Lecture 5 MACROMOLECULES 1 Carbohydrates And Lipids Sept
Lecture 5 MACROMOLECULES #1 Carbohydrates And Lipids Sept 9, 2005
Lecture outline: - Polymers -Carbohydrates monomers and polymers - Lipids
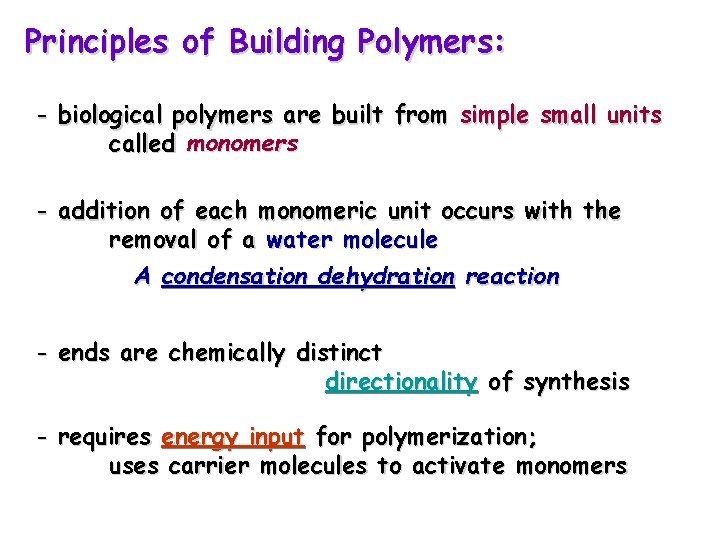
Principles of Building Polymers: - biological polymers are built from simple small units called monomers - addition of each monomeric unit occurs with the removal of a water molecule A condensation dehydration reaction - ends are chemically distinct directionality of synthesis - requires energy input for polymerization; uses carrier molecules to activate monomers
MODULAR DESIGN SIMPLICITY AND VERSATILITY ASSEMBLY-LINE MENTALITY Don’t have to make every structure from scratch Simplified chemistry, repeating link Dehydration Synthesis
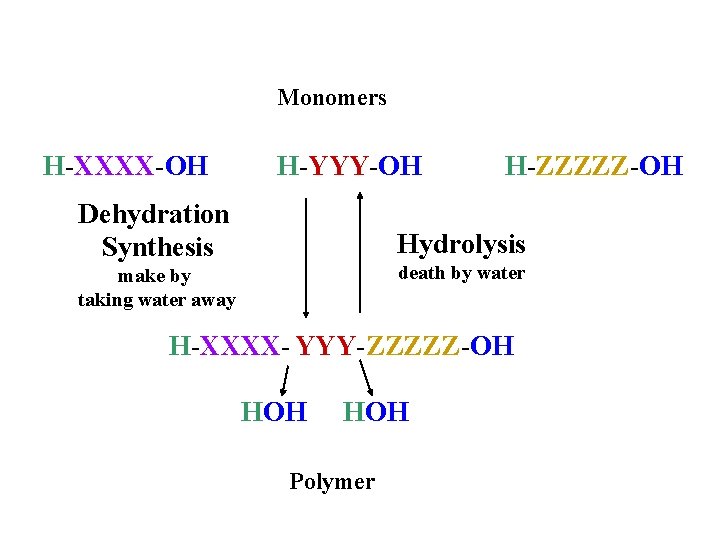
Monomers H-XXXX-OH H-YYY-OH Dehydration Synthesis H-ZZZZZ-OH Hydrolysis death by water make by taking water away H-XXXX- YYY-ZZZZZ-OH HOH Polymer
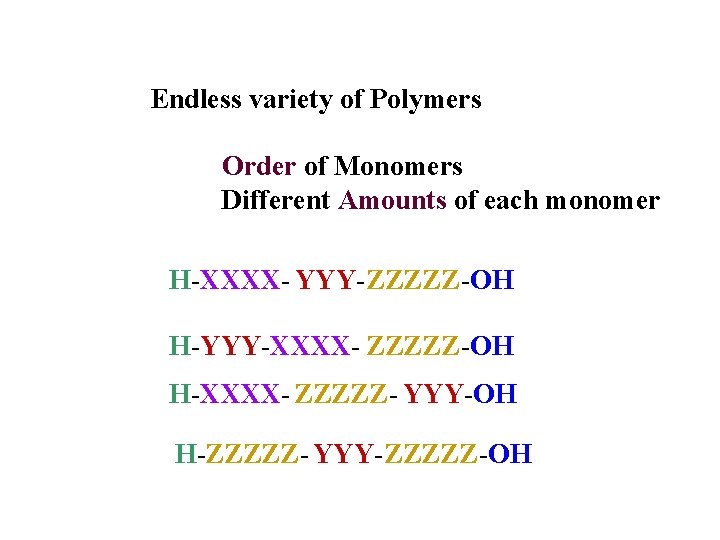
Endless variety of Polymers Order of Monomers Different Amounts of each monomer H-XXXX- YYY-ZZZZZ-OH H-YYY-XXXX- ZZZZZ-OH H-XXXX- ZZZZZ- YYY-OH H-ZZZZZ- YYY-ZZZZZ-OH
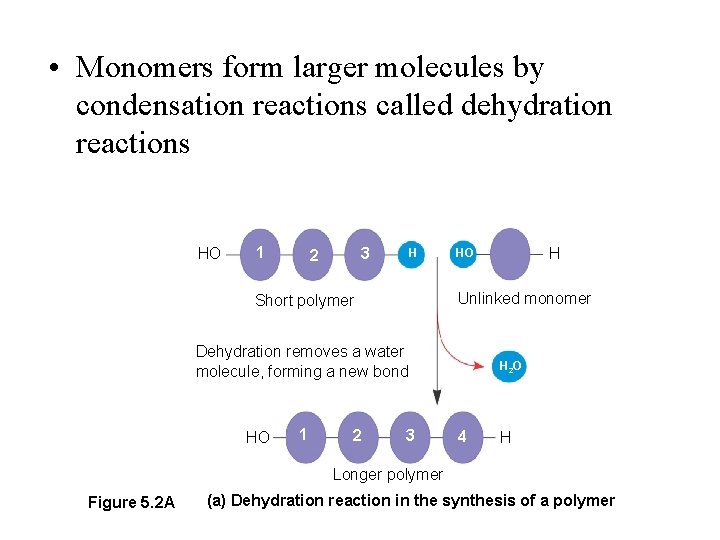
• Monomers form larger molecules by condensation reactions called dehydration reactions HO 1 3 2 H Unlinked monomer Short polymer Dehydration removes a water molecule, forming a new bond HO 1 2 H HO 3 H 2 O 4 H Longer polymer Figure 5. 2 A (a) Dehydration reaction in the synthesis of a polymer
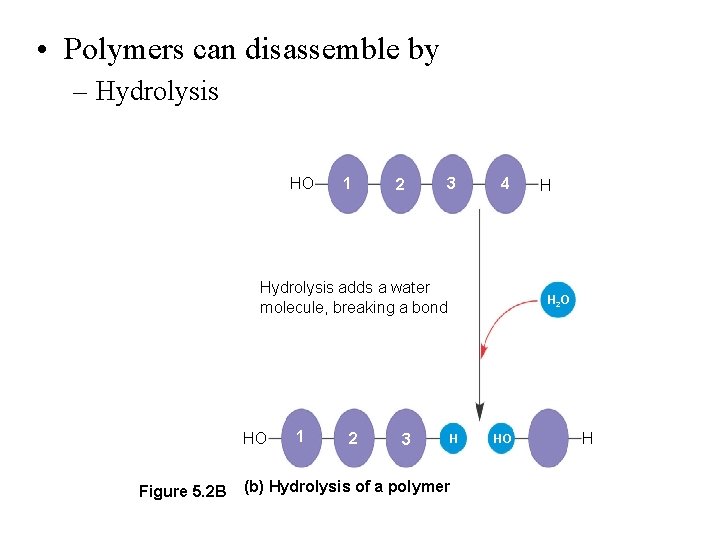
• Polymers can disassemble by – Hydrolysis HO 1 2 3 4 Hydrolysis adds a water molecule, breaking a bond HO Figure 5. 2 B 1 2 3 H H 2 O H (b) Hydrolysis of a polymer HO H

Monomers Polymers

CARBOHYDRATES Sugars and Sugar Derivatives Monomers: Polymers: Monosaccharides Polysaccharides Simple Sugars Glucose Fructose Ribose Oligosaccharides Informational structures Long chains of monomers storage starch: amylose amylopectin glycogen structure Fiber: cellulose
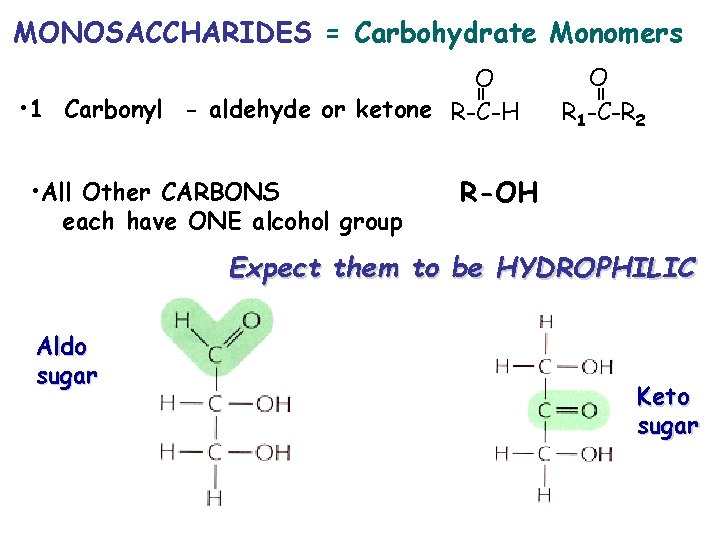
MONOSACCHARIDES = Carbohydrate Monomers • All Other CARBONS each have ONE alcohol group O R 1 -C-R 2 = = O • 1 Carbonyl - aldehyde or ketone R-C-H R-OH Expect them to be HYDROPHILIC Aldo sugar Keto sugar
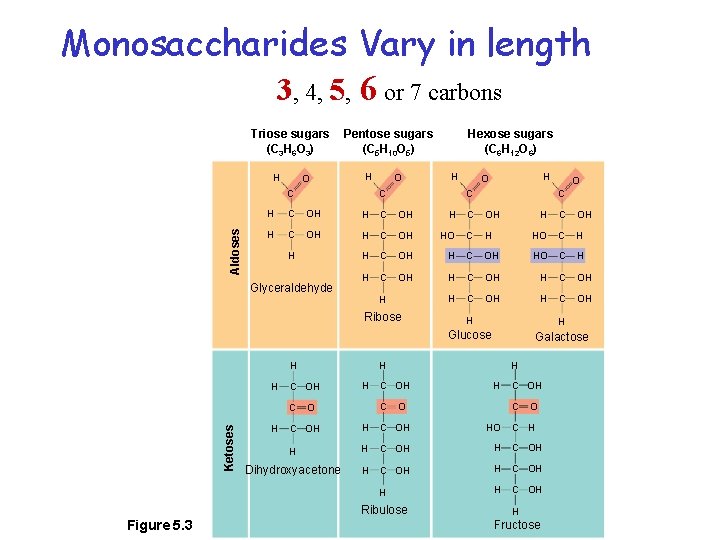
Monosaccharides Vary in length 3, 4, 5, 6 or 7 carbons Triose sugars (C 3 H 6 O 3) H O Pentose sugars (C 5 H 10 O 5) H Aldoses C O H O C C OH H C OH HO C H C OH H H H H C OH H HO C H C OH HO C H H C OH H H Glucose Galactose H C OH C O O C OH HO H H C OH Dihydroxyacetone H C OH H Ribulose O C H Ribose Ketoses H C Glyceraldehyde Figure 5. 3 Hexose sugars (C 6 H 12 O 6) C H H Fructose
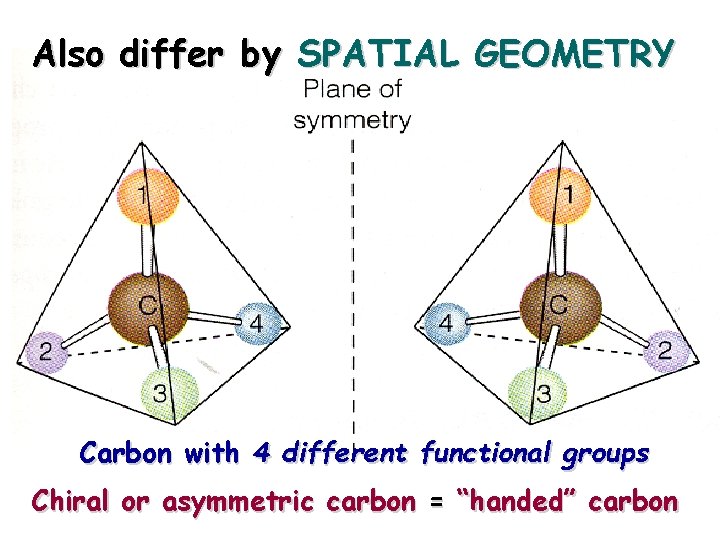
Also differ by SPATIAL GEOMETRY Carbon with 4 different functional groups Chiral or asymmetric carbon = “handed” carbon
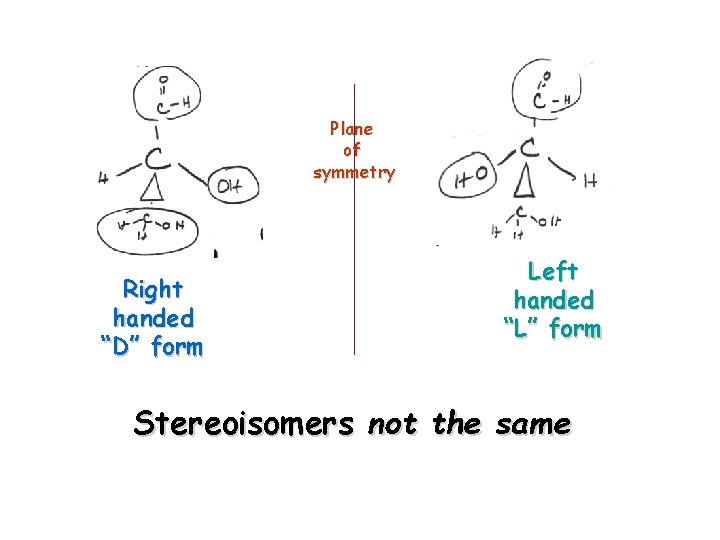
Plane of symmetry Right handed “D” form Left handed “L” form Stereoisomers not the same
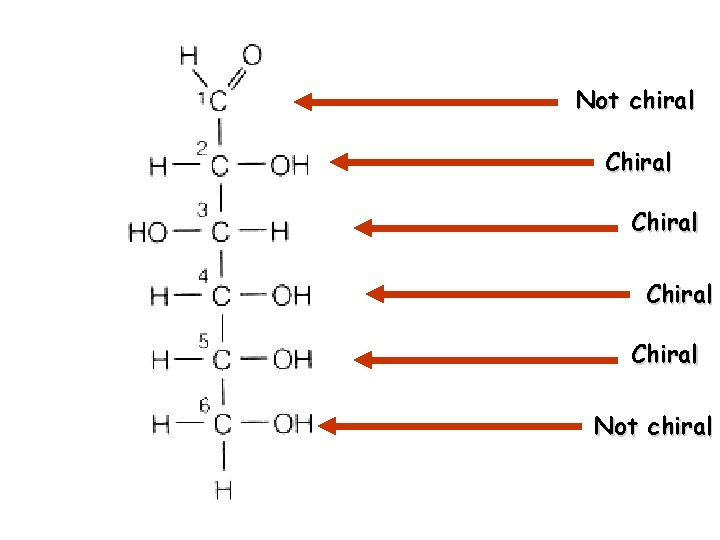
Not chiral Chiral Not chiral
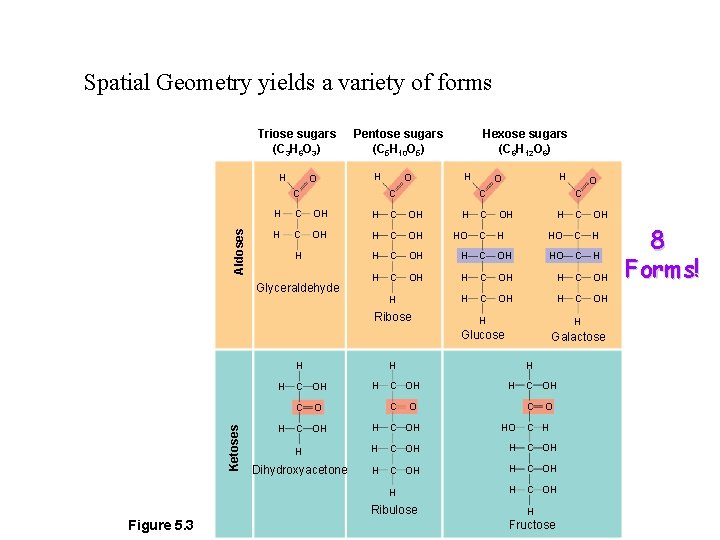
Spatial Geometry yields a variety of forms Triose sugars (C 3 H 6 O 3) H O Pentose sugars (C 5 H 10 O 5) H Aldoses C O H O C C OH H C OH HO C H C OH H H H H C OH H HO C H C OH HO C H H C OH H H Glucose Galactose H C OH C O O C OH HO H H C OH Dihydroxyacetone H C OH H Ribulose O C H Ribose Ketoses H C Glyceraldehyde Figure 5. 3 Hexose sugars (C 6 H 12 O 6) C H H Fructose 8 Forms!
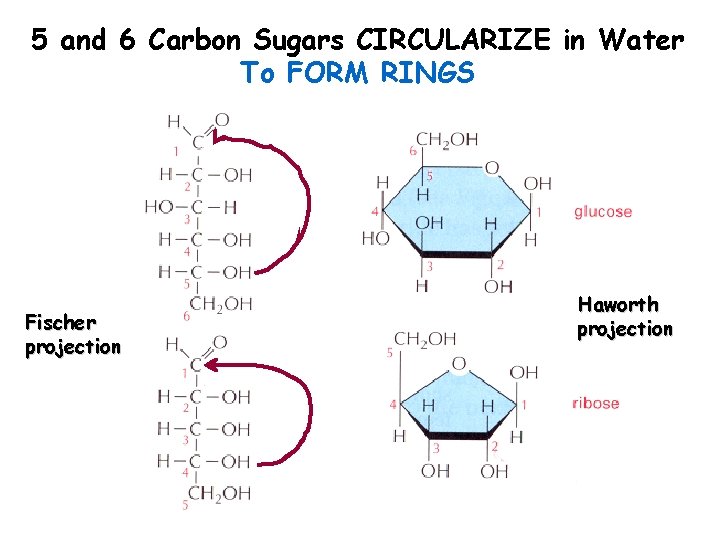
5 and 6 Carbon Sugars CIRCULARIZE in Water To FORM RINGS Fischer projection Haworth projection
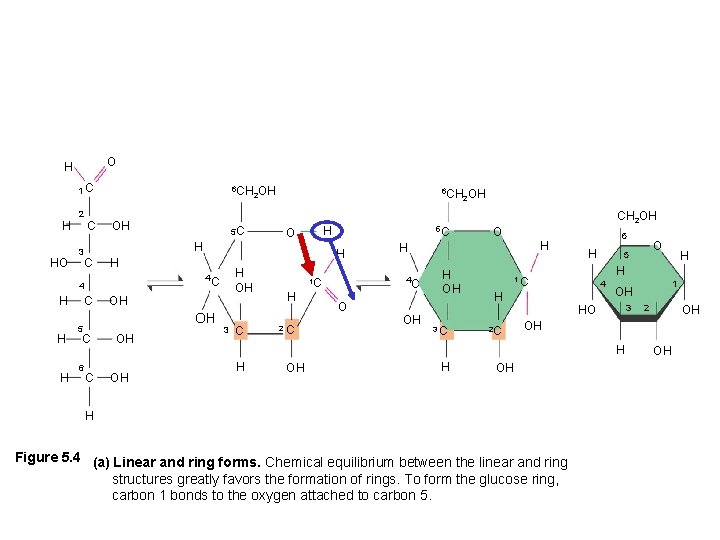
O H 1 C H HO 2 3 C 6 CH OH 2 OH H H C O H H OH OH OH 5 C C OH OH 5 C H 4 C C 6 H H 4 H 5 C 6 CH OH 2 3 C H 2 C O H H 4 C 1 C CH 2 OH O OH H OH 3 C 6 H 2 C 4 OH H 1 OH 3 HO 2 OH OH H Figure 5. 4 (a) Linear and ring forms. Chemical equilibrium between the linear and ring structures greatly favors the formation of rings. To form the glucose ring, carbon 1 bonds to the oxygen attached to carbon 5. H H 1 C H O 5 OH
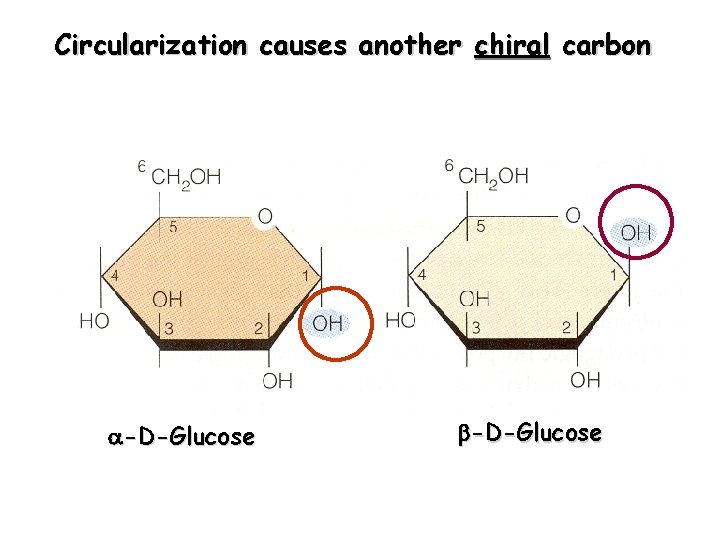
Circularization causes another chiral carbon -D-Glucose
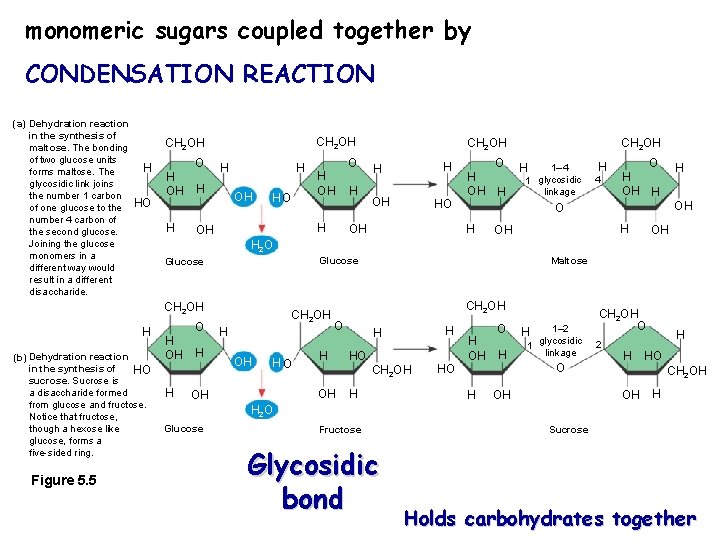
monomeric sugars coupled together by CONDENSATION REACTION (a) Dehydration reaction in the synthesis of maltose. The bonding of two glucose units H forms maltose. The glycosidic link joins the number 1 carbon of one glucose to the HO number 4 carbon of the second glucose. Joining the glucose monomers in a different way would result in a different disaccharide. CH 2 OH O H OH HO (b) Dehydration reaction in the synthesis of HO sucrose. Sucrose is a disaccharide formed from glucose and fructose. Notice that fructose, though a hexose like glucose, forms a five-sided ring. Figure 5. 5 H OH H H H OHOH HO O H OH H H 1– 4 1 glycosidic linkage H 4 O H H OH O H OH CH 2 OH H OH OH H 2 O Glucose CH 2 OH H O CH 2 OH O H OH H H CH 2 OH HO CH 2 OH O H HO CH 2 OH OH OH Maltose H HO H OH H O H 1– 2 glycosidic 1 linkage H O OH CH 2 OH O 2 H H HO CH 2 OH OH H H 2 O Glucose Fructose Glycosidic bond Sucrose Holds carbohydrates together
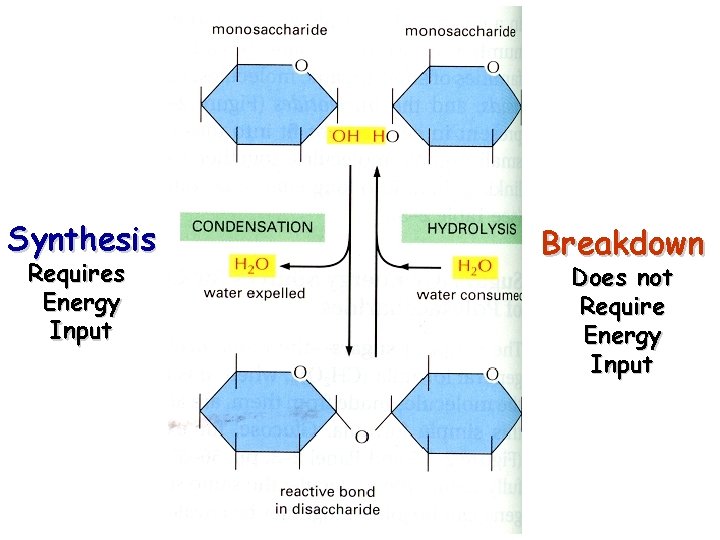
Synthesis Requires Energy Input Breakdown Does not Require Energy Input
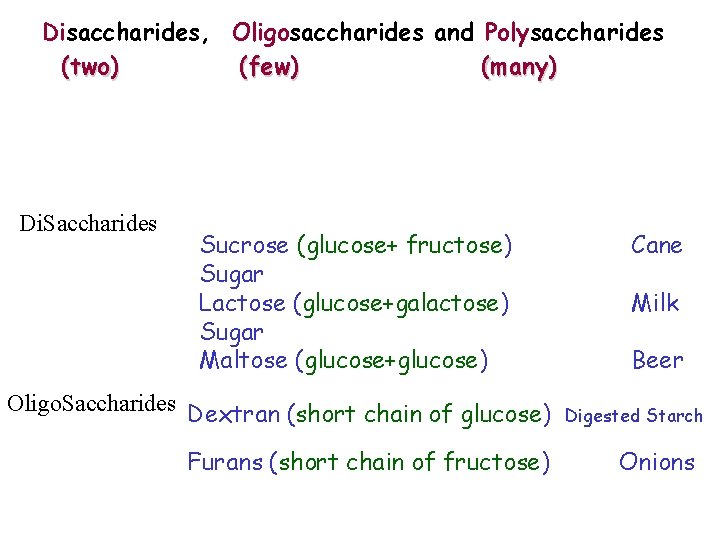
Disaccharides, Oligosaccharides and Polysaccharides (two) (few) (many) Di. Saccharides Sucrose (glucose+ fructose) Sugar Lactose (glucose+galactose) Sugar Maltose (glucose+glucose) Oligo. Saccharides Dextran (short chain of glucose) Furans (short chain of fructose) Cane Milk Beer Digested Starch Onions
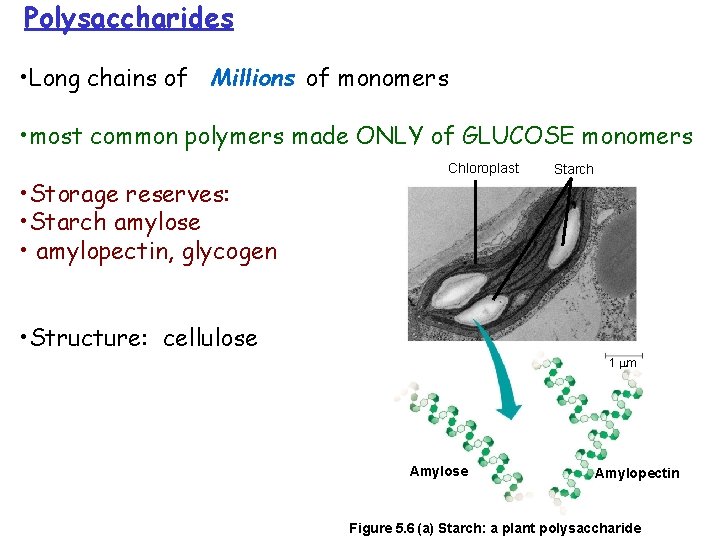
Polysaccharides • Long chains of Millions of monomers • most common polymers made ONLY of GLUCOSE monomers • Storage reserves: • Starch amylose • amylopectin, glycogen Chloroplast Starch • Structure: cellulose 1 m Amylose Amylopectin Figure 5. 6 (a) Starch: a plant polysaccharide
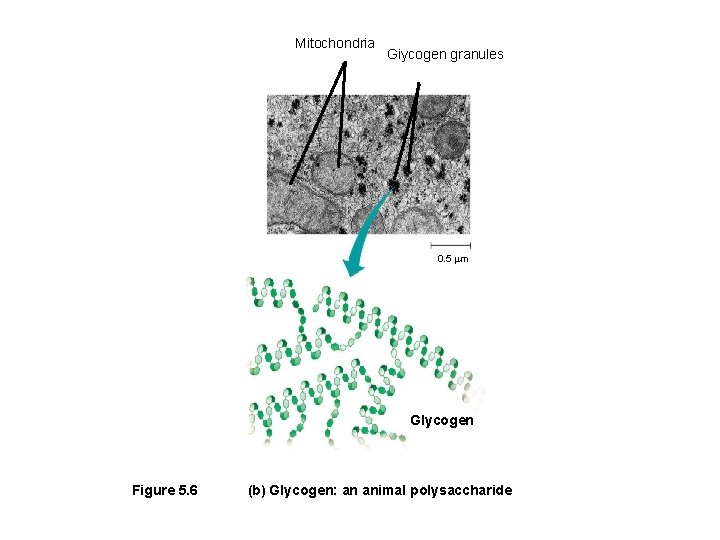
Mitochondria Giycogen granules 0. 5 m Glycogen Figure 5. 6 (b) Glycogen: an animal polysaccharide
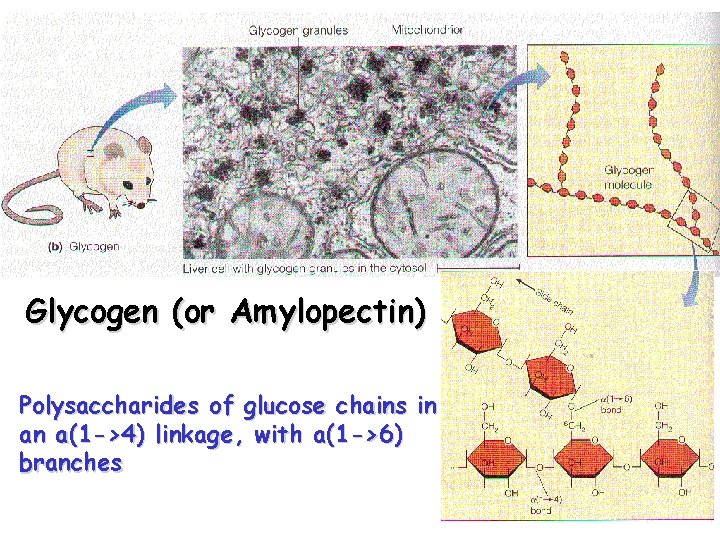
Glycogen (or Amylopectin) Polysaccharides of glucose chains in an a(1 ->4) linkage, with a(1 ->6) branches
Structural Polysaccharides • Cellulose – Is also a polymer of glucose – But has different glycosidic linkages than starch – We can readily digest starches but cannot digest cellulose

• Cellulose is indigestable to animals – Cows and termites have microbes in their stomachs to facilitate this process Figure 5. 9
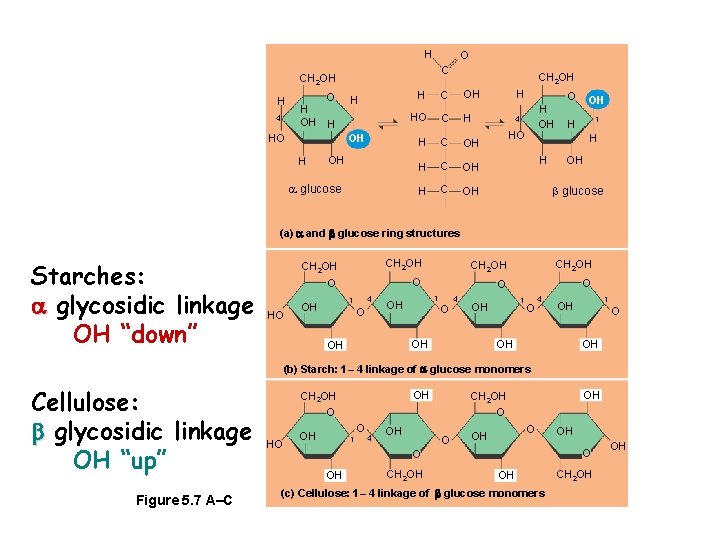
H O C CH 2 OH H 4 H OH O H OH HO H OH glucose OH C H H CH 2 OH HO C H H C OH H OH 4 HO OH 1 H H H OH glucose (a) and glucose ring structures Starches: glycosidic linkage OH “down” CH 2 OH O HO 4 1 OH O CH 2 OH O O O 1 OH O 4 OH OH 1 OH O 4 1 OH OH (b) Starch: 1– 4 linkage of glucose monomers Cellulose: glycosidic linkage OH “up” Figure 5. 7 A–C OH CH 2 OH O HO O OH 1 4 OH OH CH 2 OH O O OH CH 2 OH OH OH (c) Cellulose: 1– 4 linkage of glucose monomers CH 2 OH OH
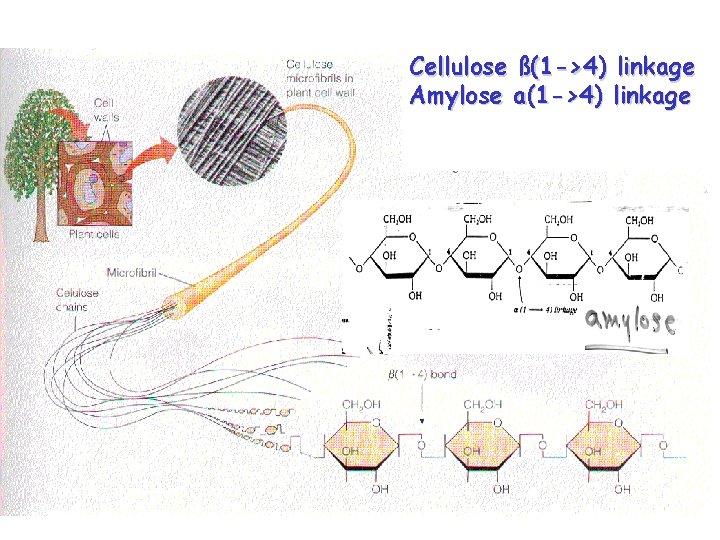
Cellulose ß(1 ->4) linkage Amylose a(1 ->4) linkage
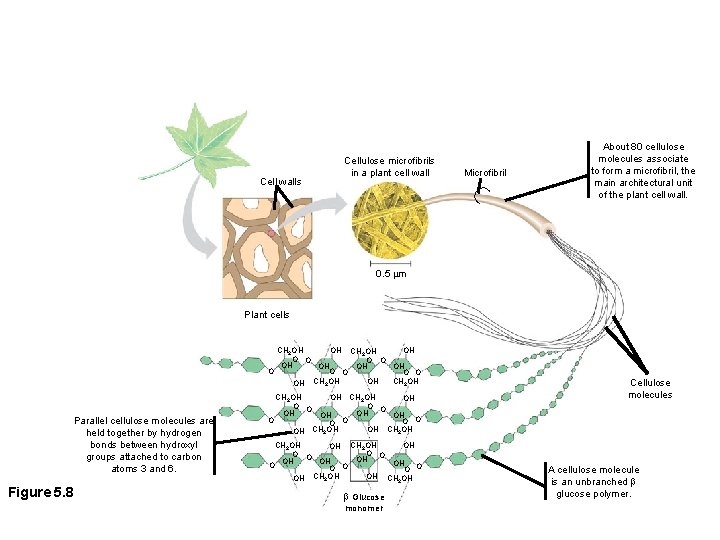
Microfibril Cell walls Cellulose microfibrils in a plant cell wall About 80 cellulose molecules associate to form a microfibril, the main architectural unit of the plant cell wall. 0. 5 m Plant cells OH CH 2 OH O O OH OH O O O CH OH OH CH 2 OH OH 2 Parallel cellulose molecules are held together by hydrogen bonds between hydroxyl groups attached to carbon atoms 3 and 6. Figure 5. 8 CH 2 OH OH O O OH OH OH O O O O CH OH OH CH 2 OH OH 2 CH 2 OH OH OH CH 2 OH O OH OH OH O O O CH OH OH CH 2 OH OH 2 Glucose monomer Cellulose molecules A cellulose molecule is an unbranched glucose polymer.
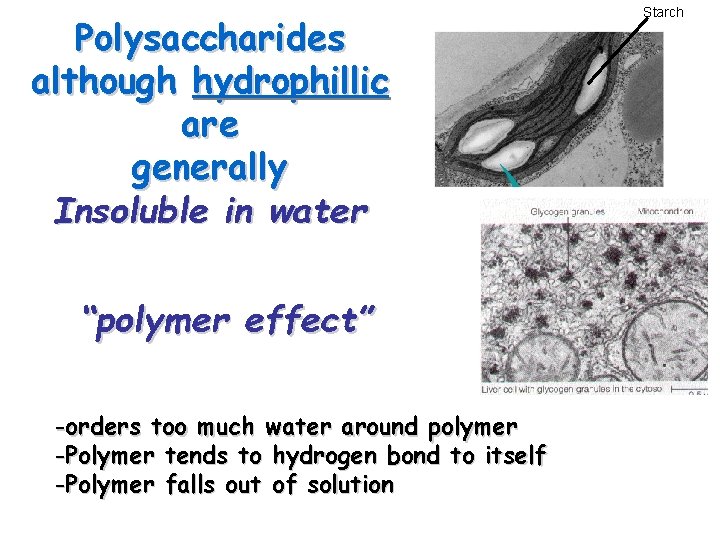
Polysaccharides although hydrophillic are generally Insoluble in water “polymer effect” -orders too much water around polymer -Polymer tends to hydrogen bond to itself -Polymer falls out of solution Starch
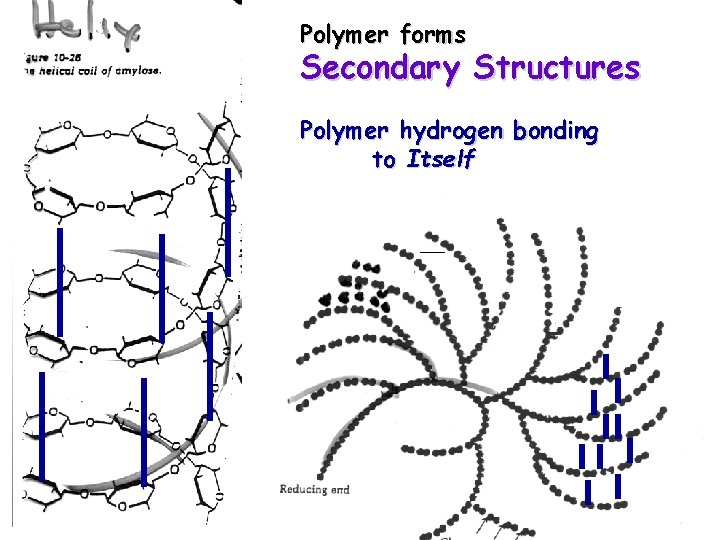
Polymer forms Secondary Structures Polymer hydrogen bonding to Itself

If Denature Secondary Structure (Break Hydrogen Bonds of Polymer with Itself) Water will Hydrogen bond With Polymer RESULT IS BOUND WATER GEL
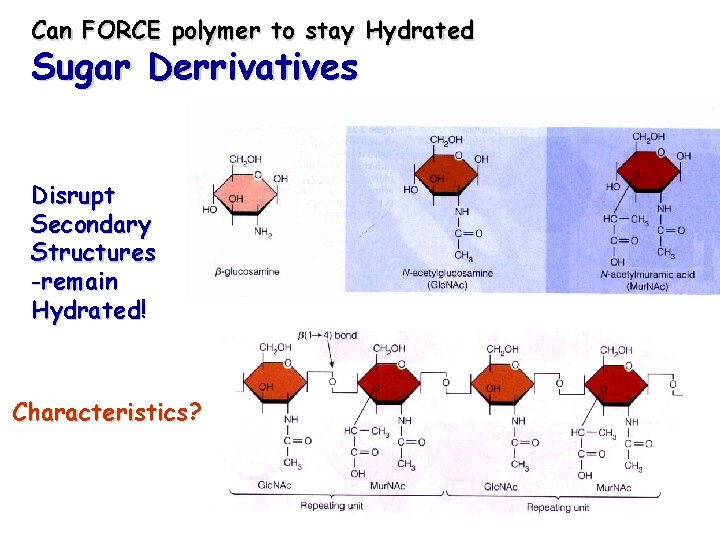
Can FORCE polymer to stay Hydrated Sugar Derrivatives Disrupt Secondary Structures -remain Hydrated! Characteristics?
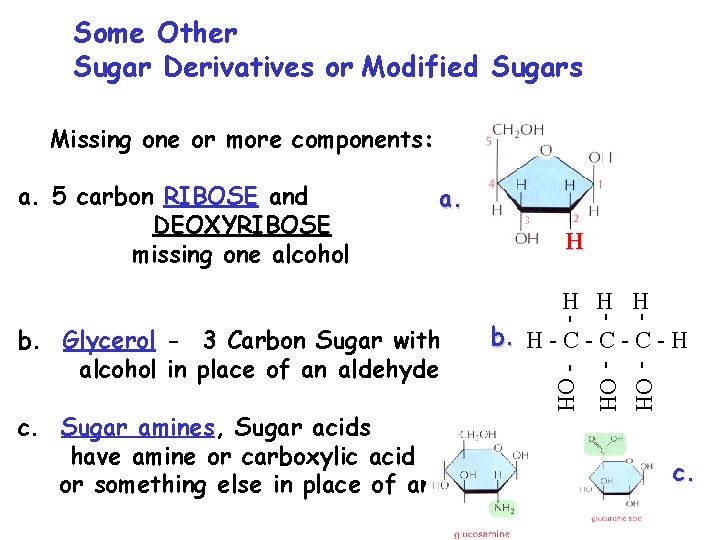
Some Other Sugar Derivatives or Modified Sugars Missing one or more components: a. 5 carbon RIBOSE and DEOXYRIBOSE missing one alcohol a. H b. H - C - C - H c. Sugar amines, Sugar acids have amine or carboxylic acid group or something else in place of an alcohol - OH b. Glycerol - 3 Carbon Sugar with alcohol in place of an aldehyde - H H H c.
Questions?
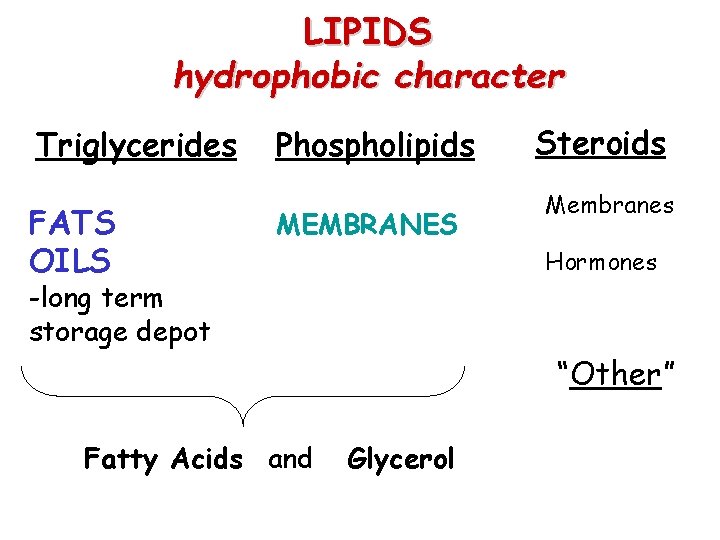
LIPIDS hydrophobic character Triglycerides FATS OILS Phospholipids MEMBRANES Steroids Membranes Hormones -long term storage depot “Other” Fatty Acids and Glycerol
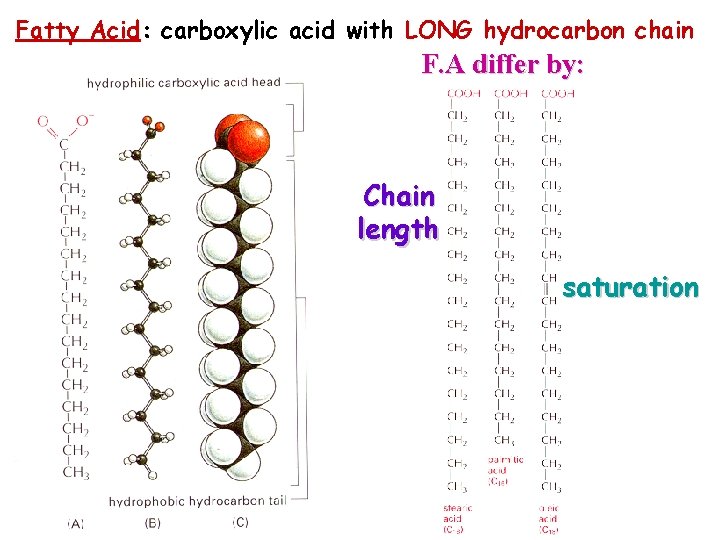
Fatty Acid: carboxylic acid with LONG hydrocarbon chain F. A differ by: Chain length saturation
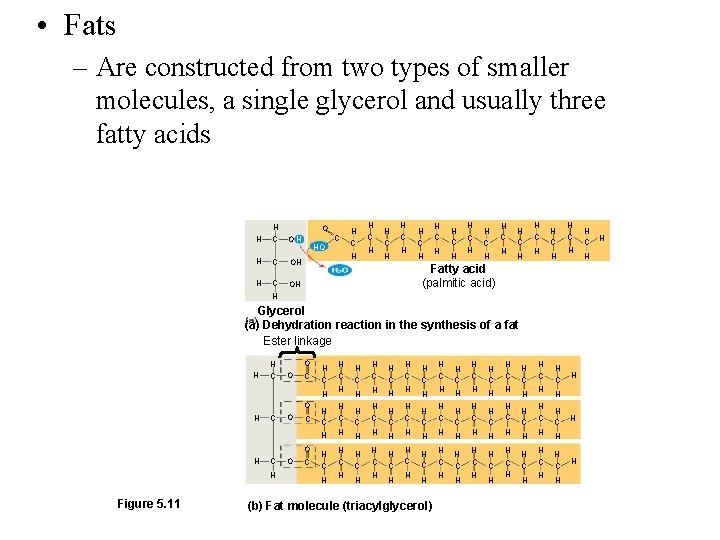
• Fats – Are constructed from two types of smaller molecules, a single glycerol and usually three fatty acids H H C O OH H C OH HO C H H C H H C H H C H H C H Fatty acid (palmitic acid) H Glycerol (a) Dehydration reaction in the synthesis of a fat Ester linkage O H H C O C H O H C H Figure 5. 11 O C H C H H H C H H C H H C H H C H (b) Fat molecule (triacylglycerol) H C H H C H H C H H C H H C H H C H H H C H H

Triglycerides: 3 fatty acids linked to Glycerol by CONDENSATION SYNTHESIS ESTER Linkage
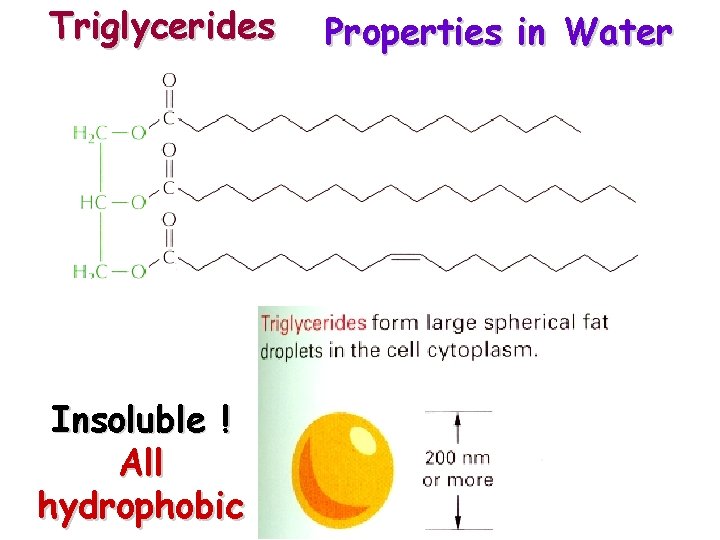
Triglycerides Insoluble ! All hydrophobic Properties in Water
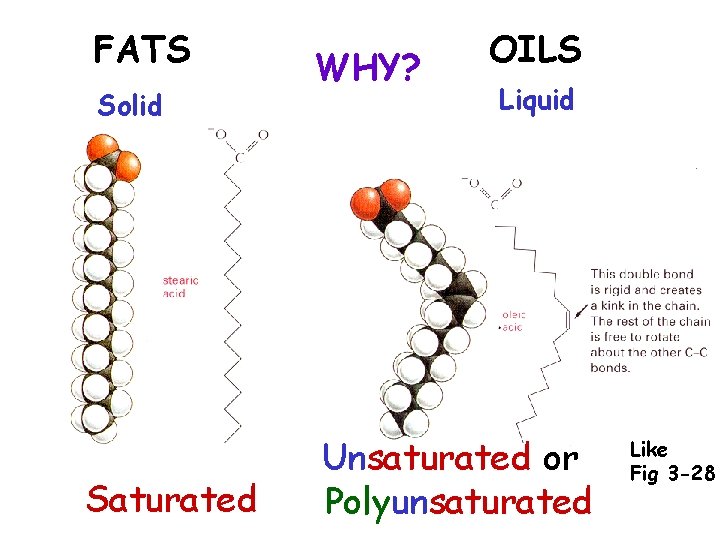
FATS Solid Saturated WHY? OILS Liquid Unsaturated or Polyunsaturated Like Fig 3 -28
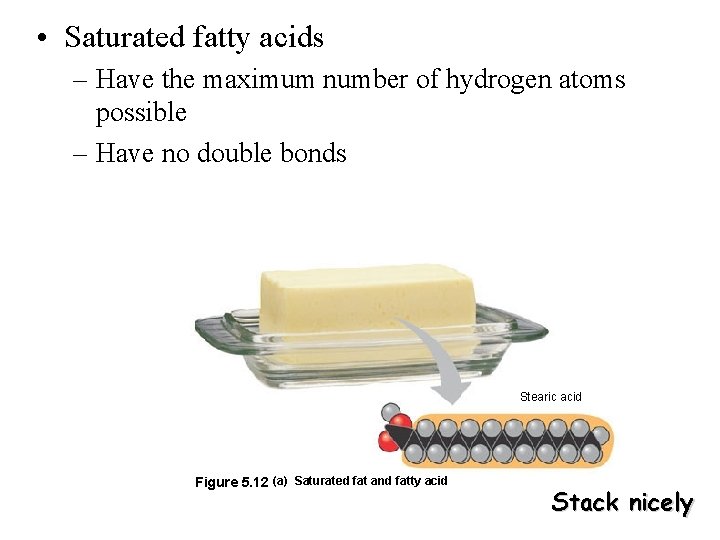
• Saturated fatty acids – Have the maximum number of hydrogen atoms possible – Have no double bonds Stearic acid Figure 5. 12 (a) Saturated fat and fatty acid Stack nicely
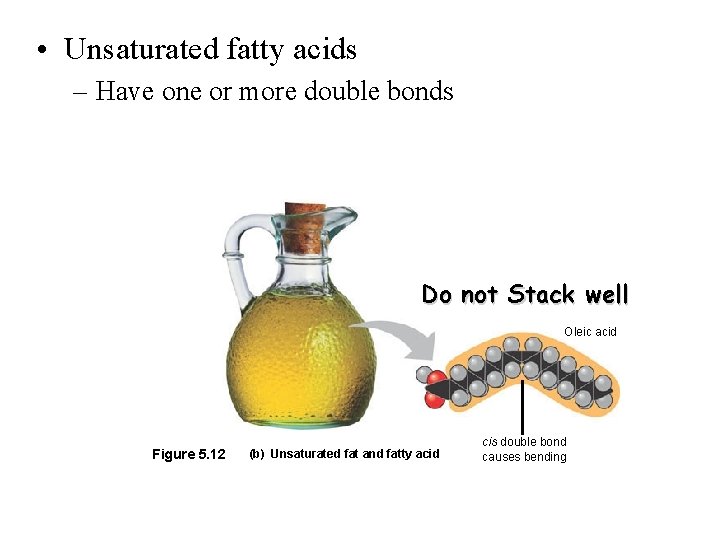
• Unsaturated fatty acids – Have one or more double bonds Do not Stack well Oleic acid Figure 5. 12 (b) Unsaturated fat and fatty acid cis double bond causes bending
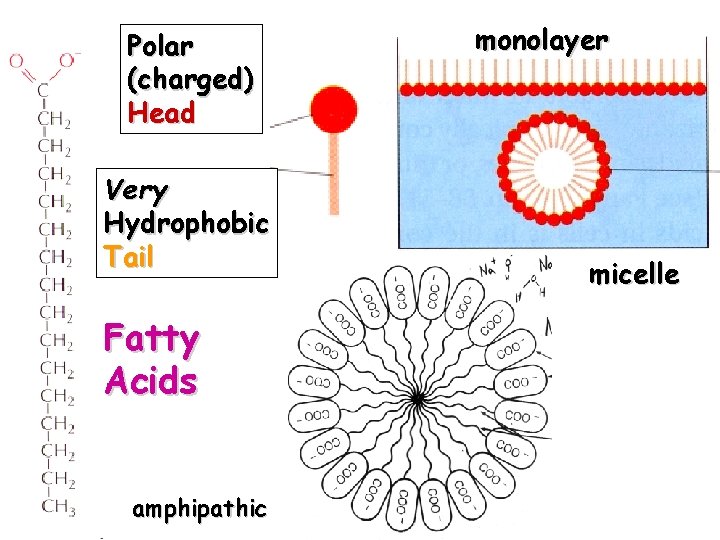
Polar (charged) Head Very Hydrophobic Tail Fatty Acids amphipathic monolayer Free Fatty Acids Hydrolyzed Triglycerides micelle
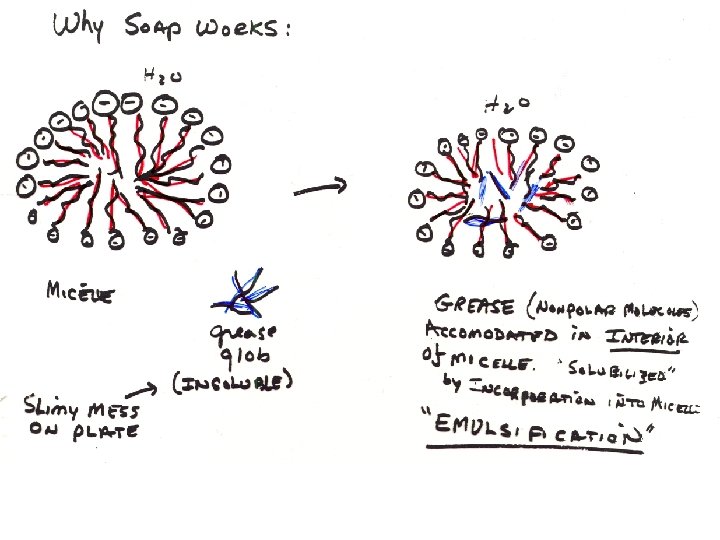
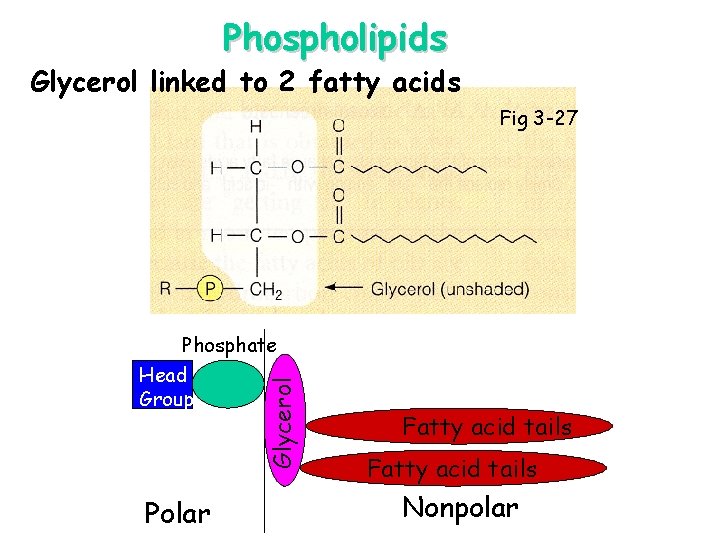
Phospholipids Glycerol linked to 2 fatty acids Fig 3 -27 Glycerol Phosphate Head Group Polar Fatty acid tails Nonpolar
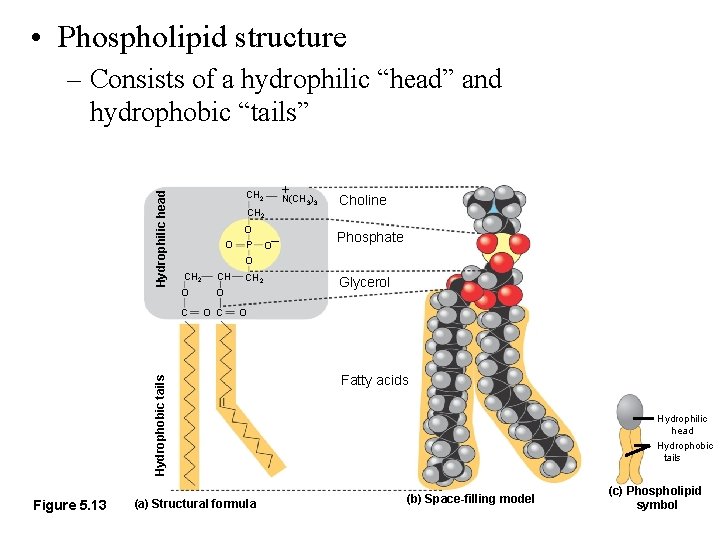
• Phospholipid structure CH 2 O O P Figure 5. 13 O– + N(CH ) 3 3 Choline Phosphate O CH 2 CH O O C CH 2 Glycerol O Hydrophobic tails Hydrophilic head – Consists of a hydrophilic “head” and hydrophobic “tails” (a) Structural formula Fatty acids Hydrophilic head Hydrophobic tails (b) Space-filling model (c) Phospholipid symbol
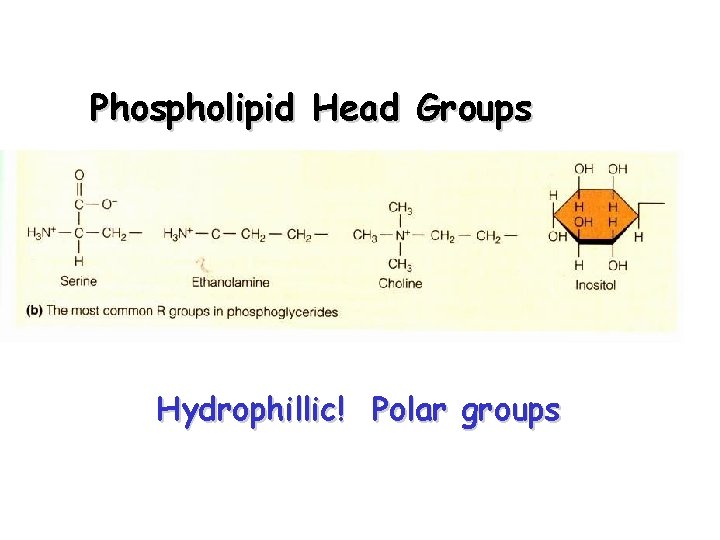
Phospholipid Head Groups Hydrophillic! Polar groups
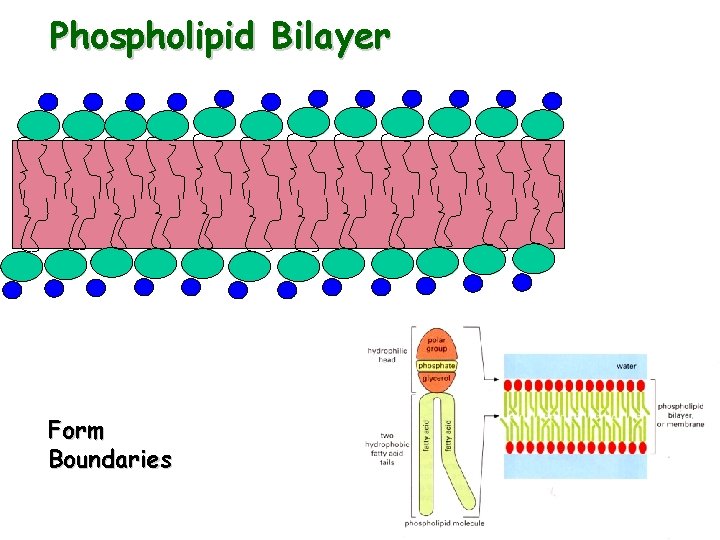
Phospholipid Bilayer Form Boundaries
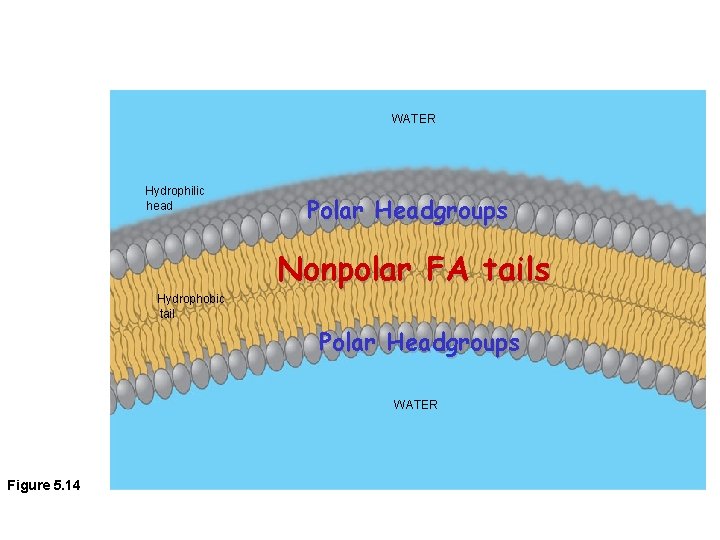
WATER Hydrophilic head Polar Headgroups Nonpolar FA tails Hydrophobic tail Polar Headgroups WATER Figure 5. 14
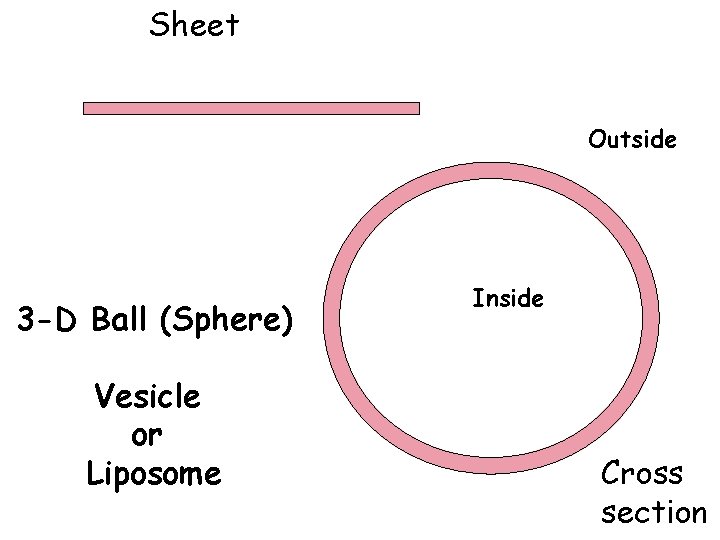
Sheet Outside 3 -D Ball (Sphere) Vesicle or Liposome Inside Cross section
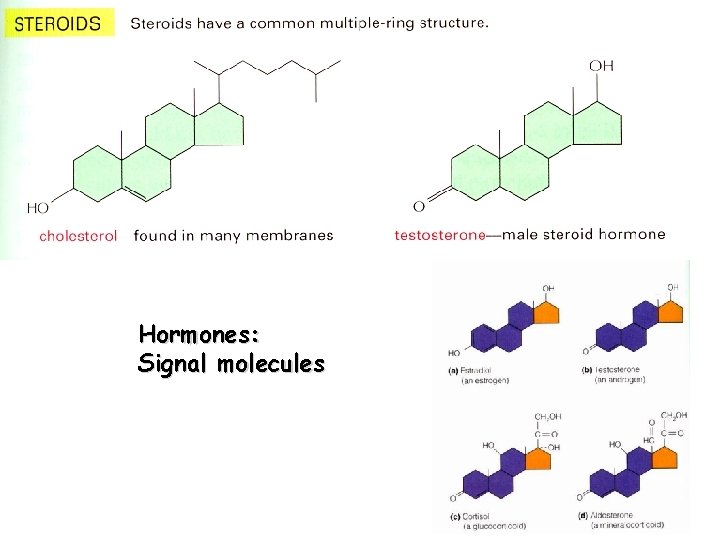
Hormones: Signal molecules
Summary • Principles of Building Polymers Directional assembly from simple units Requires energy input Condensation dehydration reactions • Carbohydrates monosaccharides polysaccharides • Lipids Triglycerides phospholipids steroids
Next Time: More Macromolecules Proteins Nucleic Acids
- Slides: 55