Lecture 5 Origin of the Elements Immediately after



















- Slides: 45

Lecture 5 Origin of the Elements

Immediately after the Big Bang • Temperatures are so high that no atoms exist, only fundamental particles even smaller than neutrons and protons. There are no atoms or elements yet. • How does the universe then pass to this state, completely unfamiliar to us, to the collection of elements we find in the universe today?

Planetary Reality “Atoms are neither created nor destroyed” But where do the elements come from? How were they formed?

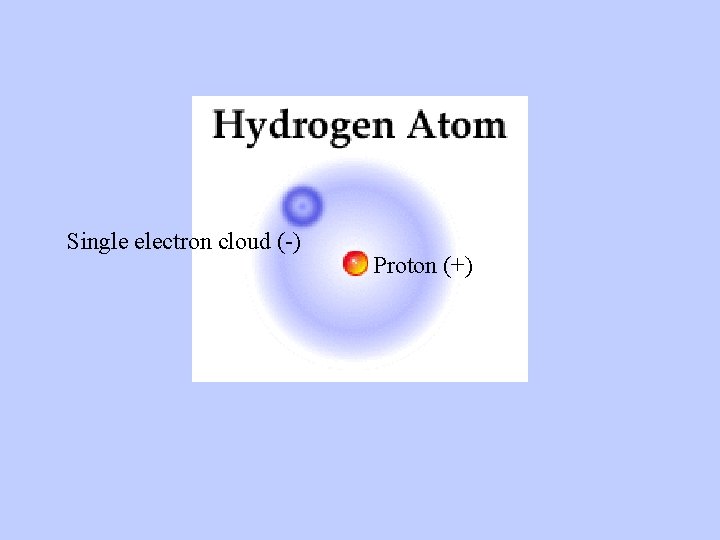
Single electron cloud (-) Proton (+)



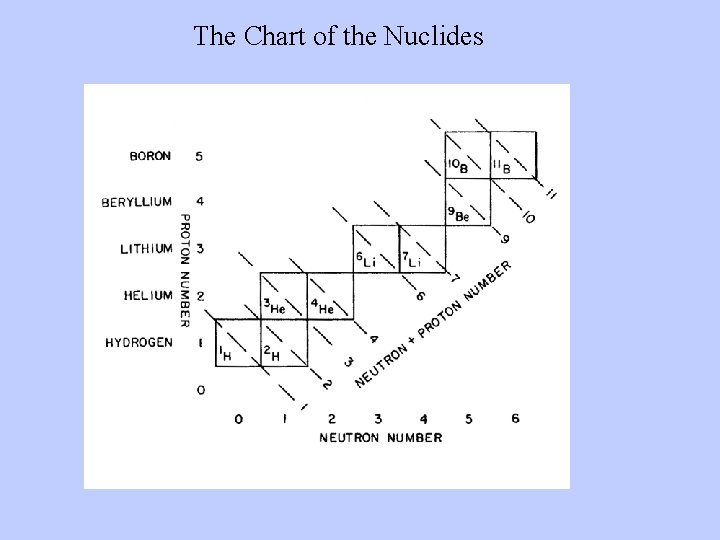
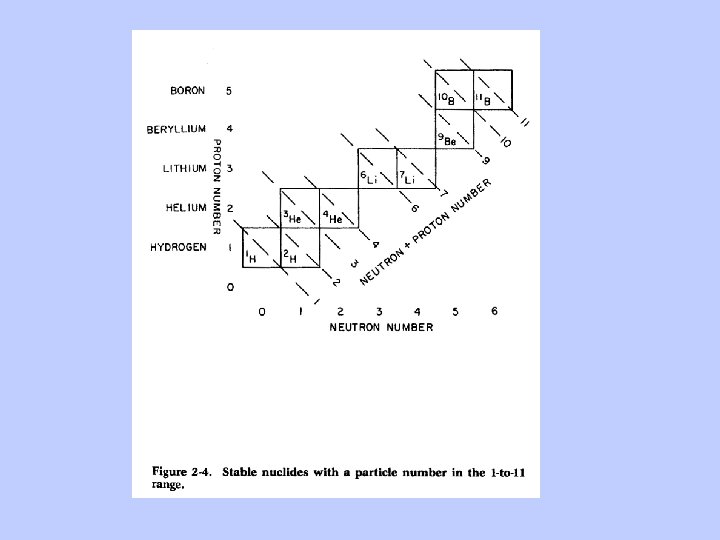
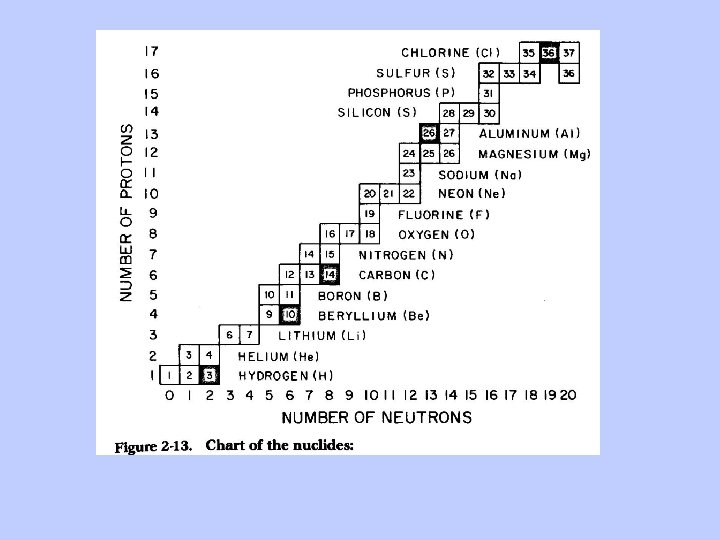
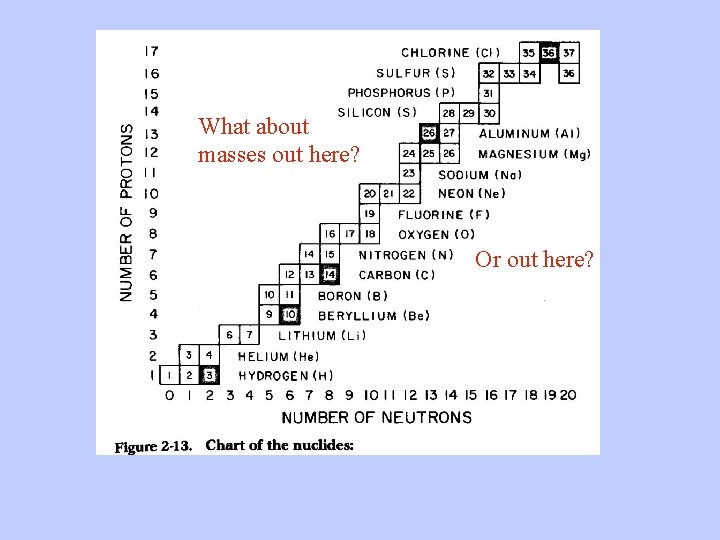
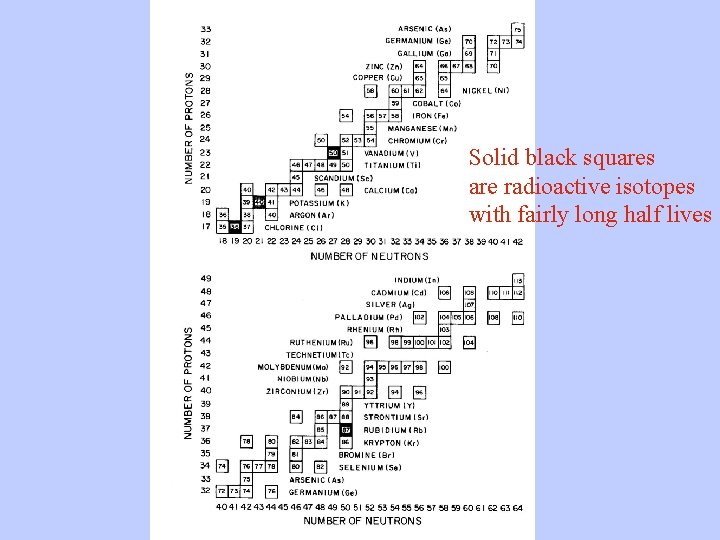
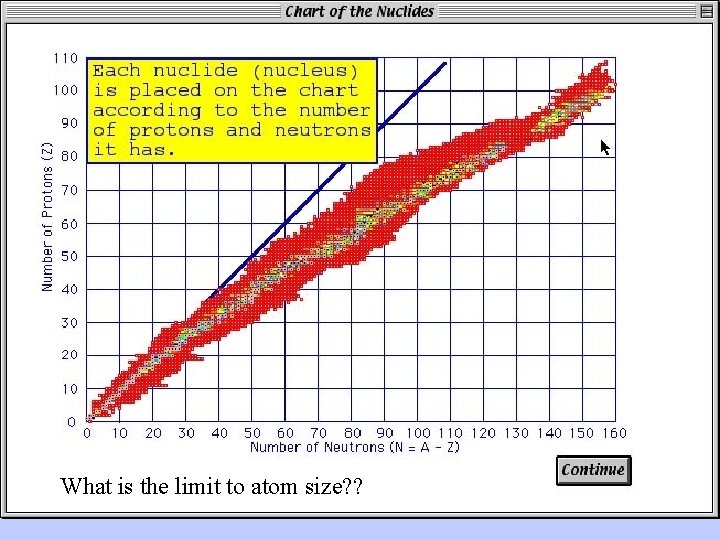
The Chart of the Nuclides

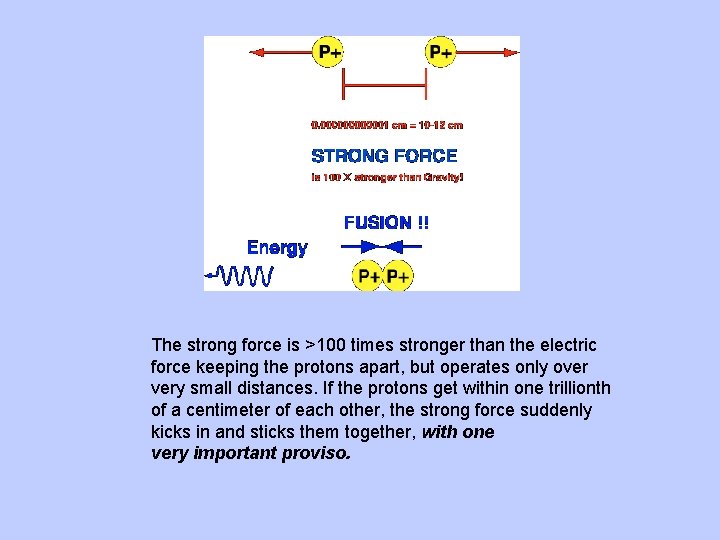
The strong force is >100 times stronger than the electric force keeping the protons apart, but operates only over very small distances. If the protons get within one trillionth of a centimeter of each other, the strong force suddenly kicks in and sticks them together, with one very important proviso.

E= 2 MC If there is a loss of mass, then energy is released. If there is a gain in mass, energy needs to be added to make the reaction happen.

Important Concepts: The total mass of a nucleus depends on its energy as well as the number of protons and neutrons Nuclear Fusion happens as long as mass of the product nucleus is lower than the reacting nucelii AND, nuclear fusion requires the nucleii to “touch” so the strong force can operate, and this requires very high temperatures. A confined environment helps.

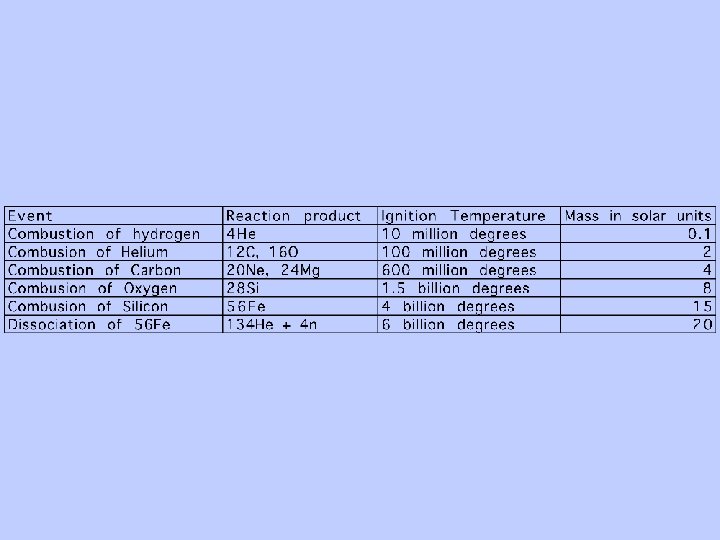
Mass of 4 hydrogen atoms: 6. 696 *10 -24 gm Mass of 1 helium atom: 6. 648 * 10 -24 gm Mass LOSS of. 048 *10 -24 gm Converting one gram of hydrogen to helium releases enough energy to boil 4. 4 million pounds of water BUT, temperatures of 10 million degrees are required for the protons to be forced together

During the Big Bang, the universe is rapidly expanding so temperatures decline very rapidly. There is a very short time window in which nuclear reactions can occur.
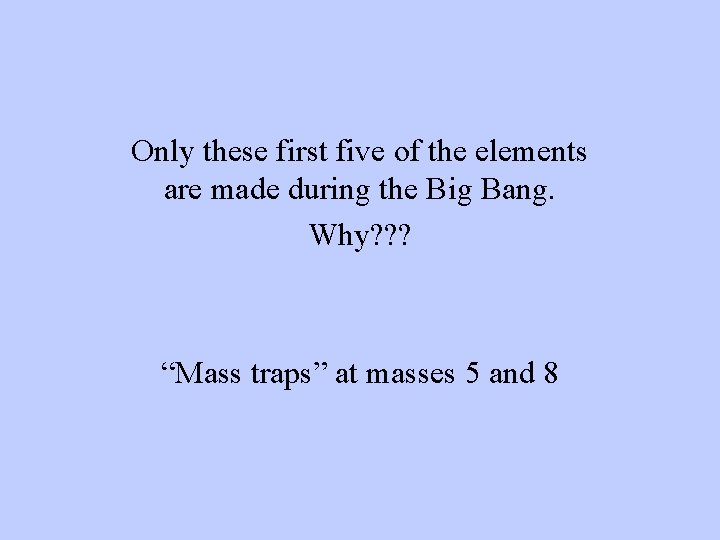
Only these first five of the elements are made during the Big Bang. Why? ? ? “Mass traps” at masses 5 and 8


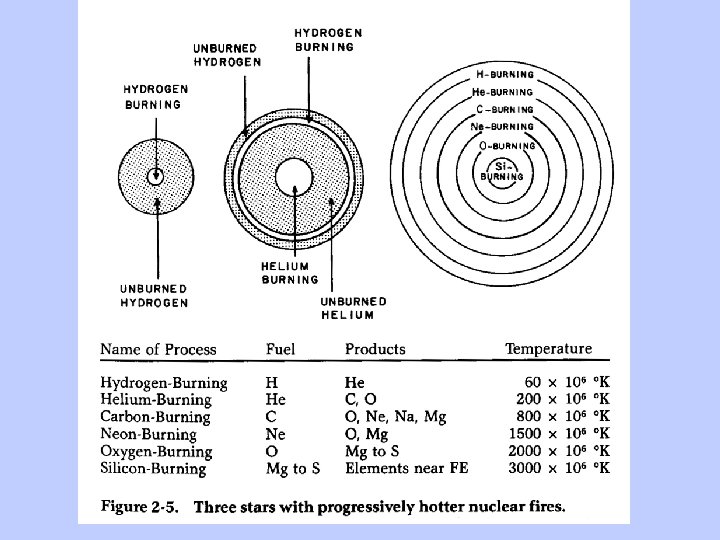
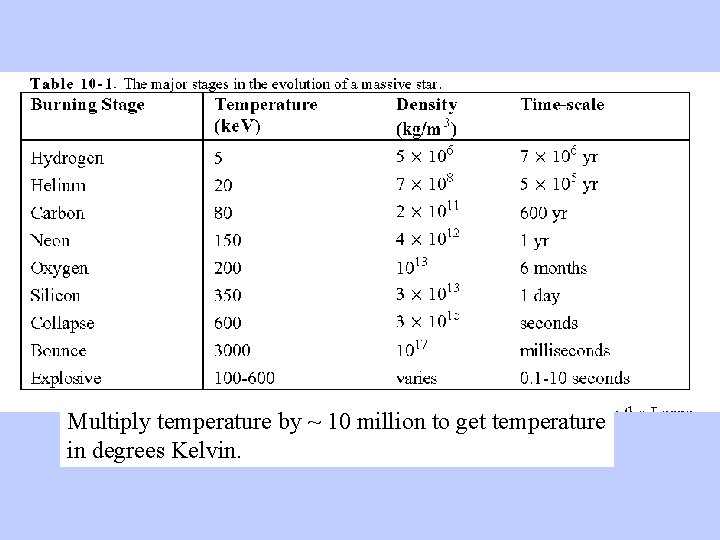
Important Concepts: Stars form by contraction of massive clouds of gas, driven by gravity As the cloud contracts, all its energy is confined to a smaller volume and it heats up It keeps contracting and keeps heating up until some force operates to offset the gravitational contraction. That force is the heat generated by nuclear fusion. The bigger the star, the stronger the gravity, and the more heat is required to keep it from contracting

12 In easier units, the mass of C is defined as 12. 00, The mass of 6 protons and 6 neutrons is 12. 102 converts to 12. 00 LOSS OF MASS-- the reaction “burns” The mass of 56 Fe is 55. 935 28 the mass of two Si is 55. 954 converts to 55. 935 LOSS of MASS-- the reaction still “burns” But since the repulsive force is strong, these reactions require very high temperatures indeed.




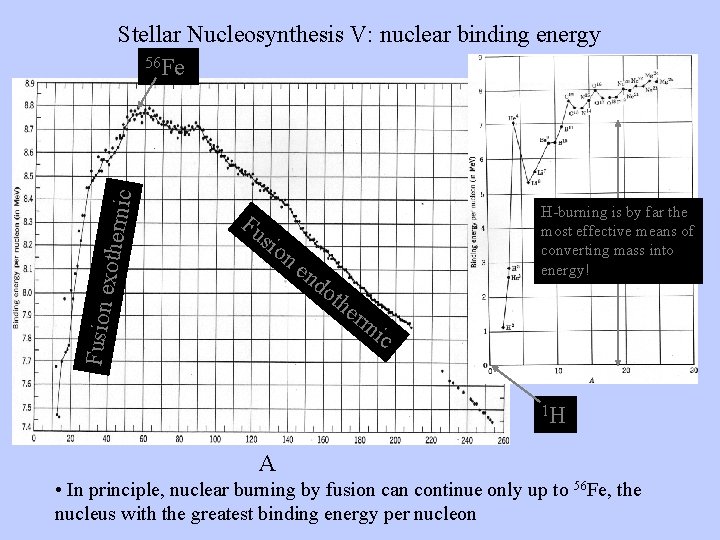
Mass loss through fusion STOPS at 56 Fe. Heavier masses have more mass per nucleon So we face two puzzles: § How do stars make all the heavier elements? §How do the elements then get distributed from stellar interiors to the galaxy to make planets like Earth possible?

Fu sio Fusion exother m ic Stellar Nucleosynthesis V: nuclear binding energy 56 Fe H-burning is by far the most effective means of converting mass into energy! ne nd oth erm ic 1 H A • In principle, nuclear burning by fusion can continue only up to 56 Fe, the nucleus with the greatest binding energy per nucleon

What about masses out here? Or out here?

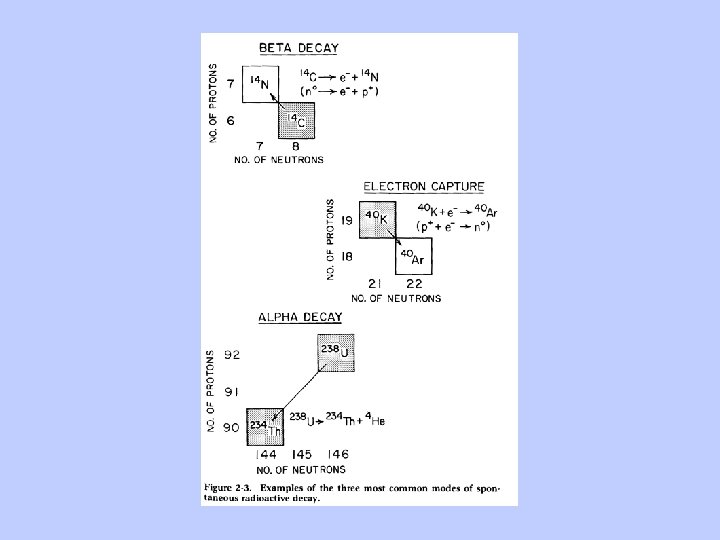
Important Concepts: The total mass of a nucleus depends on its energy as well as the number of protons and neutrons Nuclear Fusion cannot proceed beyond 56 Fe. For a given number of neutrons and protons, the lowest mass nucleus is the most stable. Neutrons and protons are convertible! The reaction: n p+ + e. Can go in both directions to obtain minimum mass. For mass 1, it proceeds to the right with a half life of 10. 3 minutes



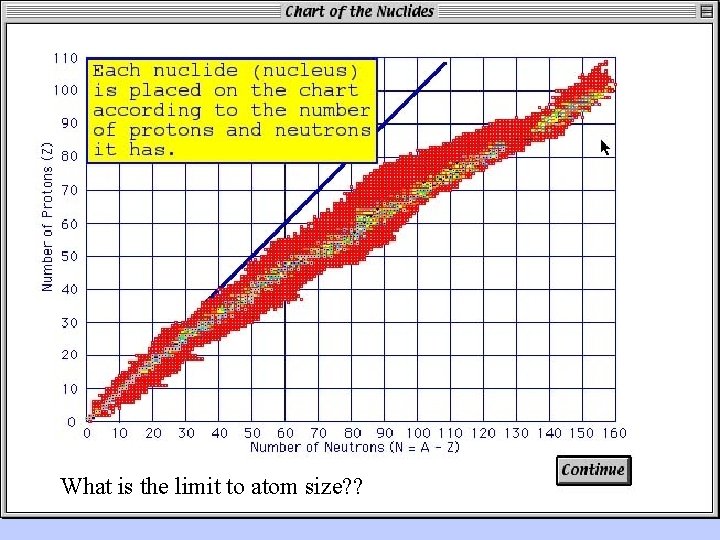
What is the limit to atom size? ?

Conceptual understanding of radioactive decay and maximum size of atomic nucleii can come from the realization that: (1) protons repel each other; (2) neutrons left to themselves decay.

Solid black squares are radioactive isotopes with fairly long half lives

Multiply temperature by ~ 10 million to get temperature in degrees Kelvin.






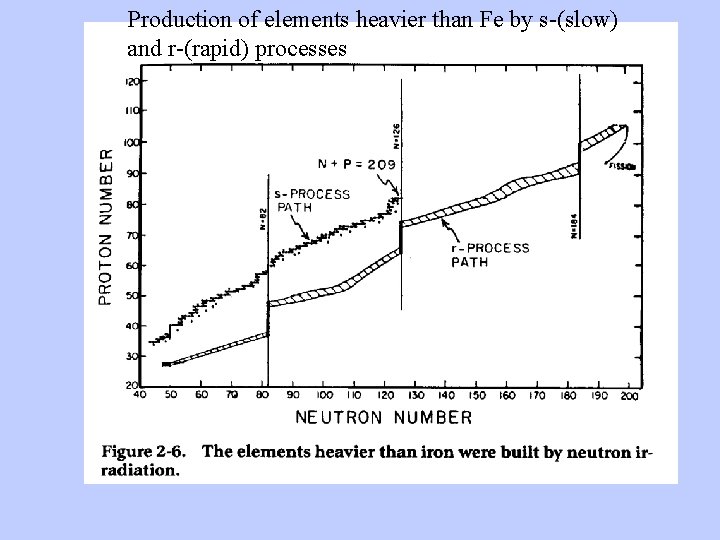
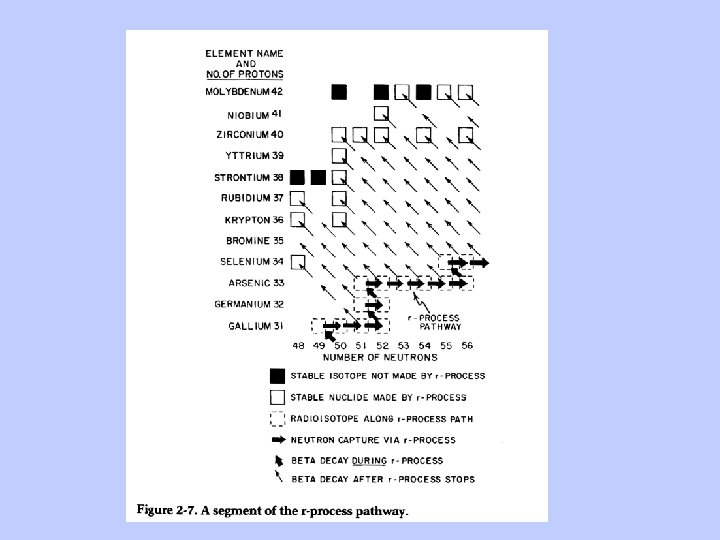
Production of elements heavier than Fe by s-(slow) and r-(rapid) processes


What is the limit to atom size? ?




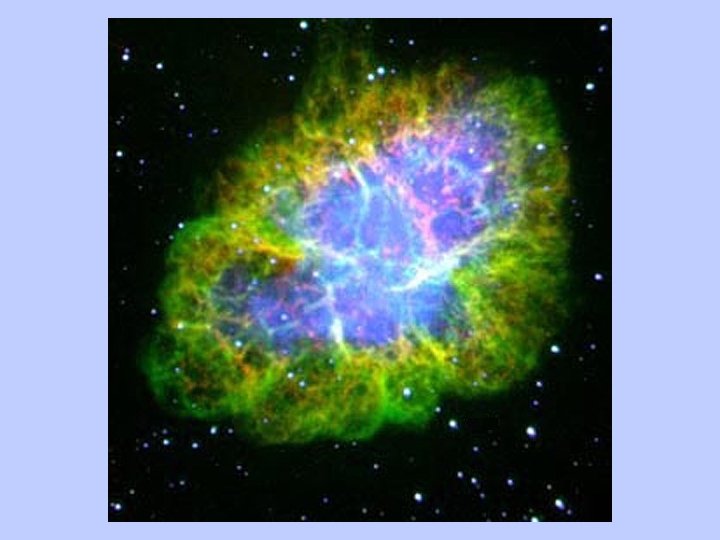
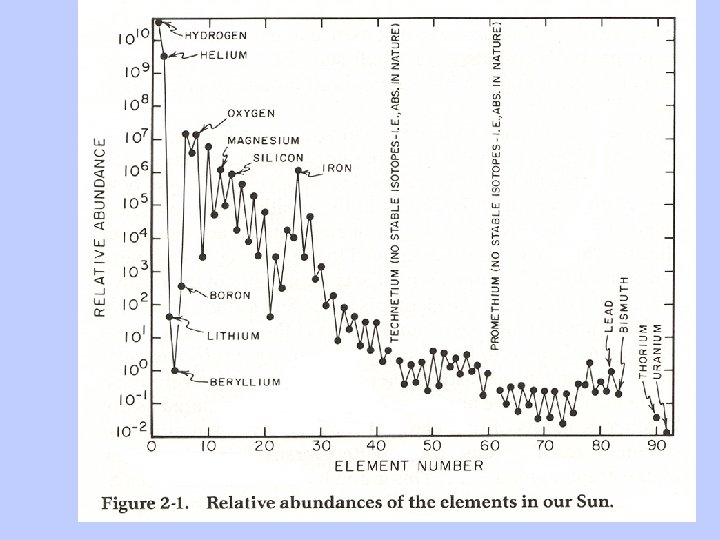
Is there concrete evidence for these processes? • How can stars be so hot for so long? • Super Nova explosions are observed • Calculations of relative element abundances correspond to observations, in detail • Technetium lines observed in supernova debris • Evidence for short-lived radionuclides in solar system materials


At the beginning… Formation of the elements Big Bang Our solar system forms at this time

Galaxies

Element Formation depends on position within a galaxy