Lecture 5 THE MOLE Avogadros number The mole

Lecture 5

THE MOLE Avogadro's number The mole is used when we're talking about numbers of atoms and molecules (tiny particles). The mole is the “chemist’s dozen 1 dozen particles = 12 particles 1 mole particles = 6. 022 x 1023 particles Mole is the amount of a substance which contains 6. 022 x 1023 “items” could be: atoms, molecules, ions, The mole is used to describe a huge amount of any extremely small particle.
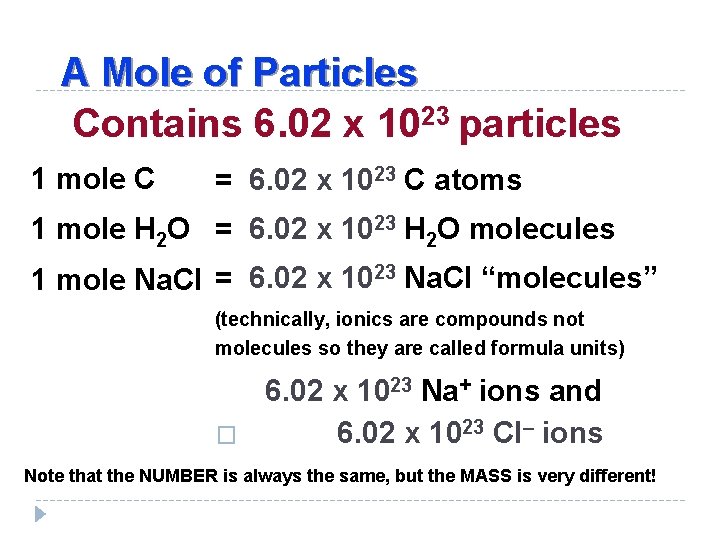
A Mole of Particles Contains 6. 02 x 1023 particles 1 mole C = 6. 02 x 1023 C atoms 1 mole H 2 O = 6. 02 x 1023 H 2 O molecules 1 mole Na. Cl = 6. 02 x 1023 Na. Cl “molecules” (technically, ionics are compounds not molecules so they are called formula units) � 6. 02 x 1023 Na+ ions and 6. 02 x 1023 Cl– ions Note that the NUMBER is always the same, but the MASS is very different!
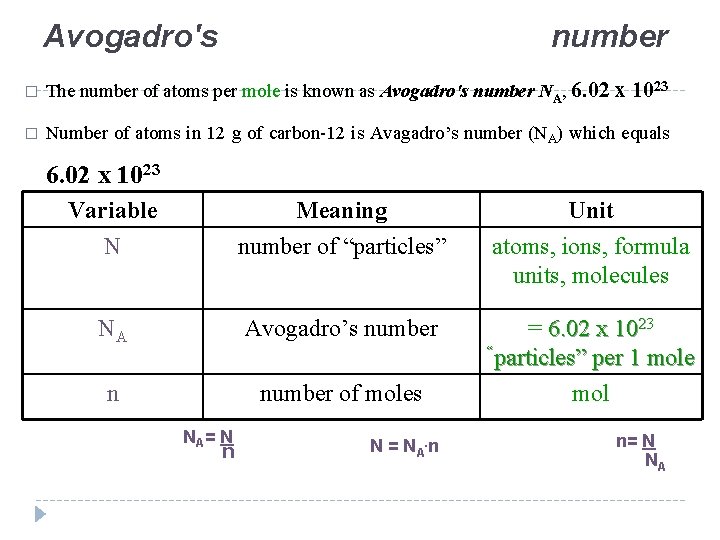
Avogadro's number � The number of atoms per mole is known as Avogadro's number NA, 6. 02 x 1023 � Number of atoms in 12 g of carbon-12 is Avagadro’s number (NA) which equals 6. 02 x 1023 Variable N Meaning number of “particles” Unit atoms, ions, formula units, molecules NA Avogadro’s number n number of moles = 6. 02 x 1023 “particles” per 1 mole mol NA = N n N = NA. n n= N NA

Mole Calculations To convert from atoms (or molecules) to moles, divide the # of atoms (or molecules) by Avogadro’s # Example: How many moles are 1. 0 x 1024 atoms? 1. n= N NA To convert from moles to atoms (or molecules), multiply the # of moles by Avogadro’s # Example: How many molecules are in 2. 5 moles? 2. N = NA. n
Molar Mass (MM) (g/mol) • • MOLE IS quantity of substance that contains the same number of molecules as exactly 12 g of carbon Molar mass is the mass of one mole of any element or compound. (in grams) The molar mass for elements is found by looking at the atomic masses on the Periodic Table. The units for molar mass are “grams per mole”
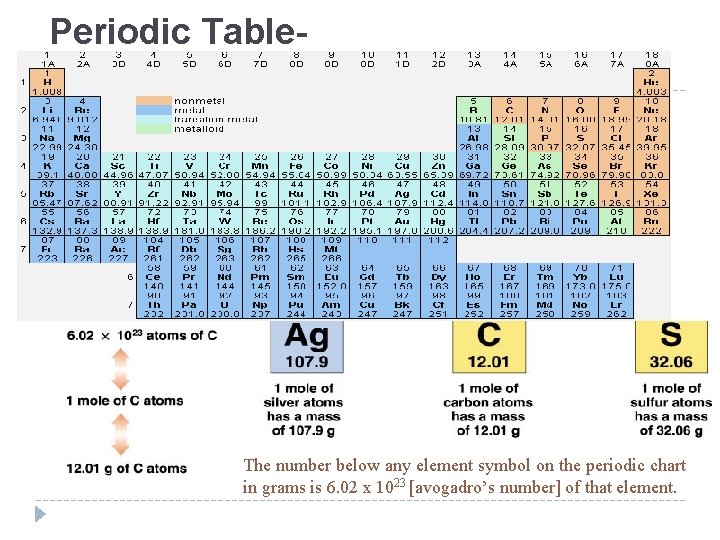
Periodic Table- The number below any element symbol on the periodic chart in grams is 6. 02 x 1023 [avogadro’s number] of that element.
Molar Mass for Compounds • Molar Masses are equal to the sums of the molar masses of all atoms in one molecule of that chemical compound Ex. Na. Cl Contains one atom of sodium and one atom of chlorine. (1 atom x 23. 0 g/mole Na) + (1 atom x 35. 5 g/mole Cl) = 58. 5 g/mole Na. Cl � (FW) =(C 58. 5 amu/f. u. The. Formula mass of 1 Weight mole of Glucose 6 H 12 O 6) is _180. 15 g/mole. MM of C = 6 x 12= 72 g MM of H = 12 x 1= 12 g MM of O = 6 x 16 = 96 -----180 g/ mole Molecular Weight (MW) =180. 15 amu/molecule

Molar Mass of Compounds �Another example: H 2 O H: 2 x 1. 00794 = 2. 01588 g O: 1 x 15. 9994 = 15. 9994 g The molar mass of H 2 O = 18. 0153 g/mol �Example: 1 Ca: 1 S: 4 O: Ca. SO 4 x 40. 1 g x 32. 1 g x 16. 0 g = = = Then add masses of all elements together 40. 1 g 32. 1 g 64. 0 g 136 g/mol

Mole-Mass Calculations Mole-Mass Relationship g mol g/mol n=m M g = mol x g/mol = g g/mol To convert from moles to grams, multiply the # of moles by atomic mass Example: How many grams in 2. 5 moles of carbon? 1. To convert from grams to moles, divide the mass in grams by atomic mass Example: How many moles are in 2. 5 g of lithium? 2.
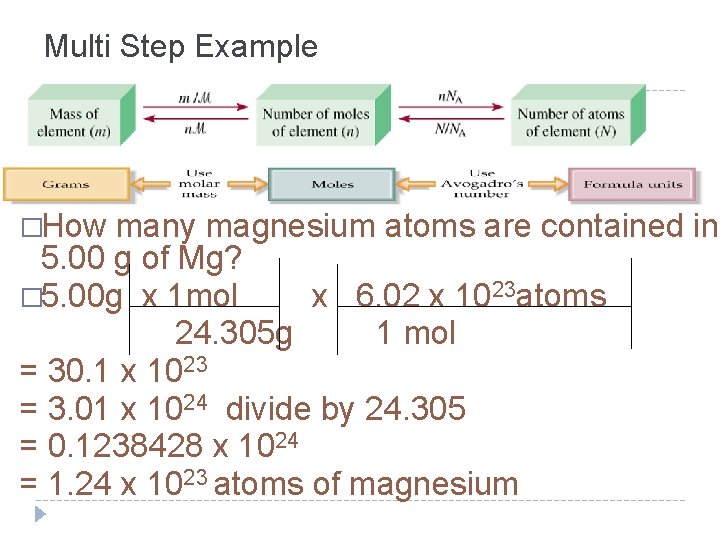
Multi Step Example �How many magnesium atoms are contained in 5. 00 g of Mg? � 5. 00 g x 1 mol x 6. 02 x 1023 atoms 24. 305 g 1 mol = 30. 1 x 1023 = 3. 01 x 1024 divide by 24. 305 = 0. 1238428 x 1024 = 1. 24 x 1023 atoms of magnesium
- Slides: 11