Lecture 5 Topic 3 Human beta globin gene

Lecture 5 Topic 3
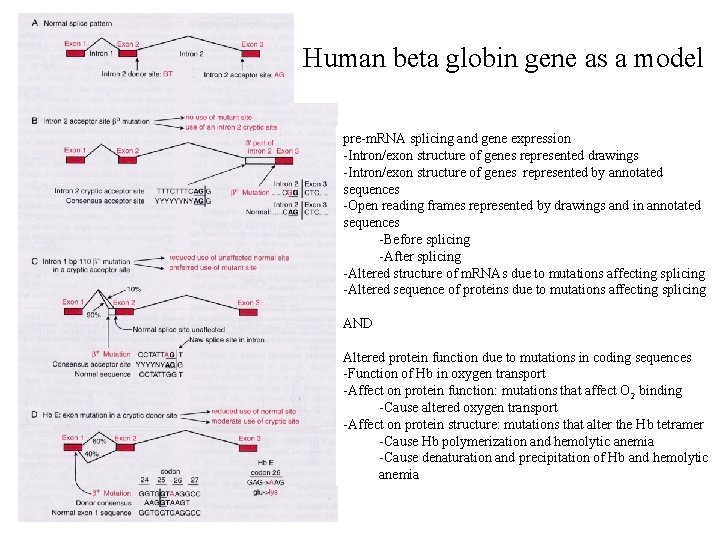
Human beta globin gene as a model pre-m. RNA splicing and gene expression -Intron/exon structure of genes represented drawings -Intron/exon structure of genes represented by annotated sequences -Open reading frames represented by drawings and in annotated sequences -Before splicing -After splicing -Altered structure of m. RNAs due to mutations affecting splicing -Altered sequence of proteins due to mutations affecting splicing AND Altered protein function due to mutations in coding sequences -Function of Hb in oxygen transport -Affect on protein function: mutations that affect O 2 binding -Cause altered oxygen transport -Affect on protein structure: mutations that alter the Hb tetramer -Cause Hb polymerization and hemolytic anemia -Cause denaturation and precipitation of Hb and hemolytic anemia

Other material from Chapter 3 • Codons, degeneracy, anticodons, wobble rules, silent mutations • Dominance and Recessiveness – Refer to Traits (phenotypes) – Molecular Basis: • Loss of function mutations, partial loss of function mutations, gain of function mutations, dominant negative mutations • Haplosufficiency and Haploinsufficiency
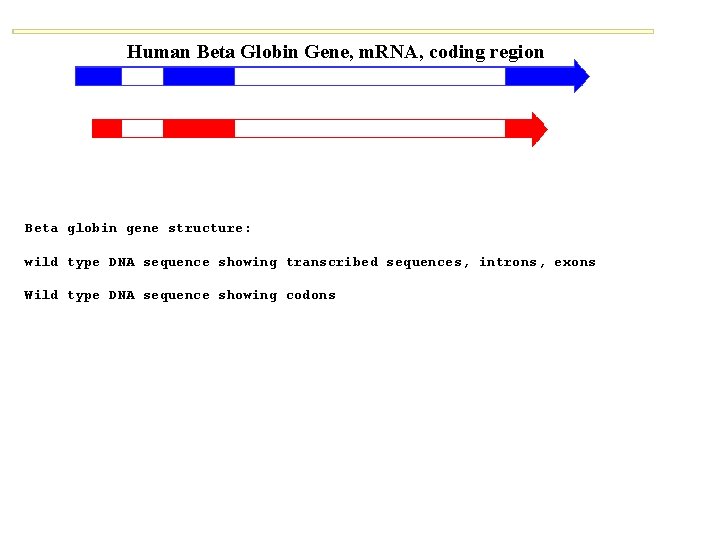
Human Beta Globin Gene, m. RNA, coding region Beta globin gene structure: wild type DNA sequence showing transcribed sequences, introns, exons Wild type DNA sequence showing codons
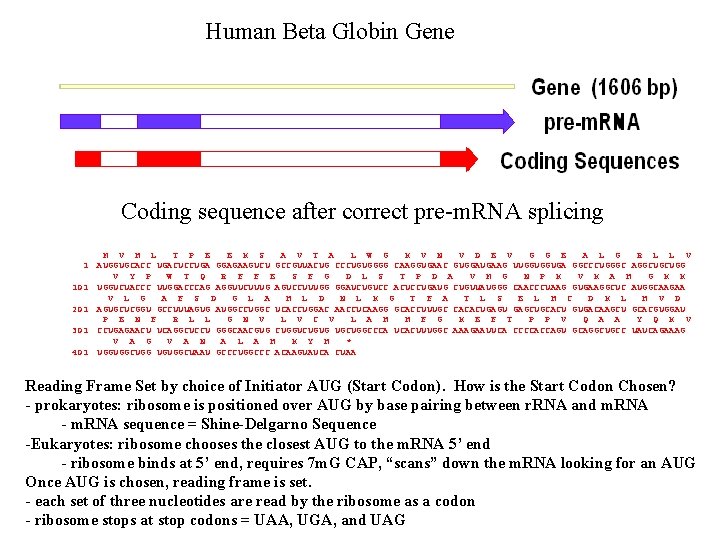
Human Beta Globin Gene Coding sequence after correct pre-m. RNA splicing 1 101 201 301 401 M V H L T P E E K S A V T A L W G K V N V D E V G G E A L G R L L V AUGGUGCACC UGACUCCUGA GGAGAAGUCU GCCGUUACUG CCCUGUGGGG CAAGGUGAAC GUGGAUGAAG UUGGUGGUGA GGCCCUGGGC AGGCUGCUGG · V Y P W T Q R F F E S F G D L S T P D A V M G N P K V K A H G K K · UGGUCUACCC UUGGACCCAG AGGUUCUUUG AGUCCUUUGG GGAUCUGUCC ACUCCUGAUG CUGUUAUGGG CAACCCUAAG GUGAAGGCUC AUGGCAAGAA · V L G A F S D G L A H L D N L K G T F A T L S E L H C D K L H V D AGUGCUCGGU GCCUUUAGUG AUGGCCUGGC UCACCUGGAC AACCUCAAGG GCACCUUUGC CACACUGAGU GAGCUGCACU GUGACAAGCU GCACGUGGAU P E N F R L L G N V L V C V L A H H F G K E F T P P V Q A A Y Q K V CCUGAGAACU UCAGGCUCCU GGGCAACGUG CUGGUCUGUG UGCUGGCCCA UCACUUUGGC AAAGAAUUCA CCCCACCAGU GCAGGCUGCC UAUCAGAAAG · V A G V A N A L A H K Y H * UGGUGGCUGG UGUGGCUAAU GCCCUGGCCC ACAAGUAUCA CUAA Reading Frame Set by choice of Initiator AUG (Start Codon). How is the Start Codon Chosen? - prokaryotes: ribosome is positioned over AUG by base pairing between r. RNA and m. RNA - m. RNA sequence = Shine-Delgarno Sequence -Eukaryotes: ribosome chooses the closest AUG to the m. RNA 5’ end - ribosome binds at 5’ end, requires 7 m. G CAP, “scans” down the m. RNA looking for an AUG Once AUG is chosen, reading frame is set. - each set of three nucleotides are read by the ribosome as a codon - ribosome stops at stop codons = UAA, UGA, and UAG
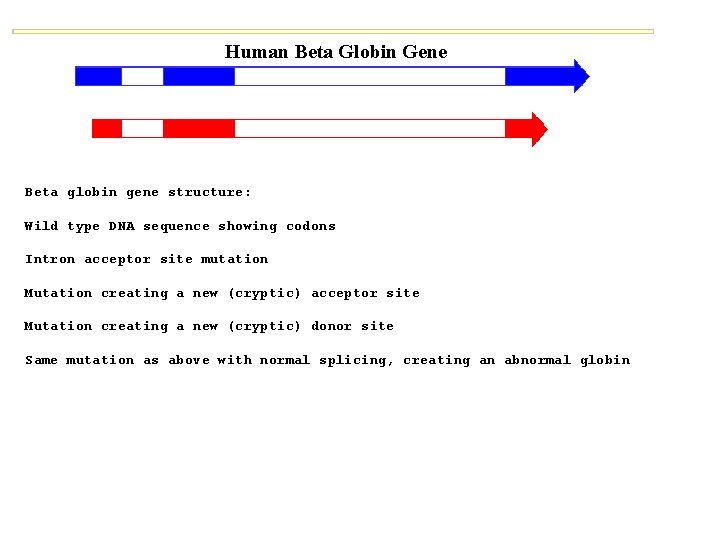
Human Beta Globin Gene Beta globin gene structure: Wild type DNA sequence showing codons Intron acceptor site mutation Mutation creating a new (cryptic) acceptor site Mutation creating a new (cryptic) donor site Same mutation as above with normal splicing, creating an abnormal globin
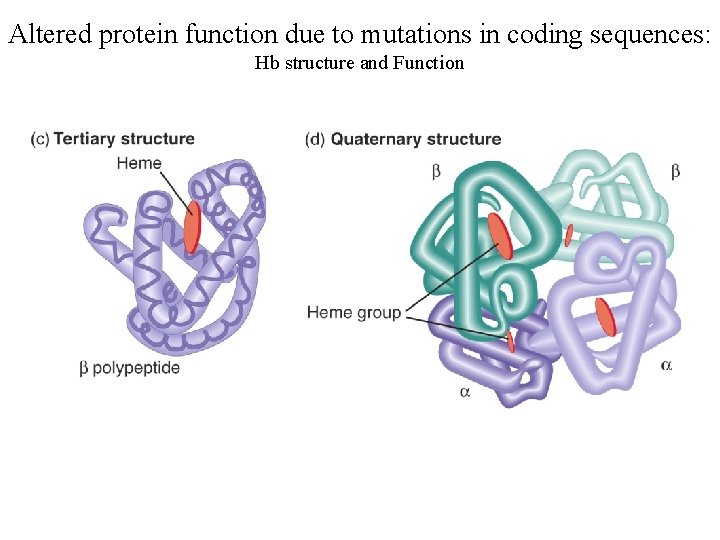
Altered protein function due to mutations in coding sequences: Hb structure and Function
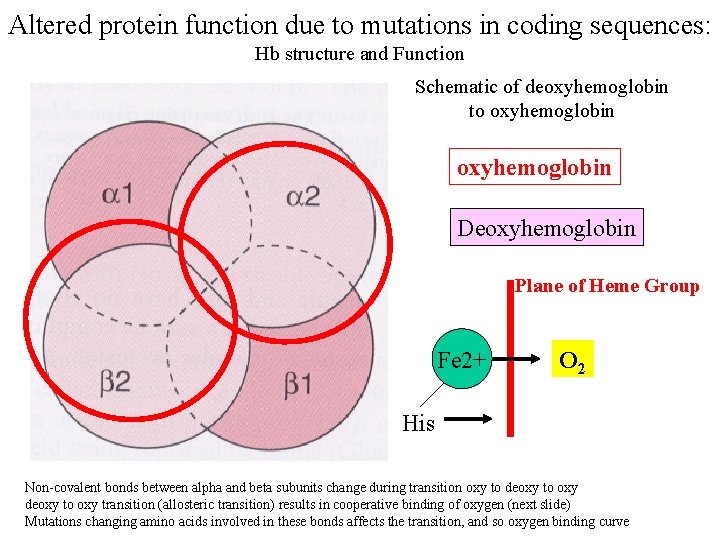
Altered protein function due to mutations in coding sequences: Hb structure and Function Schematic of deoxyhemoglobin to oxyhemoglobin Deoxyhemoglobin Plane of Heme Group Fe 2+ O 2 His Non-covalent bonds between alpha and beta subunits change during transition oxy to deoxy to oxy transition (allosteric transition) results in cooperative binding of oxygen (next slide) Mutations changing amino acids involved in these bonds affects the transition, and so oxygen binding curve
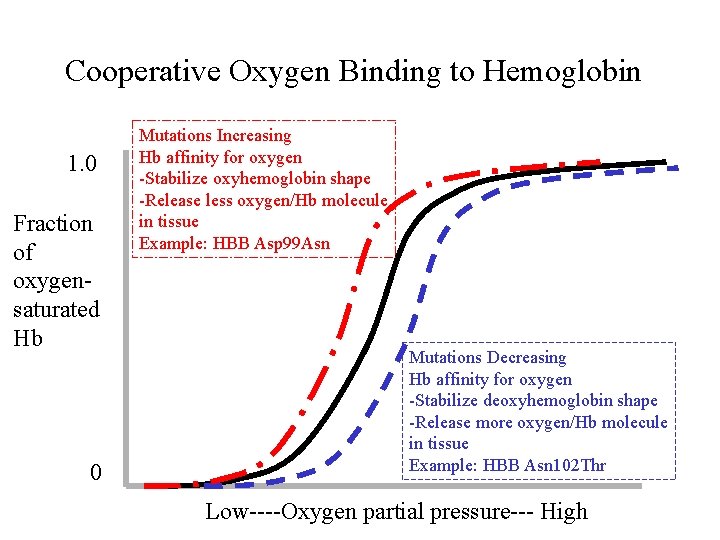
Cooperative Oxygen Binding to Hemoglobin 1. 0 Fraction of oxygensaturated Hb 0 Mutations Increasing Hb affinity for oxygen -Stabilize oxyhemoglobin shape -Release less oxygen/Hb molecule in tissue Example: HBB Asp 99 Asn Mutations Decreasing Hb affinity for oxygen -Stabilize deoxyhemoglobin shape -Release more oxygen/Hb molecule in tissue Example: HBB Asn 102 Thr Low----Oxygen partial pressure--- High

Mutations in Human Beta Globin Oxygen Transport HBB Asp 99 Asn HBB Asn 102 Thr Structure of Hb Tetramer: - Sickling HBB Glu 6 Val HBB Glu 6 Lys - Hb precipitation HBB Phe 42 Ser
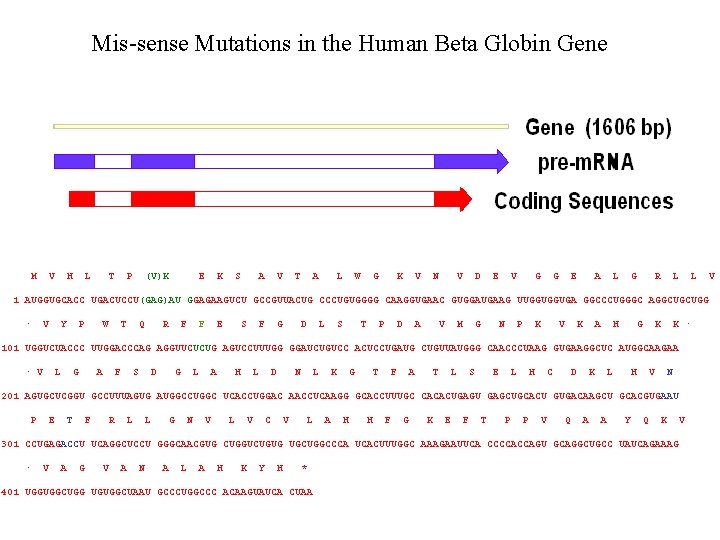
Mis-sense Mutations in the Human Beta Globin Gene M V H L T P (V)K E K S A V T A L W G K V N V D E V G G E A L G R L L 1 AUGGUGCACC UGACUCCU(GAG)AU GGAGAAGUCU GCCGUUACUG CCCUGUGGGG CAAGGUGAAC GUGGAUGAAG UUGGUGGUGA GGCCCUGGGC AGGCUGCUGG V · V Y P W T Q R F F E S F G D L S T P D A V M G N P K V K A H G K K · 101 UGGUCUACCC UUGGACCCAG AGGUUCUCUG AGUCCUUUGG GGAUCUGUCC ACUCCUGAUG CUGUUAUGGG CAACCCUAAG GUGAAGGCUC AUGGCAAGAA · V L G A F S D G L A H L D N L K G T F A T L S E L H C D K L H V N 201 AGUGCUCGGU GCCUUUAGUG AUGGCCUGGC UCACCUGGAC AACCUCAAGG GCACCUUUGC CACACUGAGU GAGCUGCACU GUGACAAGCU GCACGUGAAU P E T F R L L G N V L V C V L A H H F G K E F T P P V Q A A Y Q K 301 CCUGAGACCU UCAGGCUCCU GGGCAACGUG CUGGUCUGUG UGCUGGCCCA UCACUUUGGC AAAGAAUUCA CCCCACCAGU GCAGGCUGCC UAUCAGAAAG V · V A G V A N A L A H K Y H * 401 UGGUGGCUGG UGUGGCUAAU GCCCUGGCCC ACAAGUAUCA CUAA
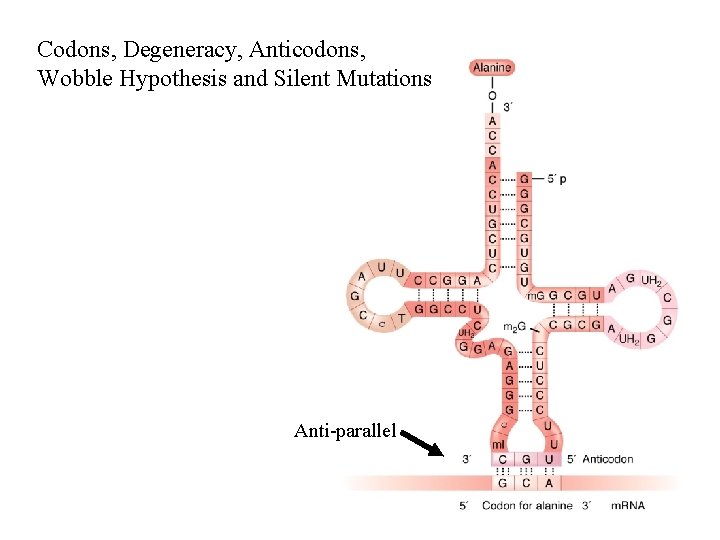
Codons, Degeneracy, Anticodons, Wobble Hypothesis and Silent Mutations Anti-parallel
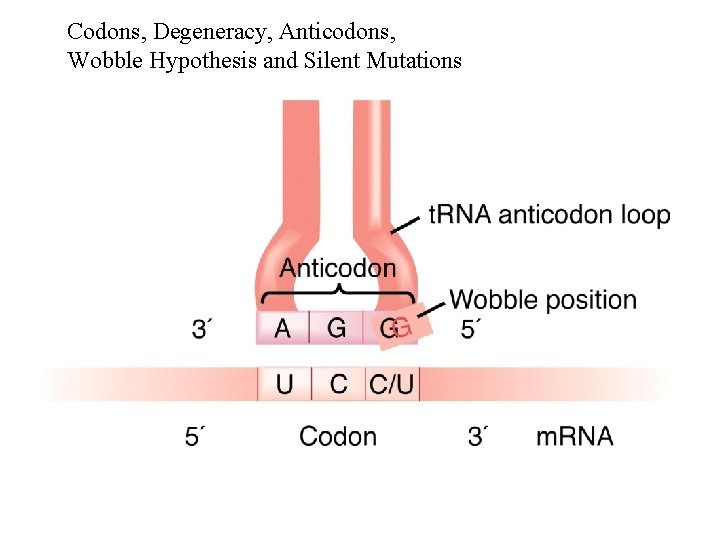
Codons, Degeneracy, Anticodons, Wobble Hypothesis and Silent Mutations
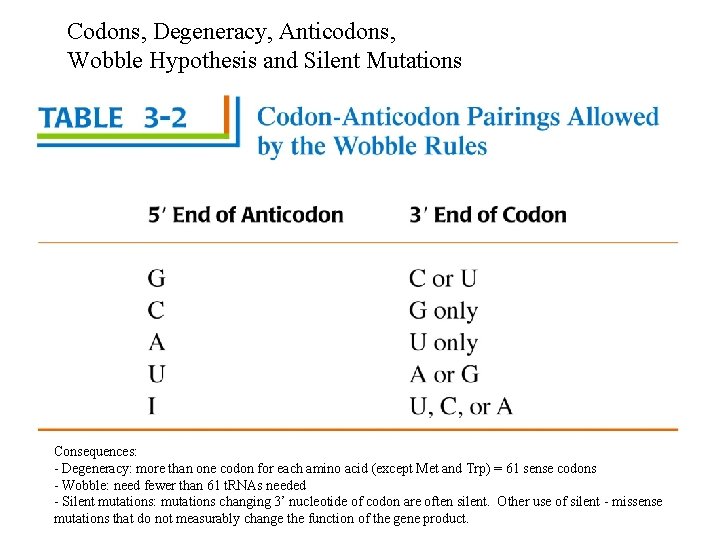
Codons, Degeneracy, Anticodons, Wobble Hypothesis and Silent Mutations Consequences: - Degeneracy: more than one codon for each amino acid (except Met and Trp) = 61 sense codons - Wobble: need fewer than 61 t. RNAs needed - Silent mutations: mutations changing 3’ nucleotide of codon are often silent. Other use of silent - missense mutations that do not measurably change the function of the gene product.
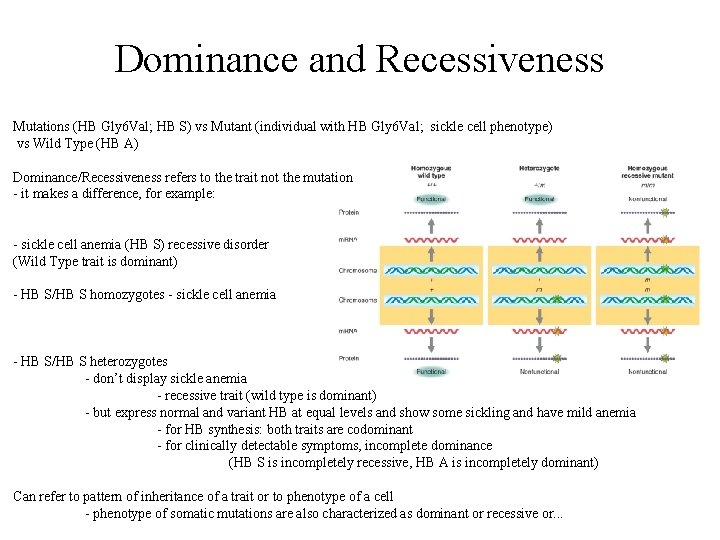
Dominance and Recessiveness Mutations (HB Gly 6 Val; HB S) vs Mutant (individual with HB Gly 6 Val; sickle cell phenotype) vs Wild Type (HB A) Dominance/Recessiveness refers to the trait not the mutation - it makes a difference, for example: - sickle cell anemia (HB S) recessive disorder (Wild Type trait is dominant) - HB S/HB S homozygotes - sickle cell anemia - HB S/HB S heterozygotes - don’t display sickle anemia - recessive trait (wild type is dominant) - but express normal and variant HB at equal levels and show some sickling and have mild anemia - for HB synthesis: both traits are codominant - for clinically detectable symptoms, incomplete dominance (HB S is incompletely recessive, HB A is incompletely dominant) Can refer to pattern of inheritance of a trait or to phenotype of a cell - phenotype of somatic mutations are also characterized as dominant or recessive or. . .
- Slides: 15