LECTURE 6 QUANTUM PHYSICS II Instructor ShihChieh Hsu











- Slides: 54

LECTURE 6 QUANTUM PHYSICS II Instructor: Shih-Chieh Hsu

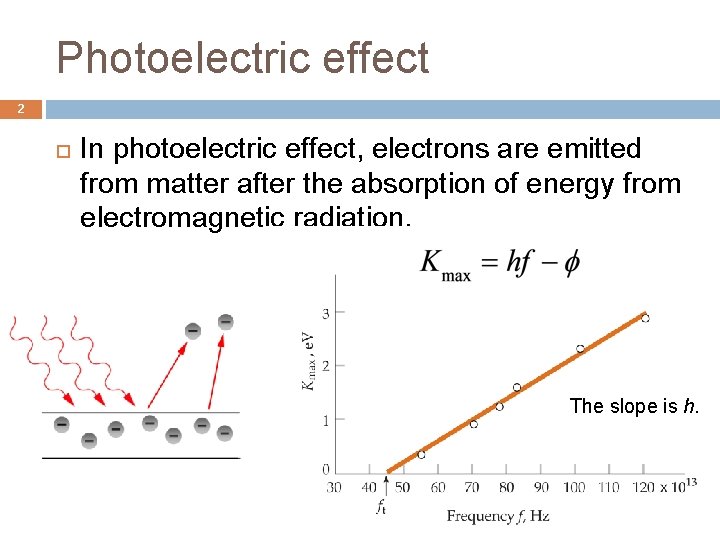
Photoelectric effect 2 In photoelectric effect, electrons are emitted from matter after the absorption of energy from electromagnetic radiation. The slope is h.

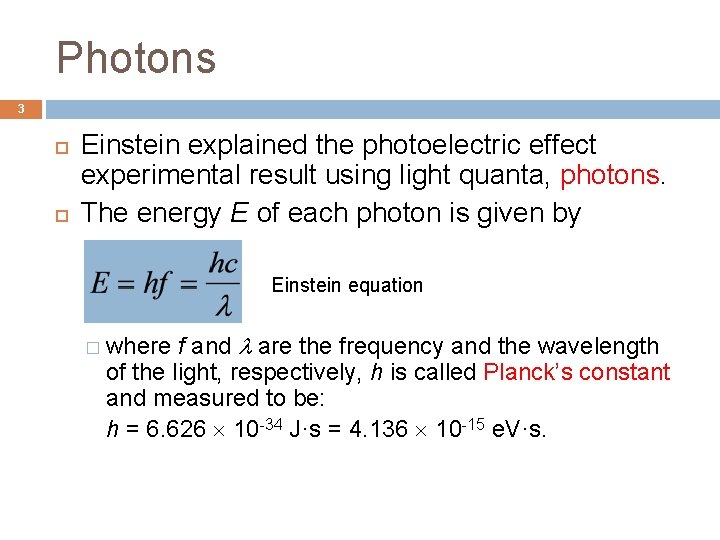
Photons 3 Einstein explained the photoelectric effect experimental result using light quanta, photons. The energy E of each photon is given by Einstein equation � where f and are the frequency and the wavelength of the light, respectively, h is called Planck’s constant and measured to be: h = 6. 626 10 -34 J·s = 4. 136 10 -15 e. V·s.

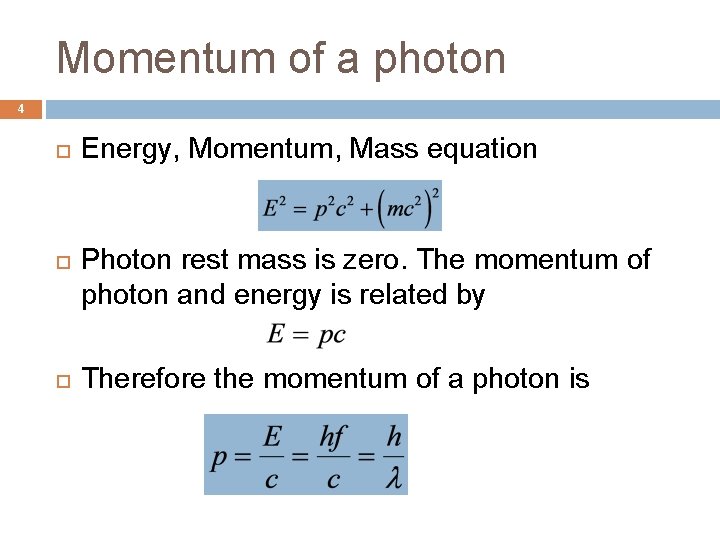
Momentum of a photon 4 Energy, Momentum, Mass equation Photon rest mass is zero. The momentum of photon and energy is related by Therefore the momentum of a photon is

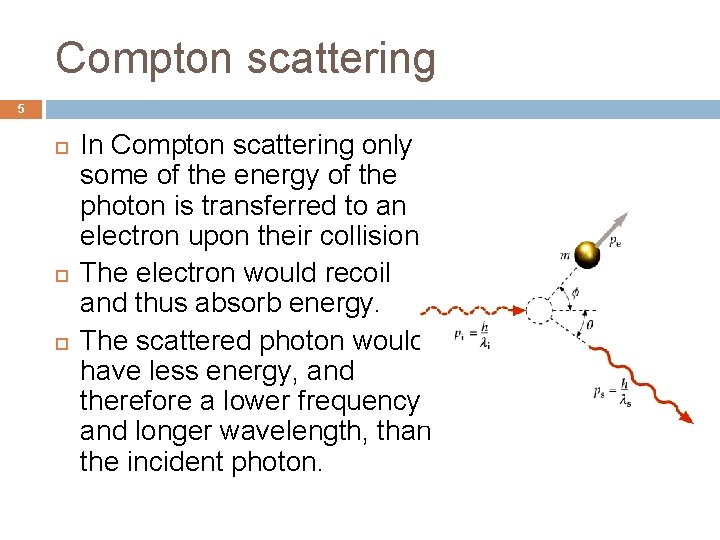
Compton scattering 5 In Compton scattering only some of the energy of the photon is transferred to an electron upon their collision. The electron would recoil and thus absorb energy. The scattered photon would have less energy, and therefore a lower frequency and longer wavelength, than the incident photon.

Matter Waves 6 Tipler chapter 34 -5 to 34 -10 � � � Electrons and Matter waves The Interpretation of the Wave function Wave-Particle Duality A Particle in a box Energy quantization in Other system

7 Development of Quantum Mechanics In 1862, Kirchhoff coined black body radiation or known as cavity radiation � The experiments raised the question of the failure of classical EM theories In 1887, Heinrich Hertz discovers photoelectric effect � Another experiment raised the concern of the wave and particle nature of the light.

Theory of quantization of light 8 In 1900, Max Planck resolves the blackbody radiation issues by introducing “quantum” concept of the discrete energy element � � The energy element is discrete and the energy is proportional to the frequency The invention of Planck constant h In 1905, Einstein explained photoelectric effect by using Max Planck’s light quantization concept � Photon is introduced by Gilbert N. Lewis in 1926

More Quantization System 9 In 1909, Robert Millikan conducted oildrop experiment and showed that electric charge is quantized. In 1911, Ernest Rutherford’s Gold Foil Experiment disproved the plum pudding model of the atom.

Old Quantum Theory 10 In 1913, Niels Bohr explains the spectra line of the hydrogen atom – using quantization ideas. 1918 -1923 expansion of quantum mechanics research works! e. g. Stern-Gerlach demonstrated spin property of electrons in 1920 Stern Gerlach

New Quantum Theory 11 In 1924, Louise de Broglie proposes matter wave theory. In 1925, Matrix Mechanics is invented � Heisenberg Uncertainty was proposed in 1927 Werner Heisenberg Max Born Pascual Jord

Completion of Quantum Mechanics 12 In 1925, Erwin Schrödinger invented wave mechanics and non-relativistic Schrödinger equation as generalization of de Broglie’s theory 1927, Paul Dirac began the process of unifying quantum mechanics with special relativity by proposing the Dirac equation for the electron.

Black-Body Radiation 13 "Blackbody radiation" or "cavity radiation" refers to an object or system which absorbs all radiation incident upon it. It re-radiates energy which is characteristic of this radiating system only, not dependent upon the type of radiation which is incident upon it.

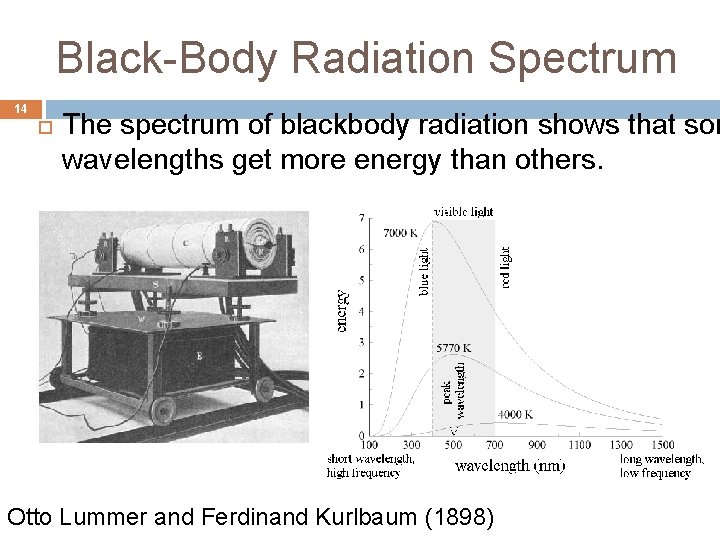
Black-Body Radiation Spectrum 14 The spectrum of blackbody radiation shows that som wavelengths get more energy than others. Otto Lummer and Ferdinand Kurlbaum (1898)

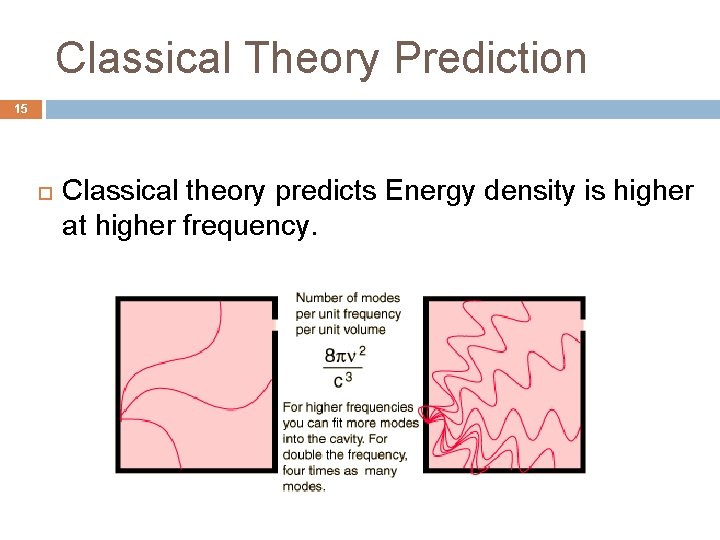
Classical Theory Prediction 15 Classical theory predicts Energy density is higher at higher frequency.

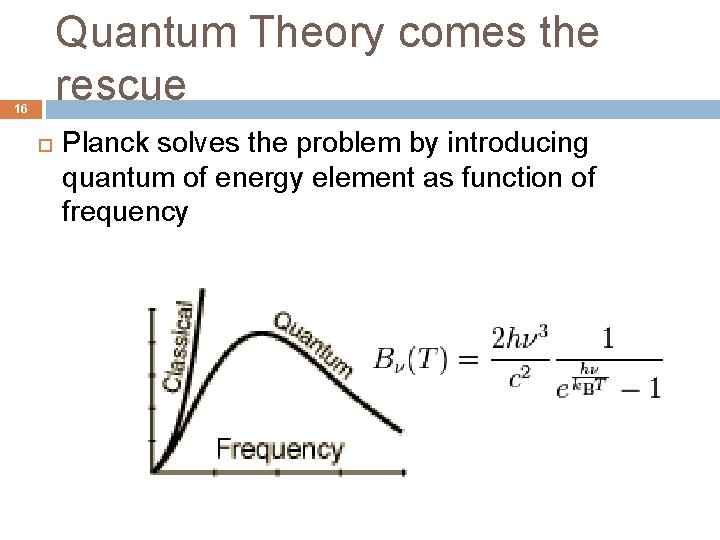
Quantum Theory comes the rescue 16 Planck solves the problem by introducing quantum of energy element as function of frequency

Discovery of electron 17 J. J. Thomson discovered electrons using a cathode-ray tube in 1897. By applying electric and magnetic fields to the ray and observing that it deflects, he concluded that the ray is negatively charged. By measuring the amount of deflection, he measured the charge-to-mass ratio. The particles in the ray always had the same ratio, so he concluded that they are fundamental particles.

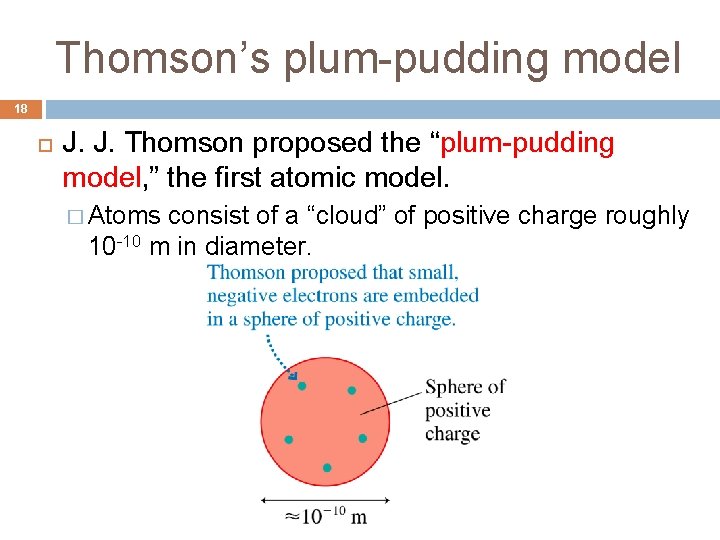
Thomson’s plum-pudding model 18 J. J. Thomson proposed the “plum-pudding model, ” the first atomic model. � Atoms consist of a “cloud” of positive charge roughly 10 -10 m in diameter.

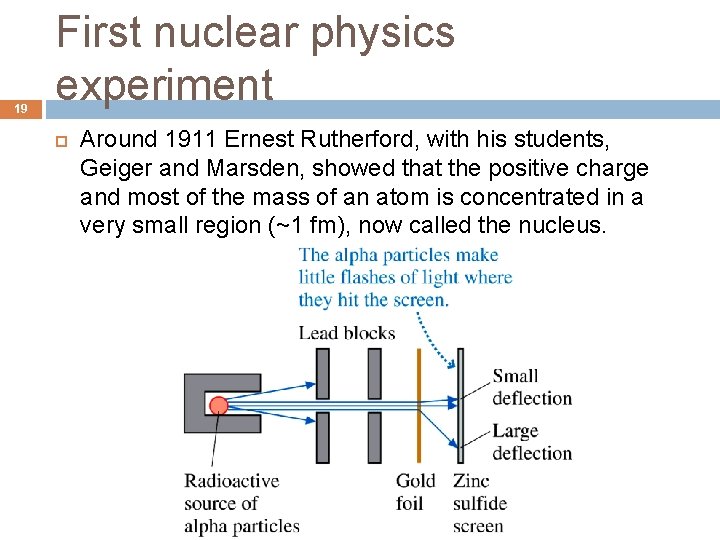
19 First nuclear physics experiment Around 1911 Ernest Rutherford, with his students, Geiger and Marsden, showed that the positive charge and most of the mass of an atom is concentrated in a very small region (~1 fm), now called the nucleus.

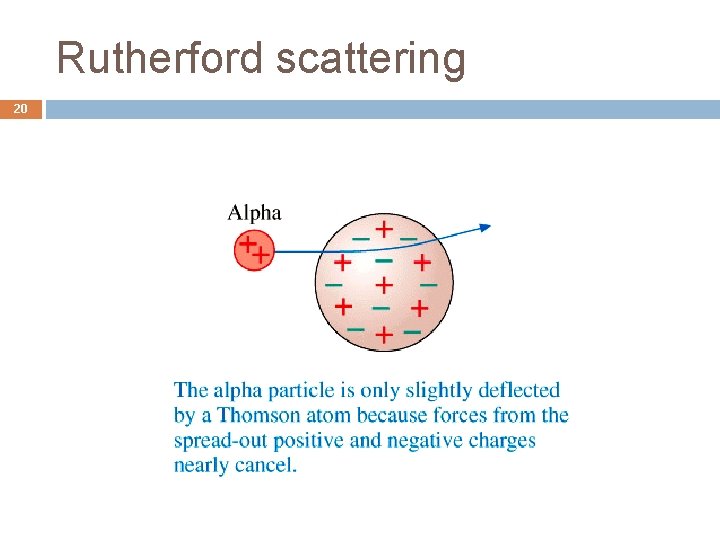
Rutherford scattering 20

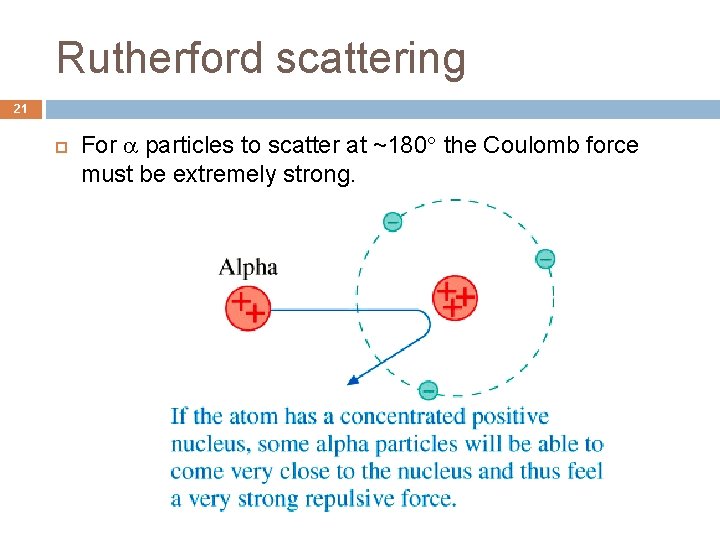
Rutherford scattering 21 For particles to scatter at ~180 the Coulomb force must be extremely strong.

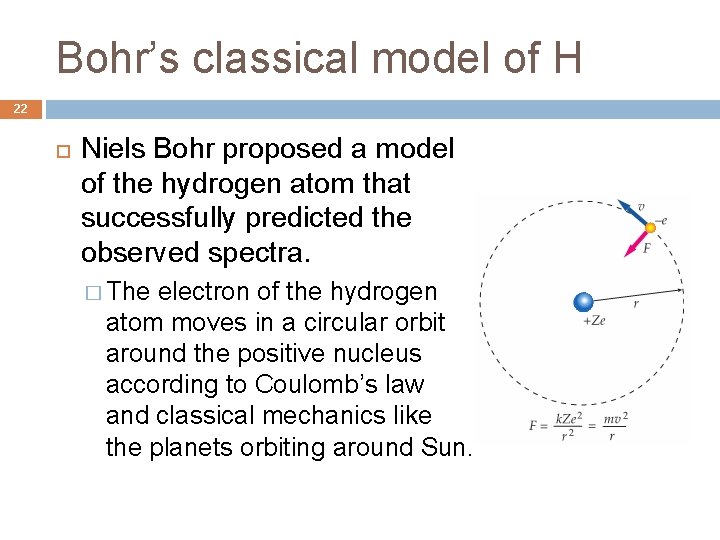
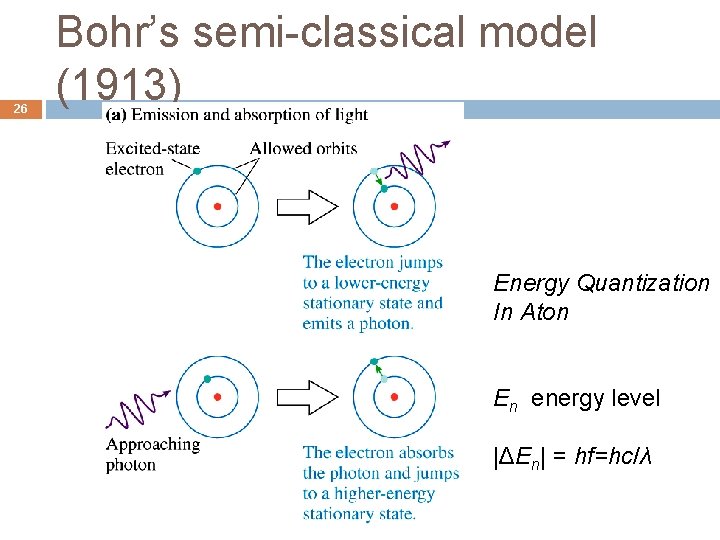
Bohr’s classical model of H 22 Niels Bohr proposed a model of the hydrogen atom that successfully predicted the observed spectra. � The electron of the hydrogen atom moves in a circular orbit around the positive nucleus according to Coulomb’s law and classical mechanics like the planets orbiting around Sun.

Flaw in the classical model 23 Classical EM theory says that an electron in a circular orbit is accelerating, so it would radiate an EM wave and loses its energy. This atom would quickly collapse as the electron spirals into the nucleus and radiates away the energy.

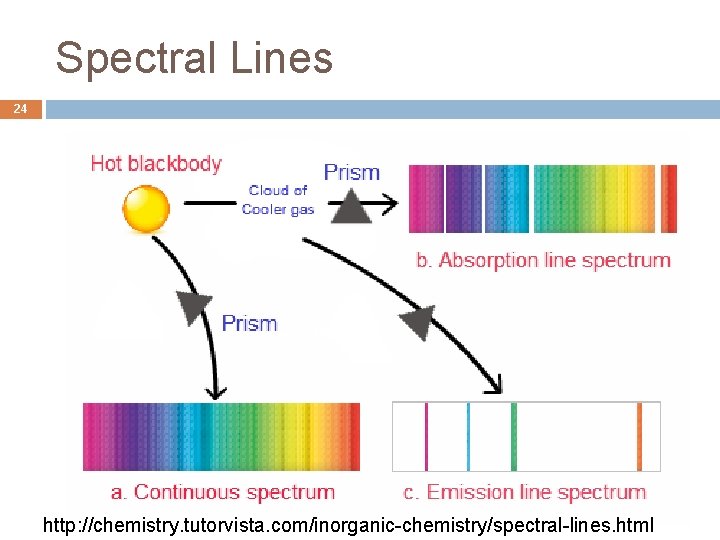
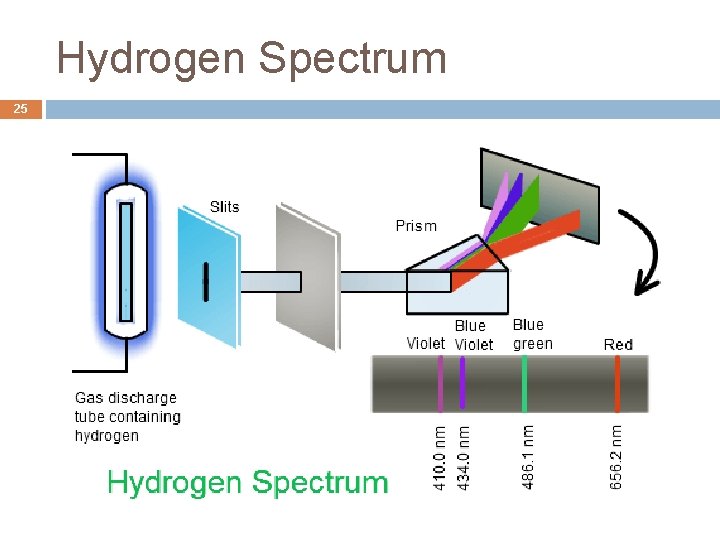
Spectral Lines 24 http: //chemistry. tutorvista. com/inorganic-chemistry/spectral-lines. html

Hydrogen Spectrum 25

26 Bohr’s semi-classical model (1913) Energy Quantization In Aton En energy level |ΔEn| = hf=hc/λ

27 Old Quantum Theory

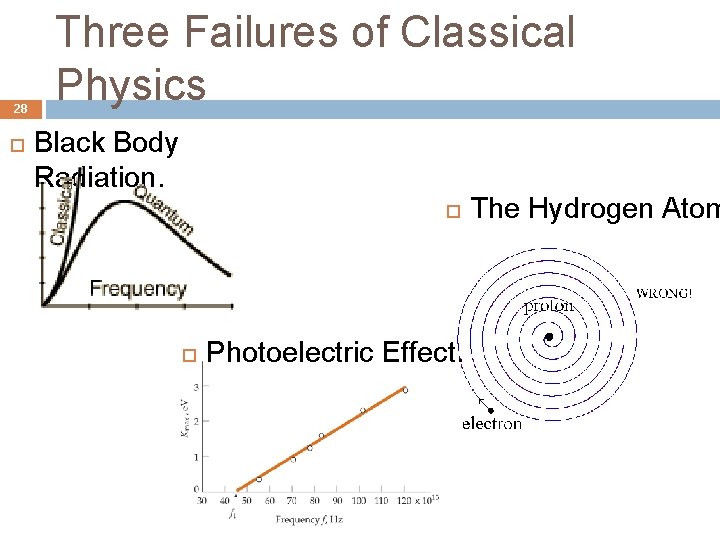
28 Three Failures of Classical Physics Black Body Radiation. Photoelectric Effect. The Hydrogen Atom

29 New Quantum Theory

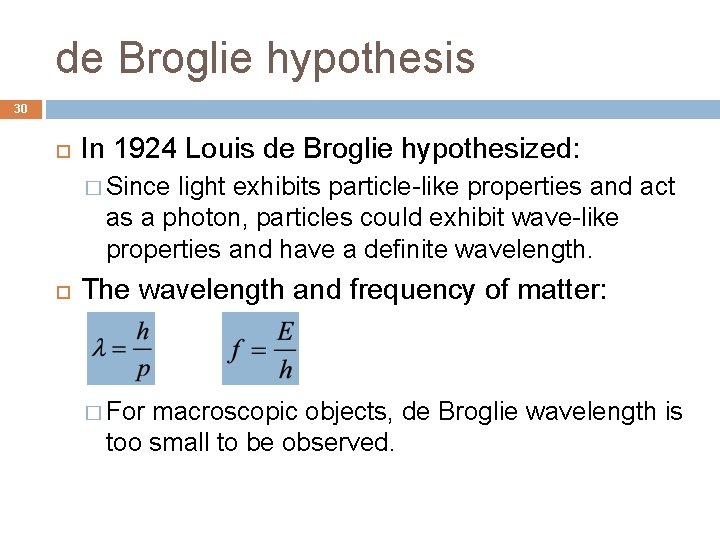
de Broglie hypothesis 30 In 1924 Louis de Broglie hypothesized: � Since light exhibits particle-like properties and act as a photon, particles could exhibit wave-like properties and have a definite wavelength. The wavelength and frequency of matter: � For macroscopic objects, de Broglie wavelength is too small to be observed.

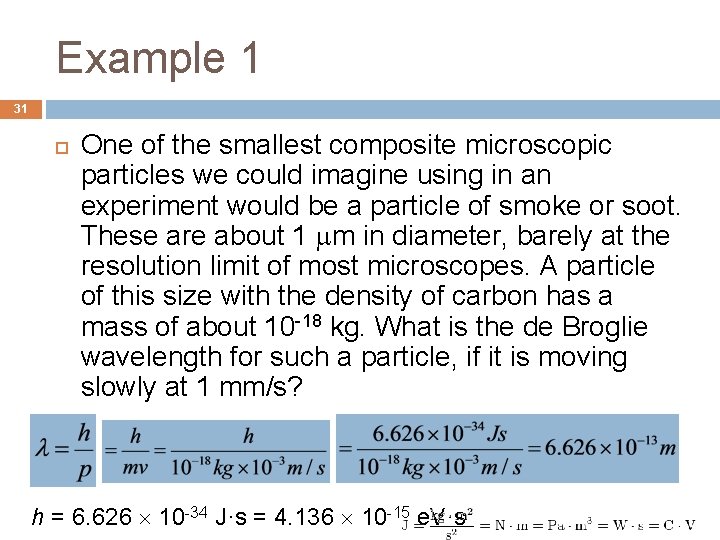
Example 1 31 One of the smallest composite microscopic particles we could imagine using in an experiment would be a particle of smoke or soot. These are about 1 m in diameter, barely at the resolution limit of most microscopes. A particle of this size with the density of carbon has a mass of about 10 -18 kg. What is the de Broglie wavelength for such a particle, if it is moving slowly at 1 mm/s? h = 6. 626 10 -34 J·s = 4. 136 10 -15 e. V·s

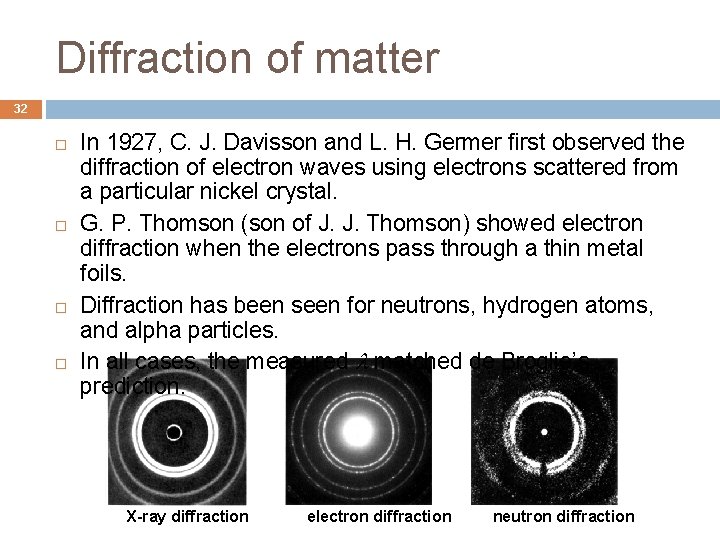
Diffraction of matter 32 In 1927, C. J. Davisson and L. H. Germer first observed the diffraction of electron waves using electrons scattered from a particular nickel crystal. G. P. Thomson (son of J. J. Thomson) showed electron diffraction when the electrons pass through a thin metal foils. Diffraction has been seen for neutrons, hydrogen atoms, and alpha particles. In all cases, the measured matched de Broglie’s prediction. X-ray diffraction electron diffraction neutron diffraction

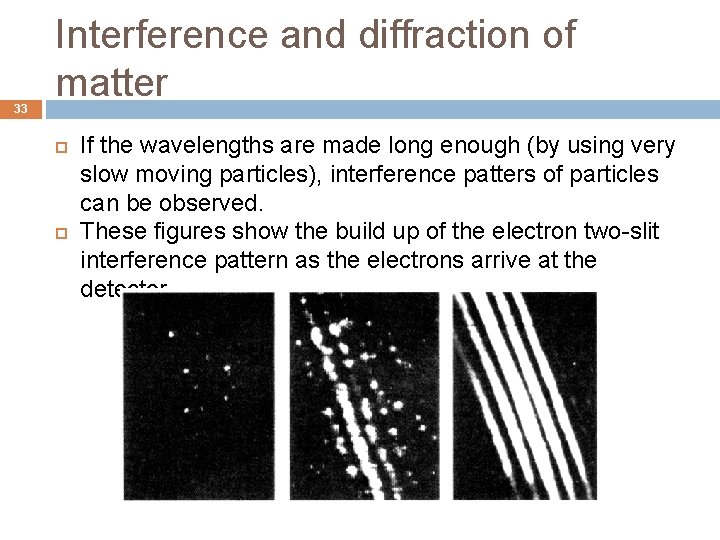
33 Interference and diffraction of matter If the wavelengths are made long enough (by using very slow moving particles), interference patters of particles can be observed. These figures show the build up of the electron two-slit interference pattern as the electrons arrive at the detector.

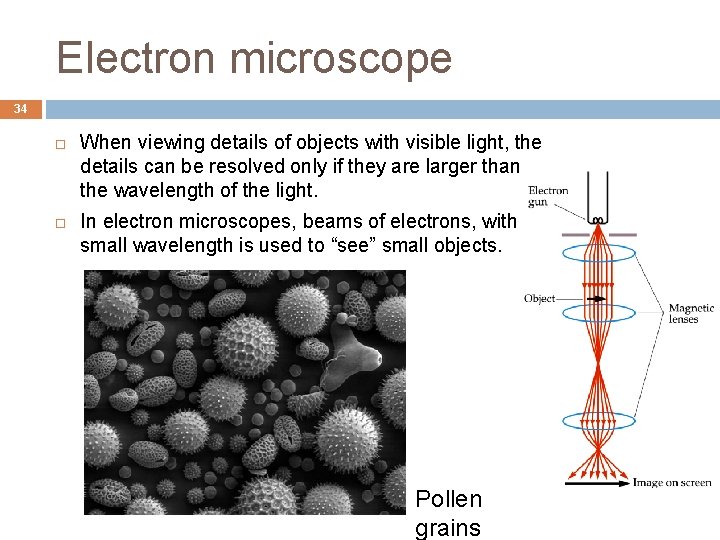
Electron microscope 34 When viewing details of objects with visible light, the details can be resolved only if they are larger than the wavelength of the light. In electron microscopes, beams of electrons, with small wavelength is used to “see” small objects. Pollen grains

Clicker Question 18 -1 The electron microscope is a welcome addition to the field of microscopy because electrons have a _____ wavelength than light, thereby increasing the _____ of the microscope. � � longer; resolving power longer; breadth of field shorter; resolving power longer; intensity

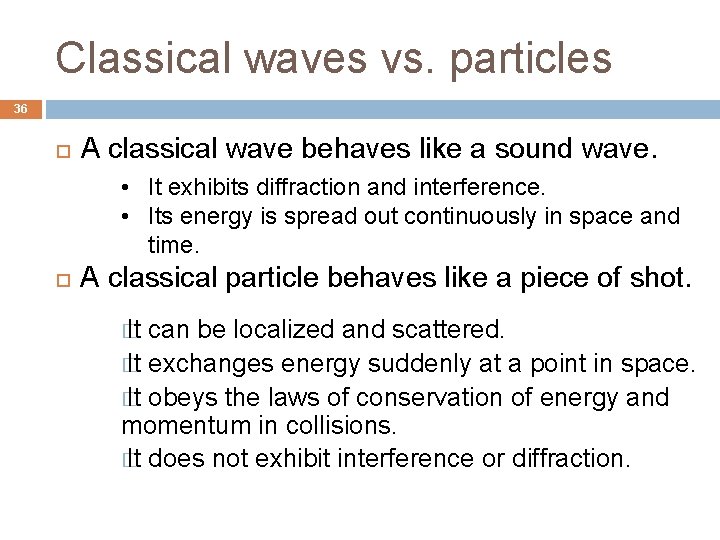
Classical waves vs. particles 36 A classical wave behaves like a sound wave. • It exhibits diffraction and interference. • Its energy is spread out continuously in space and time. A classical particle behaves like a piece of shot. � It can be localized and scattered. � It exchanges energy suddenly at a point in space. � It obeys the laws of conservation of energy and momentum in collisions. � It does not exhibit interference or diffraction.

Wave-particle duality 37 Light, normally thought of as a wave, exhibits particle properties when it interacts with matter. • photoelectric effect Electrons, normally thought of as particles, exhibit the wave properties when they pass near the edges of obstacles. • interference and diffraction All carriers of p and E exhibit both wave and particle characteristics. In classical physics, the concepts of waves and particles are mutually exclusive.

Wave-particle duality 38 The classical concepts of waves and particles do not adequately describe the complete behavior of any phenomenon. Everything propagates like a wave and exchanges energy like a particle. Often the concepts of the classical particle and the classical wave give the same results. � If is very small, diffraction and interference are not observable. � If there a lot of particles, they can be treated as a wave.

Clicker Question 19 -1 If the wavelength of an electron is equal to the wavelength of a proton, then. 1. 2. 3. 4. 5. the speed of the proton is greater than the speed of the electron the speeds of the proton and the electron are equal the speed of the proton is less than the speed of the electron the energy of the proton is greater than the energy of the electron, both (1) and (4) are correct.

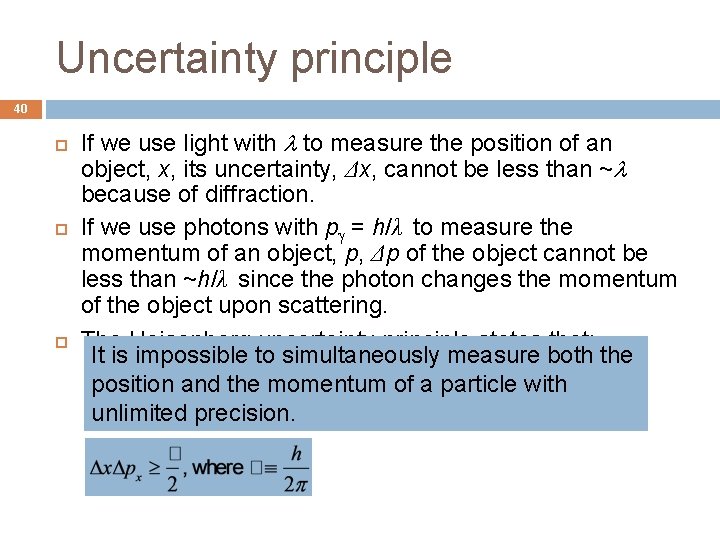
Uncertainty principle 40 If we use light with to measure the position of an object, x, its uncertainty, Δx, cannot be less than ~ because of diffraction. If we use photons with p = h/λ to measure the momentum of an object, p, Δp of the object cannot be less than ~h/λ since the photon changes the momentum of the object upon scattering. The Heisenberg uncertainty principle states that: It is impossible to simultaneously measure both the position and the momentum of a particle with unlimited precision.

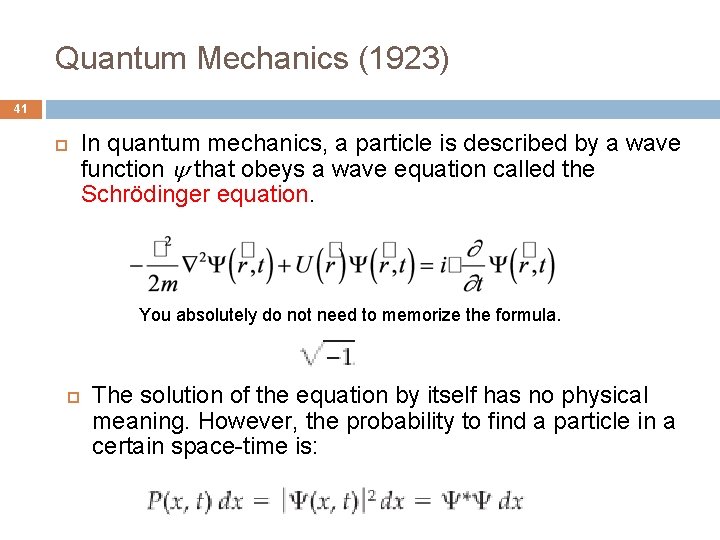
Quantum Mechanics (1923) 41 In quantum mechanics, a particle is described by a wave function that obeys a wave equation called the Schrödinger equation. You absolutely do not need to memorize the formula. The solution of the equation by itself has no physical meaning. However, the probability to find a particle in a certain space-time is:

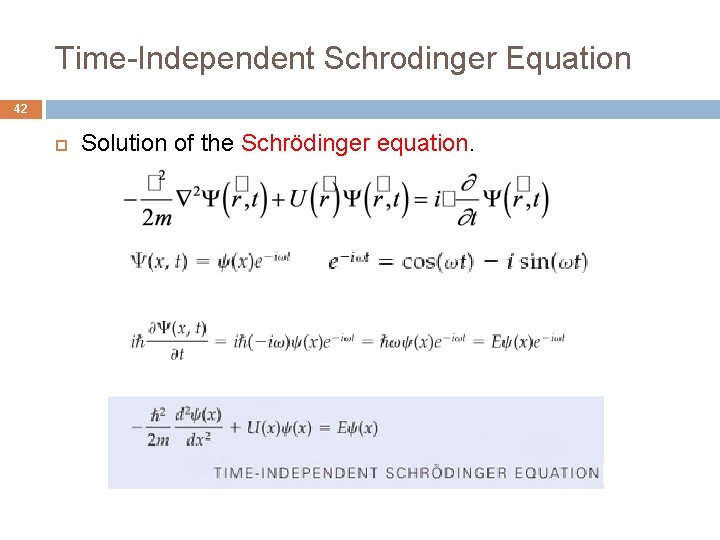
Time-Independent Schrodinger Equation 42 Solution of the Schrödinger equation.

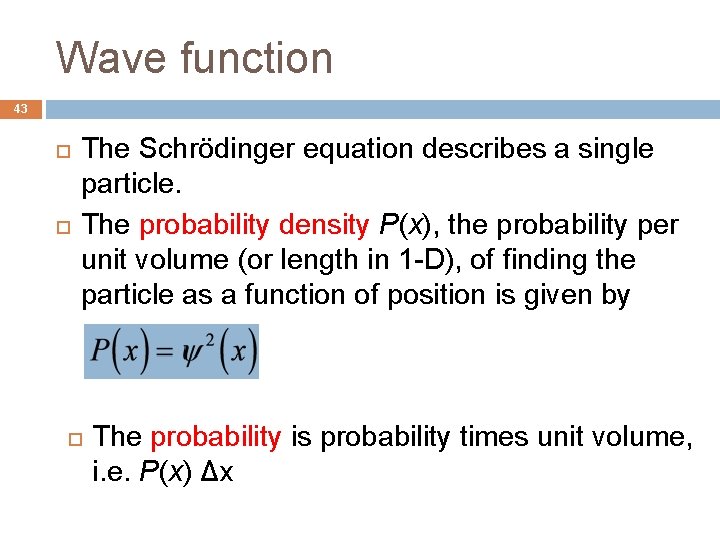
Wave function 43 The Schrödinger equation describes a single particle. The probability density P(x), the probability per unit volume (or length in 1 -D), of finding the particle as a function of position is given by The probability is probability times unit volume, i. e. P(x) Δx

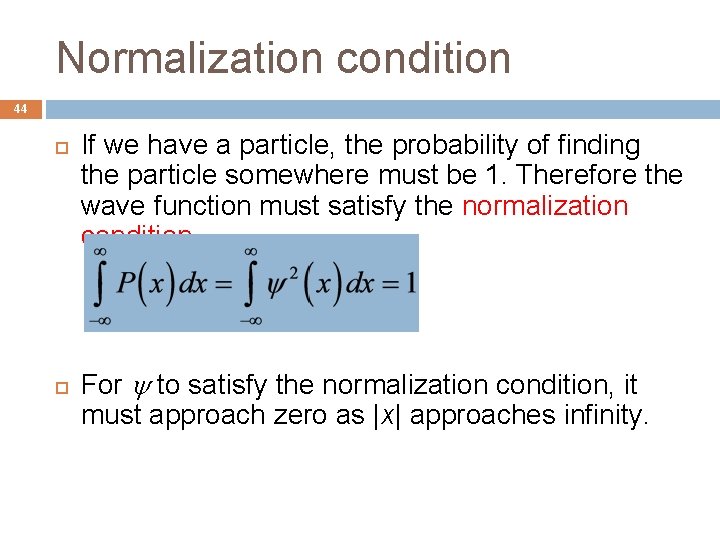
Normalization condition 44 If we have a particle, the probability of finding the particle somewhere must be 1. Therefore the wave function must satisfy the normalization condition. For to satisfy the normalization condition, it must approach zero as |x| approaches infinity.

45 Backup

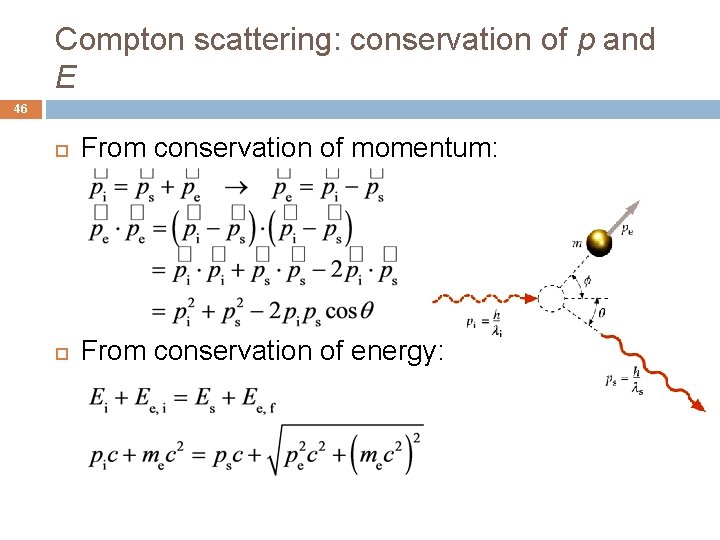
Compton scattering: conservation of p and E 46 From conservation of momentum: From conservation of energy:

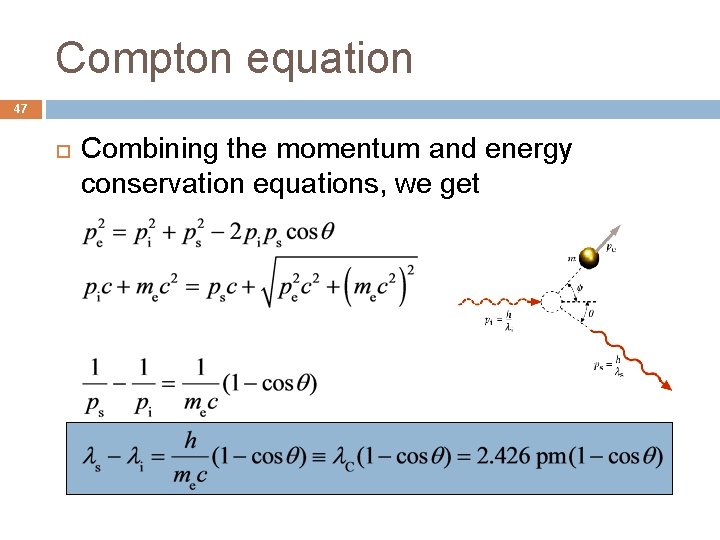
Compton equation 47 Combining the momentum and energy conservation equations, we get

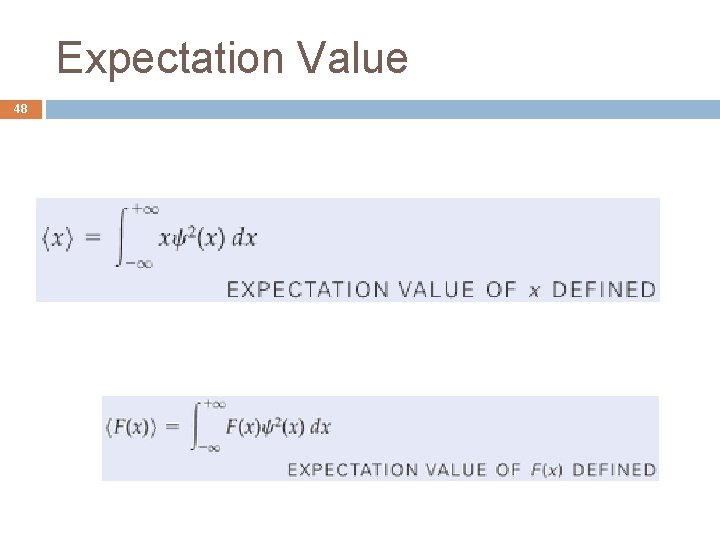
Expectation Value 48

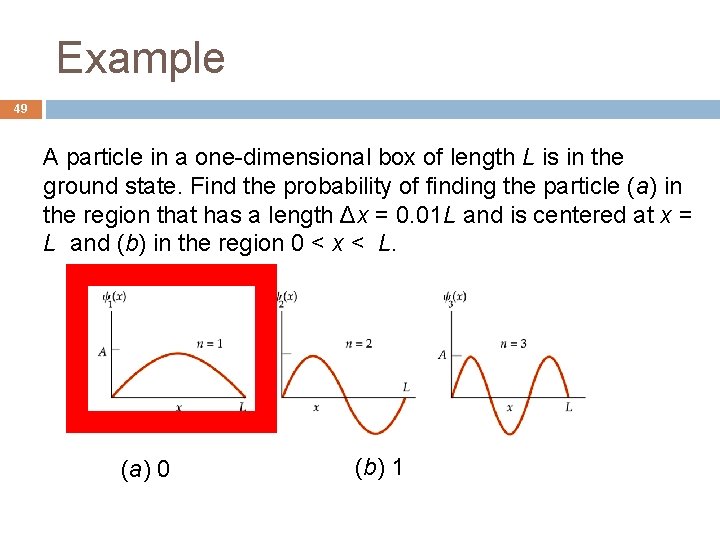
Example 49 A particle in a one-dimensional box of length L is in the ground state. Find the probability of finding the particle (a) in the region that has a length Δx = 0. 01 L and is centered at x = L and (b) in the region 0 < x < L. (a) 0 (b) 1

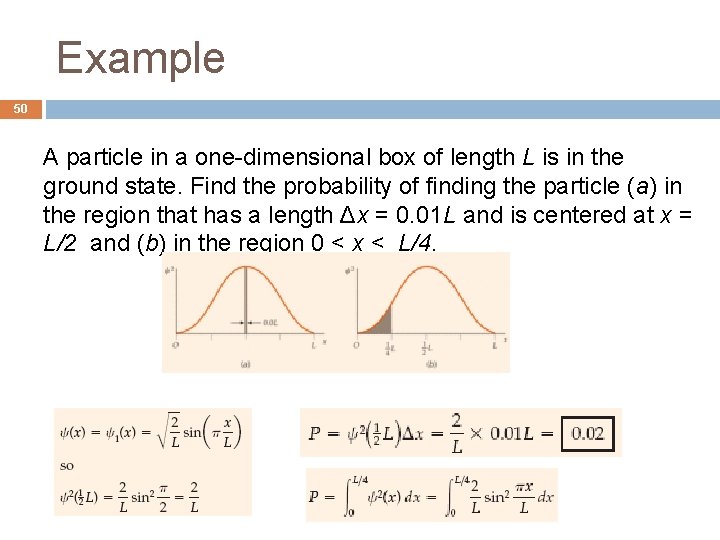
Example 50 A particle in a one-dimensional box of length L is in the ground state. Find the probability of finding the particle (a) in the region that has a length Δx = 0. 01 L and is centered at x = L/2 and (b) in the region 0 < x < L/4.

Example 3 51 The photons in a monochromatic beam are scattered by electrons. The wavelength of the photons that are scattered at an angle of 135° with the direction of the incident photon beam is 2. 3 percent more than the wavelength of the incident photons. a) b) What is the wavelength of the incident photons? What is the kinetic energy of the electron?

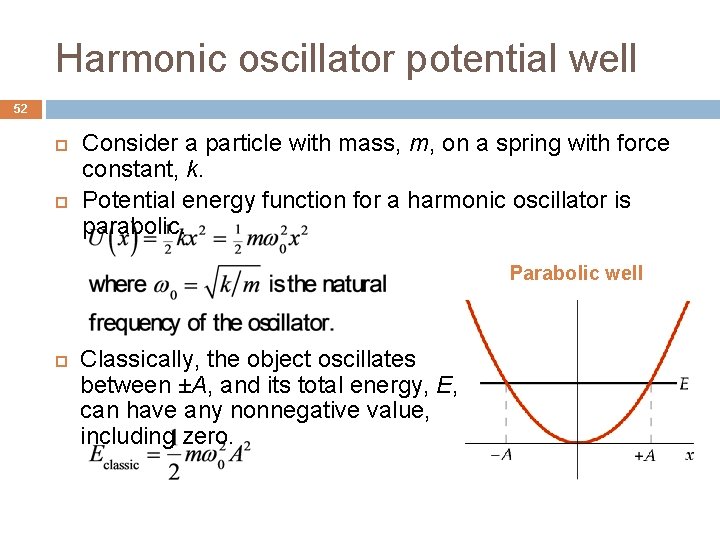
Harmonic oscillator potential well 52 Consider a particle with mass, m, on a spring with force constant, k. Potential energy function for a harmonic oscillator is parabolic. Parabolic well Classically, the object oscillates between ±A, and its total energy, E, can have any nonnegative value, including zero.

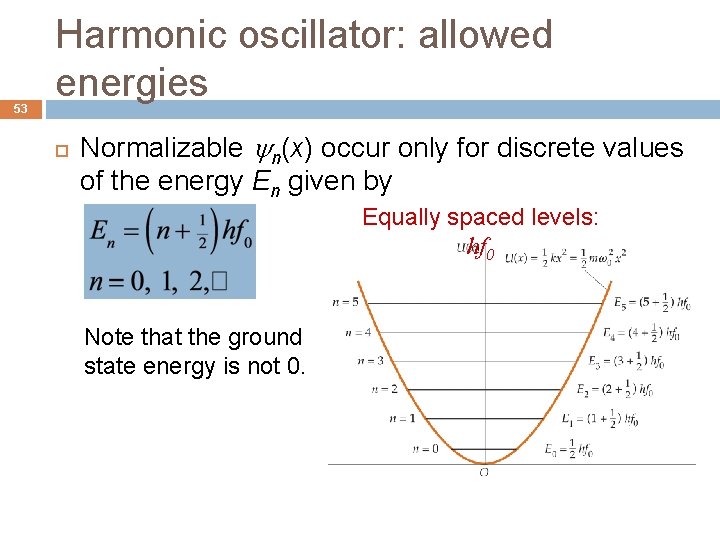
53 Harmonic oscillator: allowed energies Normalizable n(x) occur only for discrete values of the energy En given by Equally spaced levels: hf 0 Note that the ground state energy is not 0.

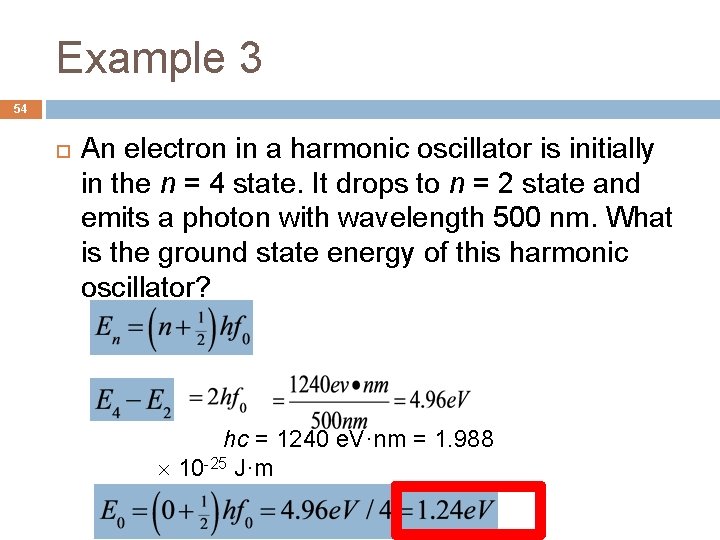
Example 3 54 An electron in a harmonic oscillator is initially in the n = 4 state. It drops to n = 2 state and emits a photon with wavelength 500 nm. What is the ground state energy of this harmonic oscillator? hc = 1240 e. V·nm = 1. 988 10 -25 J·m