of Ionic Compounds of Elements That Form More Than One Ion. Problem 6 -2: Give the systematic names for the formulas for the names of the following compounds. a) iron (III) sulfide - Fe is 3+, and S is 2 - therefore the compound is: Fe 2 S 3 b) Co. F 2 - c) stannic oxide - Stannic is the common name for tin (IV), Sn 4+, the oxide ion is O 2 -, therefore the formula of the compound is: Sn. O 2 d) Ni. Cl 3 -

Ionic Compounds with Polyatomic Ions • Polyatomic ions are assigned special names that must be memorized. • Special rules apply to anions that contain an atom of a given element and different numbers of oxygen atoms. These anions are called oxyanions.

Start learning these boldface ones.

Rules for Families of Oxoanions Families with Two Oxoanions The ion with more O atoms takes the nonmetal root and the suffix “-ate”. The ion with fewer O atoms takes the nonmetal root and the suffix “-ite”. Families with Four Oxoanions (usually a Halogen) The ion with most O atoms has the prefix “per-”, the nonmetal root and the suffix “-ate”. The ion with one less O atom has just the suffix “-ate”. The ion with two less O atoms has the just the suffix “-ite”. The ion with three less O atoms has the prefix “hypo-” and the suffix “-ite”.

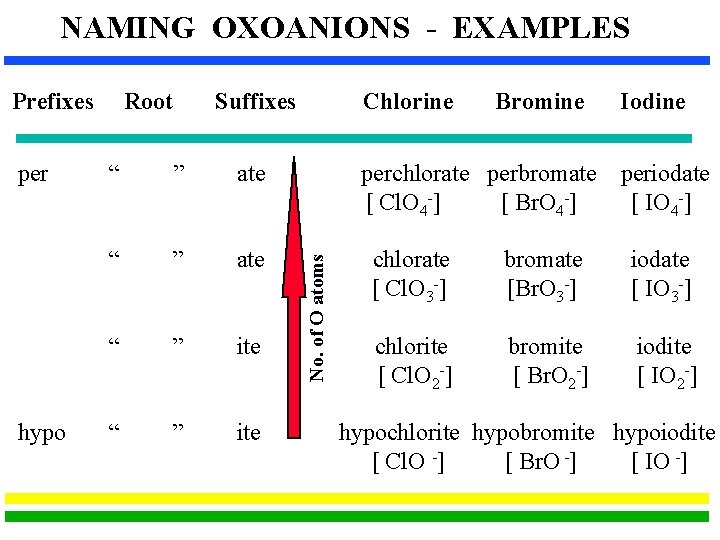
NAMING OXOANIONS - EXAMPLES per hypo Root Suffixes “ ” ate “ ” ite Chlorine Bromine perchlorate perbromate [ Cl. O 4 -] [ Br. O 4 -] No. of O atoms Prefixes Iodine periodate [ IO 4 -] chlorate [ Cl. O 3 -] bromate [Br. O 3 -] iodate [ IO 3 -] chlorite [ Cl. O 2 -] bromite [ Br. O 2 -] iodite [ IO 2 -] hypochlorite hypobromite hypoiodite [ Cl. O -] [ Br. O -] [ IO -]

Binary Compounds (Type III; Covalent – Contain Two Nonmetals • The first element in the formula is named first, using the full element name. • The second element is named as if it were an anion. • Prefixes are used to denote the numbers of atoms present. • The prefix mono- is never used for naming the first element, e. g. CO is carbon monoxide.

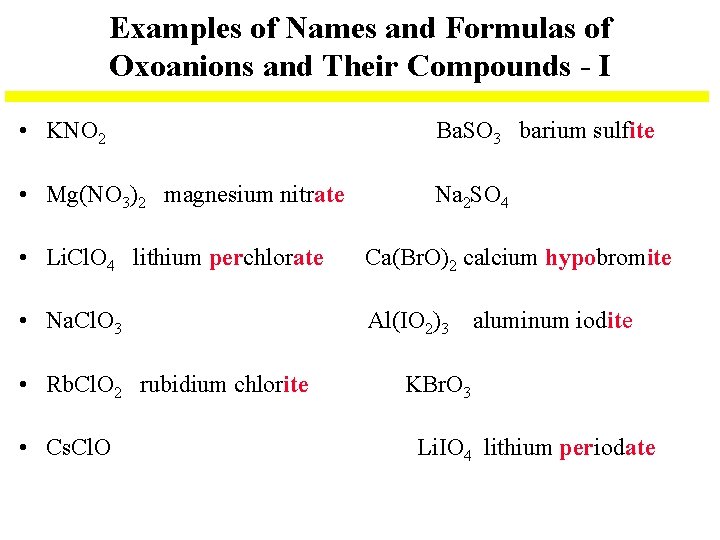
Examples of Names and Formulas of Oxoanions and Their Compounds - I • KNO 2 Ba. SO 3 barium sulfite • Mg(NO 3)2 magnesium nitrate Na 2 SO 4 • Li. Cl. O 4 lithium perchlorate Ca(Br. O)2 calcium hypobromite • Na. Cl. O 3 Al(IO 2)3 aluminum iodite • Rb. Cl. O 2 rubidium chlorite • Cs. Cl. O KBr. O 3 Li. IO 4 lithium periodate
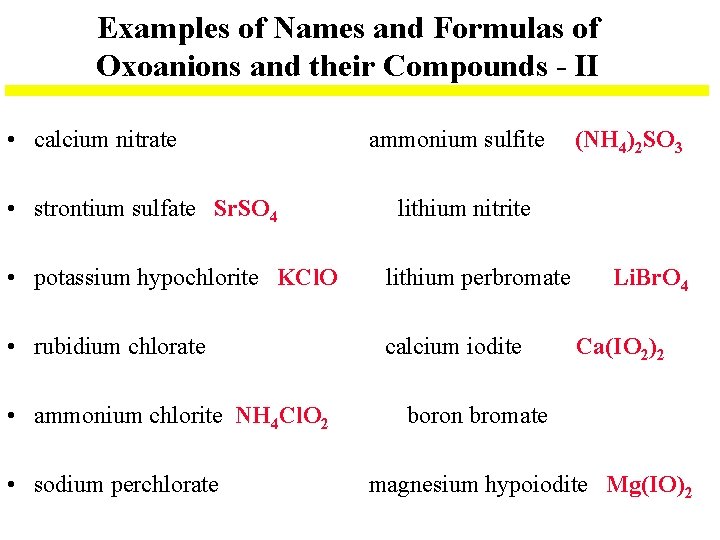
Examples of Names and Formulas of Oxoanions and their Compounds - II • calcium nitrate • strontium sulfate Sr. SO 4 ammonium sulfite lithium nitrite • potassium hypochlorite KCl. O lithium perbromate • rubidium chlorate calcium iodite • ammonium chlorite NH 4 Cl. O 2 • sodium perchlorate (NH 4)2 SO 3 Li. Br. O 4 Ca(IO 2)2 boron bromate magnesium hypoiodite Mg(IO)2
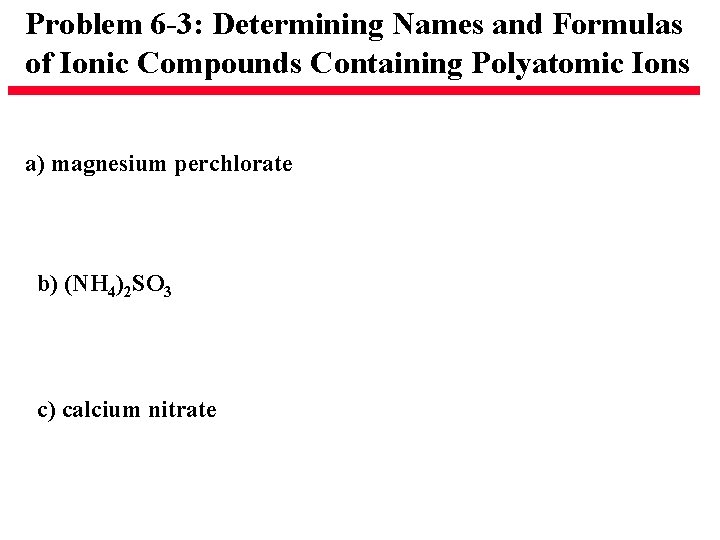
Problem 6 -3: Determining Names and Formulas of Ionic Compounds Containing Polyatomic Ions a) magnesium perchlorate b) (NH 4)2 SO 3 c) calcium nitrate
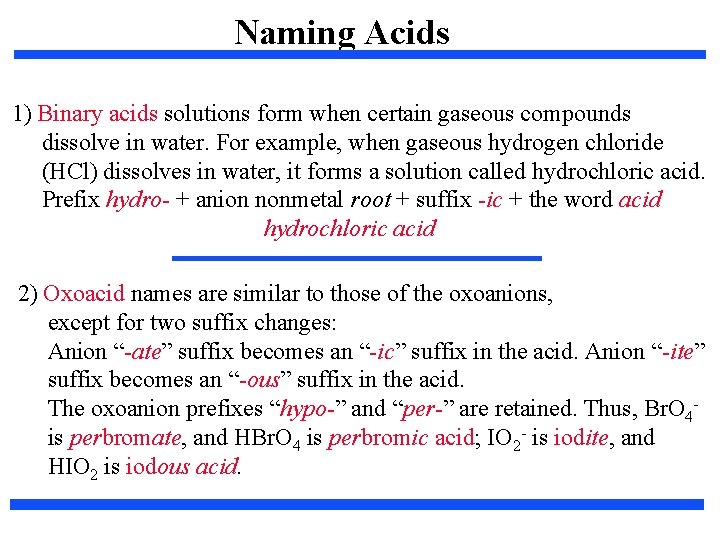
Naming Acids 1) Binary acids solutions form when certain gaseous compounds dissolve in water. For example, when gaseous hydrogen chloride (HCl) dissolves in water, it forms a solution called hydrochloric acid. Prefix hydro- + anion nonmetal root + suffix -ic + the word acid hydrochloric acid 2) Oxoacid names are similar to those of the oxoanions, except for two suffix changes: Anion “-ate” suffix becomes an “-ic” suffix in the acid. Anion “-ite” suffix becomes an “-ous” suffix in the acid. The oxoanion prefixes “hypo-” and “per-” are retained. Thus, Br. O 4 is perbromate, and HBr. O 4 is perbromic acid; IO 2 - is iodite, and HIO 2 is iodous acid.
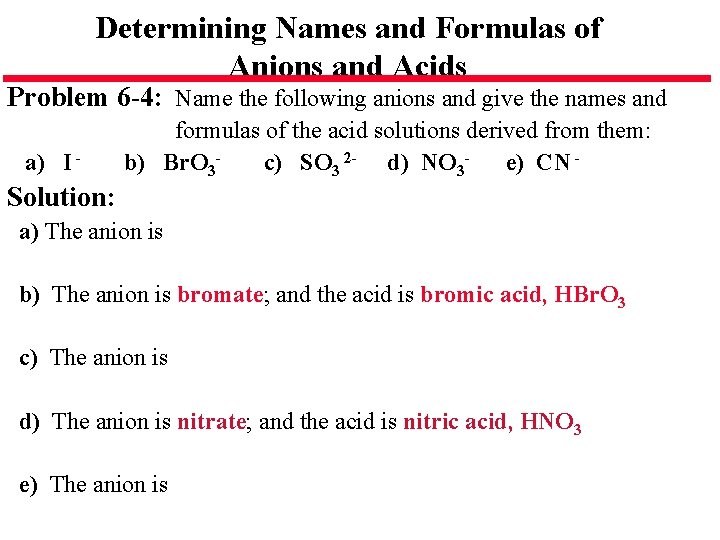
Determining Names and Formulas of Anions and Acids Problem 6 -4: Name the following anions and give the names and a) I - formulas of the acid solutions derived from them: b) Br. O 3 c) SO 3 2 - d) NO 3 e) CN - Solution: a) The anion is bromate; and the acid is bromic acid, HBr. O 3 c) The anion is d) The anion is nitrate; and the acid is nitric acid, HNO 3 e) The anion is
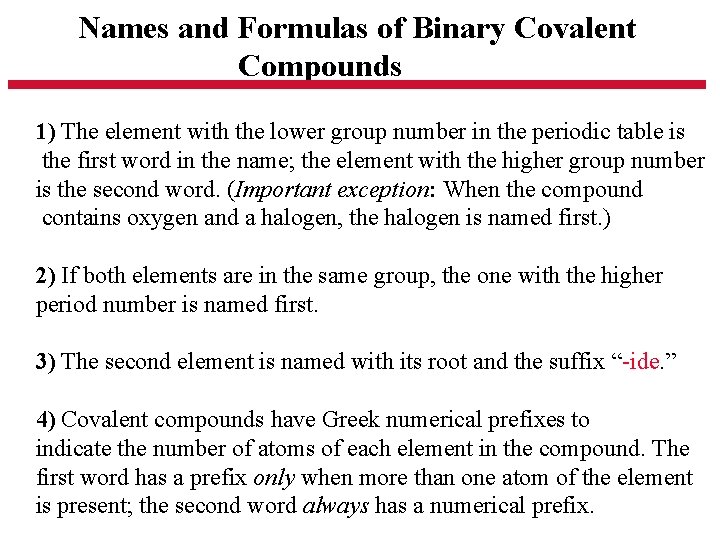
Names and Formulas of Binary Covalent Compounds 1) The element with the lower group number in the periodic table is the first word in the name; the element with the higher group number is the second word. (Important exception: When the compound contains oxygen and a halogen, the halogen is named first. ) 2) If both elements are in the same group, the one with the higher period number is named first. 3) The second element is named with its root and the suffix “-ide. ” 4) Covalent compounds have Greek numerical prefixes to indicate the number of atoms of each element in the compound. The first word has a prefix only when more than one atom of the element is present; the second word always has a numerical prefix.
Determining Names and Formulas of Binary Covalent Compounds Problem 6 -5: What are the name or chemical formulas of the following chemical compounds: a) carbon dioxide b) PCl 3 c) Give the name and chemical formula of the compound formed from two P atoms and five O atoms. Solution: a) carbon dioxide b) PCl 3 c) The compound formed from two P atoms and five O atoms

Answers to Problems in Lecture #6 1. (b) Zn. Cl 2 , Zinc Chloride; (d) Sr 3 N 2 , Strontium Nitride; (f) Sc 2 S 3 , Scandium Sulfide 2. (b) Cobalt (II) Fluoride; (d) Nickel (III) Chloride 3. (a) Mg( Cl. O 4)2 ; (b) Ammonium Sulfite; (c) Ca(NO 3)2 4. (a) iodide, hydroiodic acid, HI; (c) sulfite, sulfurous acid, H 2 SO 3 (e) cyanide, hydrocyanic acid, HCN 5. (a) CO 2 ; (b) phosphorous trichloride; (c) diphosphorous pentaoxide
- Slides: 36