Lecture 5 IC Oxygen ang chalcogens Group 16






- Slides: 35

Lecture 5 (IC) Oxygen ang chalcogens (Group 16)

Group 2: Group 1: alkaline alkali metals earth metals Group 16: Chalcogens Group 18: noble gases 4 f-elements: lanthanides, 5 f: actinides

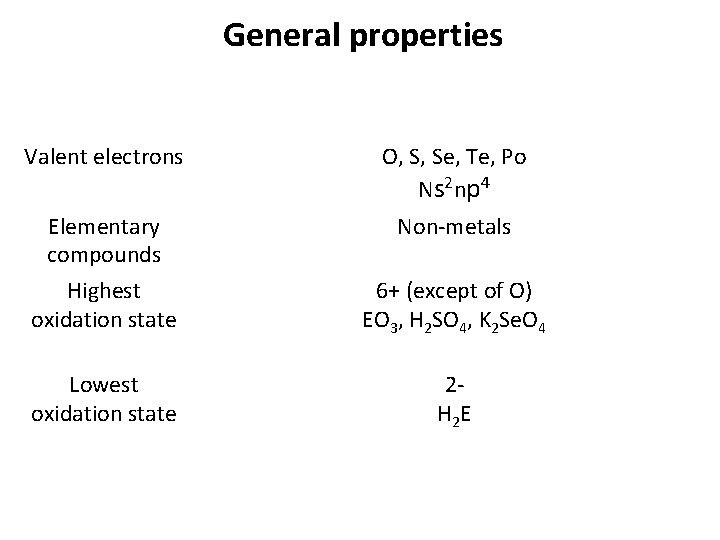
General properties Valent electrons O, S, Se, Te, Po N s 2 n p 4 Elementary compounds Highest oxidation state Non-metals 6+ (except of O) EО 3, H 2 SO 4, K 2 Se. O 4 Lowest oxidation state 2 Н 2 E

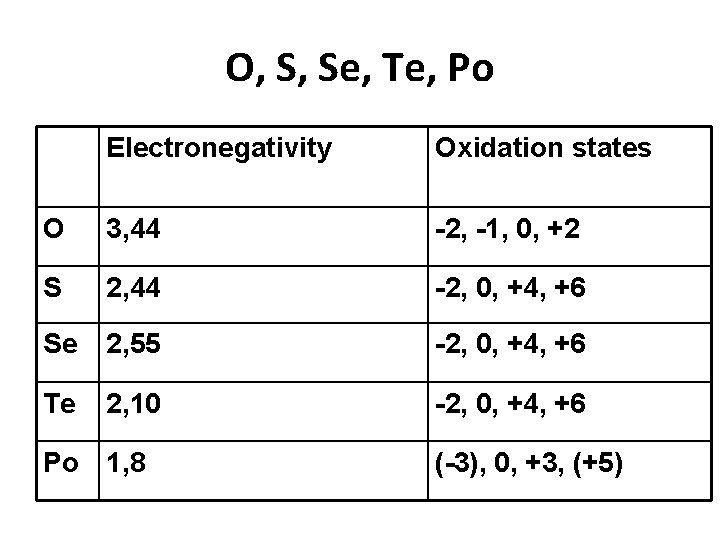
O, S, Se, Te, Po Electronegativity Oxidation states O 3, 44 -2, -1, 0, +2 S 2, 44 -2, 0, +4, +6 Se 2, 55 -2, 0, +4, +6 Te 2, 10 -2, 0, +4, +6 Po 1, 8 (-3), 0, +3, (+5)

Occurrence (earth) O: No. 1. Water, oxide minerals, air…everywhere : ) S: 14 th. Elemental S (in volcanic areas), sulfide ores (Fe. S 2 etc), sulfates (e. g. Ca. SO 4. 2 H 2 O) etc Se: 62 th. Accompanies sulfides Te: 79 th. Accompanies sulfides Po: radioactive, synthetic only

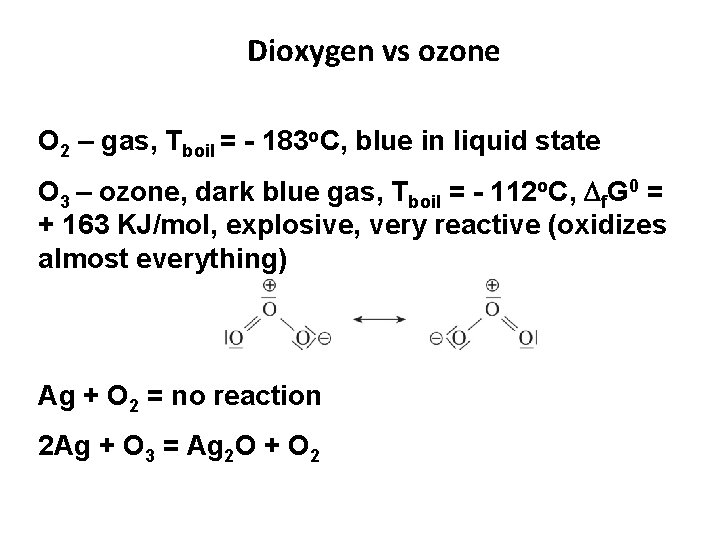
Dioxygen vs ozone O 2 – gas, Тboil = - 183 о. С, blue in liquid state О 3 – ozone, dark blue gas, Тboil = - 112 о. С, f. G 0 = + 163 KJ/mol, explosive, very reactive (oxidizes almost everything) Ag + O 2 = no reaction 2 Ag + O 3 = Ag 2 O + O 2

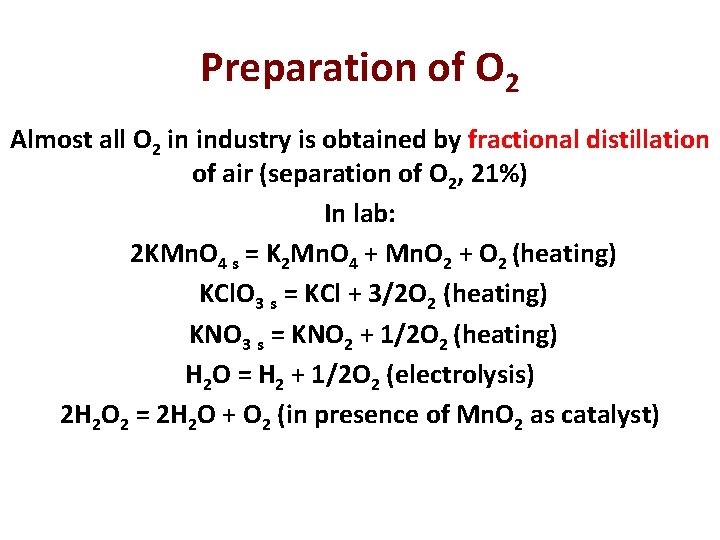
Preparation of O 2 Almost all O 2 in industry is obtained by fractional distillation of air (separation of O 2, 21%) In lab: 2 KMn. O 4 s = K 2 Mn. O 4 + Mn. O 2 + O 2 (heating) KCl. O 3 s = KCl + 3/2 O 2 (heating) KNO 3 s = KNO 2 + 1/2 O 2 (heating) H 2 O = H 2 + 1/2 O 2 (electrolysis) 2 H 2 O 2 = 2 H 2 O + O 2 (in presence of Mn. O 2 as catalyst)

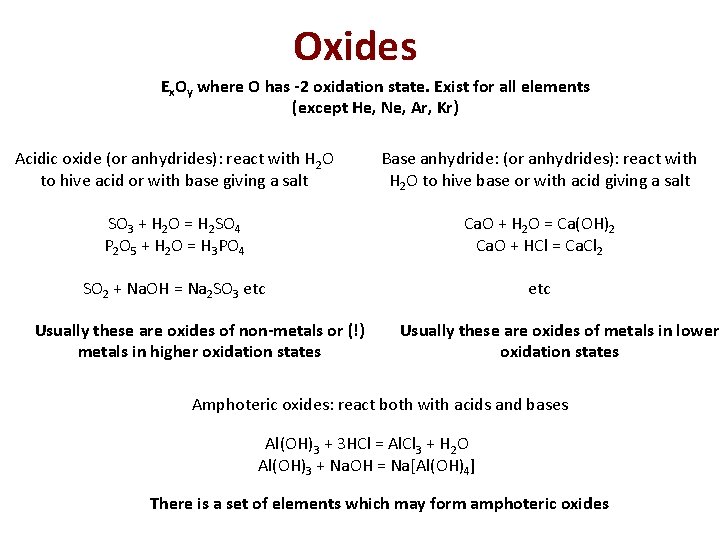
Oxides Ex. Oy where O has -2 oxidation state. Exist for all elements (except He, Ne, Ar, Kr) Acidic oxide (or anhydrides): react with H 2 O to hive acid or with base giving a salt Base anhydride: (or anhydrides): react with H 2 O to hive base or with acid giving a salt SO 3 + H 2 O = H 2 SO 4 P 2 O 5 + H 2 O = H 3 PO 4 Ca. O + H 2 O = Ca(OH)2 Ca. O + HCl = Ca. Cl 2 SO 2 + Na. OH = Na 2 SO 3 etc Usually these are oxides of non-metals or (!) metals in higher oxidation states Usually these are oxides of metals in lower oxidation states Amphoteric oxides: react both with acids and bases Al(OH)3 + 3 HCl = Al. Cl 3 + H 2 O Al(OH)3 + Na. OH = Na[Al(OH)4] There is a set of elements which may form amphoteric oxides

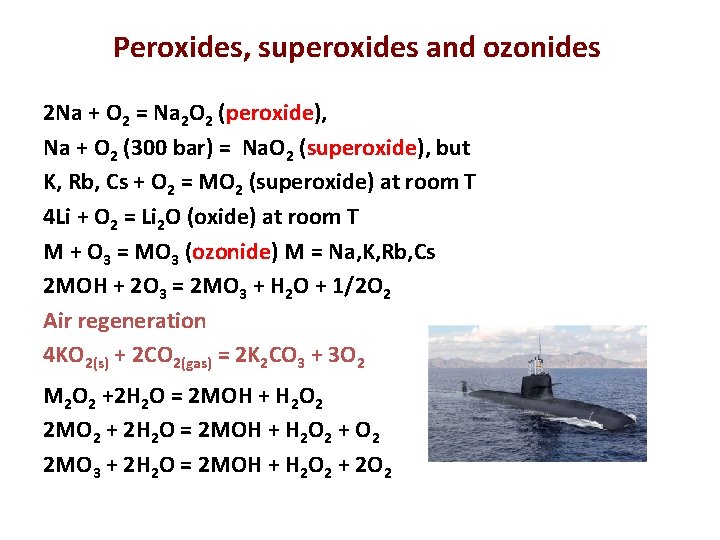
Peroxides, superoxides and ozonides 2 Na + O 2 = Na 2 O 2 (peroxide), Na + O 2 (300 bar) = Na. O 2 (superoxide), but K, Rb, Cs + O 2 = MO 2 (superoxide) at room T 4 Li + O 2 = Li 2 O (oxide) at room T M + O 3 = MO 3 (ozonide) M = Na, K, Rb, Cs 2 MOH + 2 O 3 = 2 MO 3 + H 2 O + 1/2 O 2 Air regeneration 4 KO 2(s) + 2 CO 2(gas) = 2 K 2 CO 3 + 3 O 2 M 2 O 2 +2 H 2 O = 2 MOH + H 2 O 2 2 MO 2 + 2 H 2 O = 2 MOH + H 2 O 2 + O 2 2 MO 3 + 2 H 2 O = 2 MOH + H 2 O 2 + 2 O 2

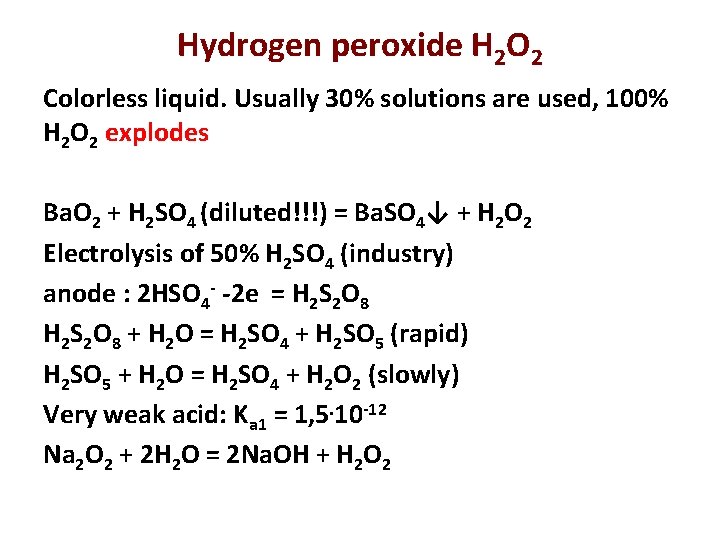
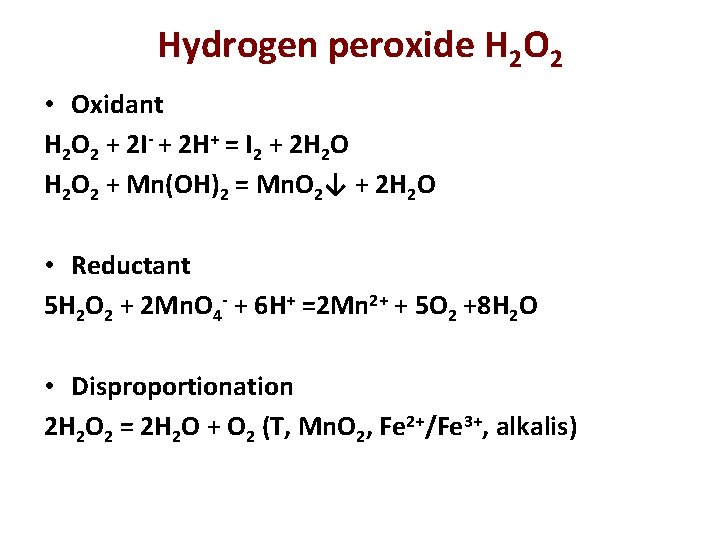
Hydrogen peroxide H 2 O 2 Colorless liquid. Usually 30% solutions are used, 100% H 2 O 2 explodes Ba. O 2 + H 2 SO 4 (diluted!!!) = Ba. SO 4↓ + H 2 O 2 Electrolysis of 50% H 2 SO 4 (industry) anode : 2 HSO 4 - -2 e = H 2 S 2 O 8 + H 2 O = H 2 SO 4 + H 2 SO 5 (rapid) H 2 SO 5 + H 2 O = H 2 SO 4 + H 2 O 2 (slowly) Very weak acid: Ka 1 = 1, 5. 10 -12 Na 2 O 2 + 2 H 2 O = 2 Na. OH + H 2 O 2

Hydrogen peroxide H 2 O 2 • Oxidant H 2 O 2 + 2 I- + 2 H+ = I 2 + 2 H 2 O 2 + Mn(OH)2 = Mn. O 2↓ + 2 H 2 O • Reductant 5 H 2 O 2 + 2 Mn. O 4 - + 6 H+ =2 Mn 2+ + 5 O 2 +8 H 2 O • Disproportionation 2 H 2 O 2 = 2 H 2 O + O 2 (Т, Mn. O 2, Fe 2+/Fe 3+, alkalis)

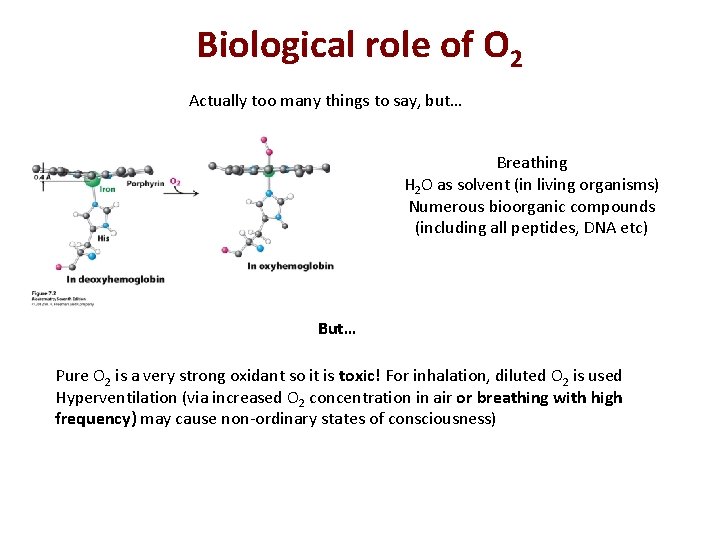
Biological role of O 2 Actually too many things to say, but… Breathing H 2 O as solvent (in living organisms) Numerous bioorganic compounds (including all peptides, DNA etc) But… Pure O 2 is a very strong oxidant so it is toxic! For inhalation, diluted O 2 is used Hyperventilation (via increased O 2 concentration in air or breathing with high frequency) may cause non-ordinary states of consciousness)

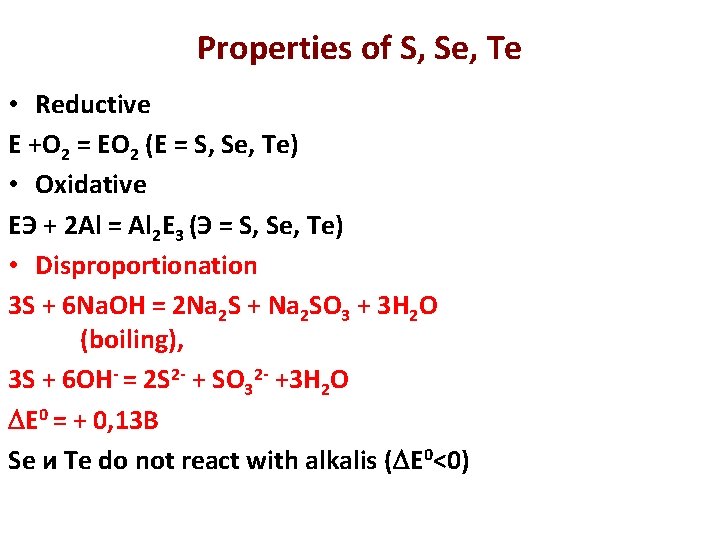
Properties of S, Se, Te • Reductive E +О 2 = EО 2 (E = S, Se, Te) • Oxidative EЭ + 2 Al = Al 2 E 3 (Э = S, Se, Te) • Disproportionation 3 S + 6 Na. OH = 2 Na 2 S + Na 2 SO 3 + 3 H 2 O (boiling), 3 S + 6 OH- = 2 S 2 - + SO 32 - +3 H 2 O E 0 = + 0, 13 B Se и Te do not react with alkalis ( E 0<0)

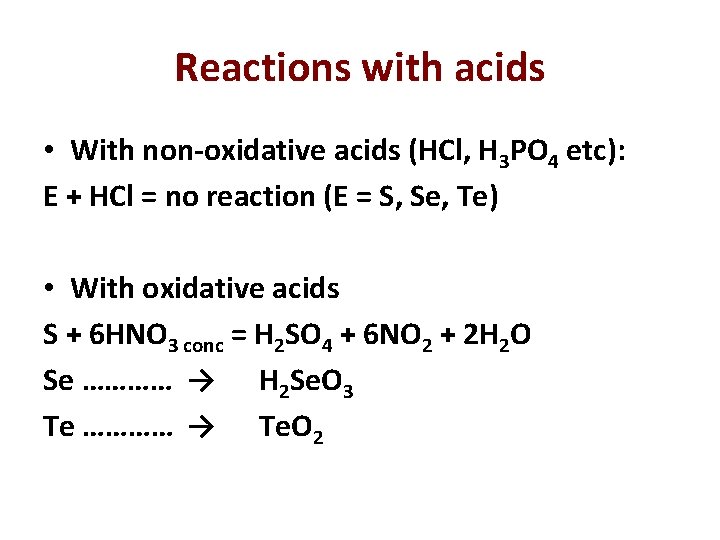
Reactions with acids • With non-oxidative acids (HCl, H 3 PO 4 etc): E + HCl = no reaction (E = S, Se, Te) • With oxidative acids S + 6 HNO 3 conc = H 2 SO 4 + 6 NO 2 + 2 H 2 O Se ………… → H 2 Se. O 3 Te ………… → Te. O 2

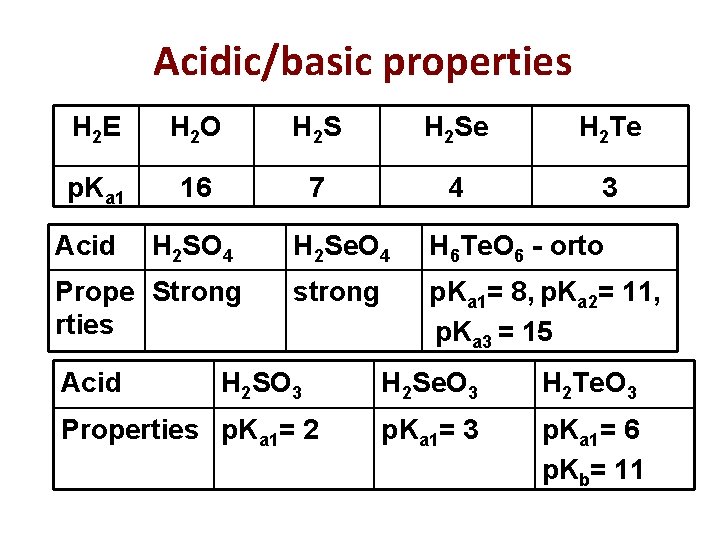
Acidic/basic properties H 2 E H 2 O H 2 Se H 2 Te p. Ka 1 16 7 4 3 Acid H 2 SO 4 Prope Strong rties Acid H 2 Se. O 4 H 6 Te. O 6 - orto strong p. Ka 1= 8, p. Ka 2= 11, p. Ka 3 = 15 H 2 SO 3 Properties p. Ka 1= 2 H 2 Se. O 3 H 2 Te. O 3 p. Ka 1= 6 p. Kb= 11

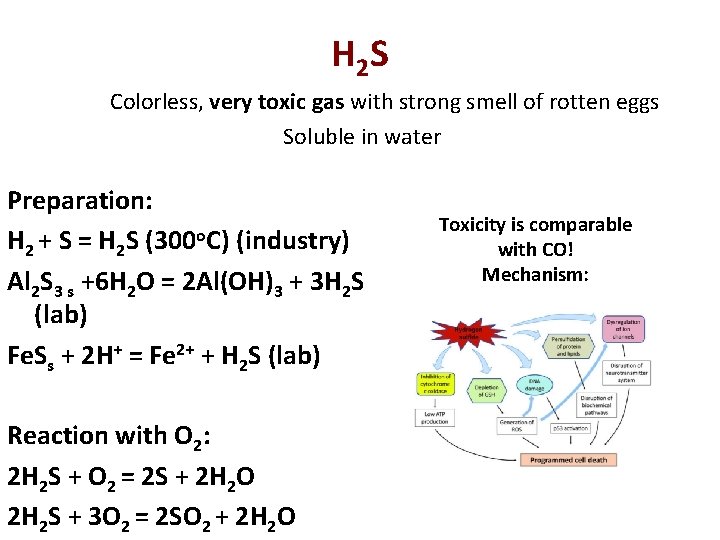
H 2 S Colorless, very toxic gas with strong smell of rotten eggs Soluble in water Preparation: H 2 + S = H 2 S (300 o. C) (industry) Al 2 S 3 s +6 H 2 O = 2 Al(OH)3 + 3 H 2 S (lab) Fe. Ss + 2 H+ = Fe 2+ + H 2 S (lab) Reaction with O 2: 2 H 2 S + O 2 = 2 S + 2 H 2 O 2 H 2 S + 3 O 2 = 2 SO 2 + 2 H 2 O Toxicity is comparable with CO! Mechanism:

H 2 S Oxidation of H 2 S in aqueous solutions: 1) To S: Mn. O 4 -, Cr 2 O 72 -, Fe 3+ 2) to SO 42 -: HNO 3 conc, Pb. O 2, Bi. O 33 -, Fe. O 42 Soluble salts are hydrolytically unstable. For weak bases: irreversible total hydrolysis (Al 2 S 3, Cr 2 S 3)

H 2 Se and H 2 Te Very toxic (mechanism is generally similar to H 2 S) H 2 Se can be obtained via reaction of selenides with non-oxidizing acids: Zn. Se + 2 HCl Zn. Cl 2 + H 2 Se H 2 Te can be obtained in a similar way, but it is highly unstable so it decomposes almost immediately (into H 2 and Te) H 2 Se and H 2 Te are very strong reductants (stronger than H 2 S)

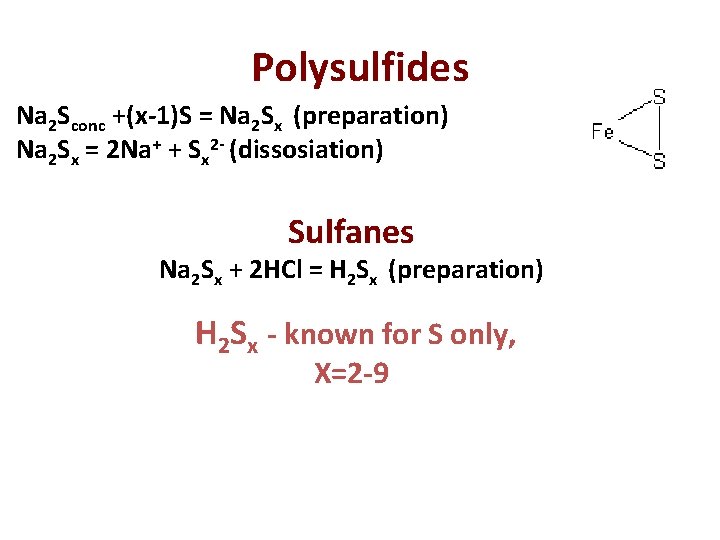
Polysulfides Na 2 Sconc +(x-1)S = Na 2 Sx (preparation) Na 2 Sx = 2 Na+ + Sx 2 - (dissosiation) Sulfanes Na 2 Sx + 2 HCl = H 2 Sx (preparation) H 2 Sx - known for S only, Х=2 -9

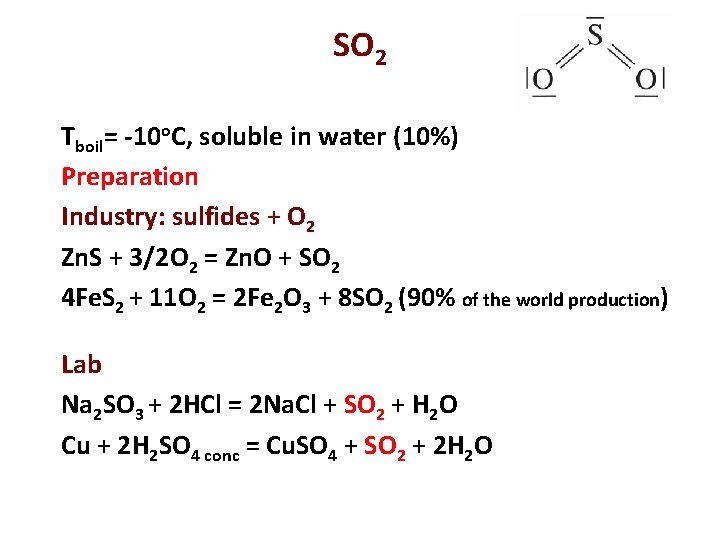
SO 2 Tboil= -10 o. C, soluble in water (10%) Preparation Industry: sulfides + O 2 Zn. S + 3/2 O 2 = Zn. O + SO 2 4 Fe. S 2 + 11 O 2 = 2 Fe 2 O 3 + 8 SO 2 (90% of the world production) Lab Na 2 SO 3 + 2 HCl = 2 Na. Cl + SO 2 + H 2 O Cu + 2 H 2 SO 4 conc = Cu. SO 4 + SO 2 + 2 H 2 O

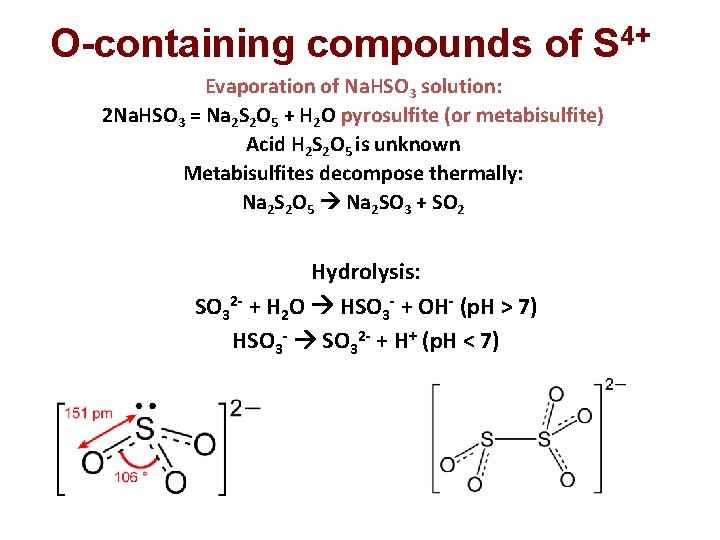
O-containing compounds of S 4+ In water: SO 2(gas)+ x. H 2 O = SO 2. x. H 2 O K 1 SO 2. x. H 2 O = H 2 SO 3 + (x-1)H 2 O K<<1 p. Ka 1 = 2; p. Ka 2 = 6 With alkalis: 2 Na. OH + SO 2 = Na 2 SO 3 + H 2 O sulfite Na. OH + SO 2 = Na. HSO 3 hydrosulfite, in solutions only

O-containing compounds of S 4+ Evaporation of Na. HSO 3 solution: 2 Na. HSO 3 = Na 2 S 2 O 5 + H 2 O pyrosulfite (or metabisulfite) Acid H 2 S 2 O 5 is unknown Metabisulfites decompose thermally: Na 2 S 2 O 5 Na 2 SO 3 + SO 2 Hydrolysis: SO 32 - + H 2 O HSO 3 - + OH- (p. H > 7) HSO 3 - SO 32 - + H+ (p. H < 7)

O-containing compounds of S 4+ • Disproportionation 4 SO 32 - = S 2 - + 3 SO 42 - (heating) • oxidation (S 4+ S 6+) SO 2 + 1/2 O 2 = SO 3 (for preparation H 2 SO 4) Na 2 SO 3 + 1/2 O 2 = Na 2 SO 4 (slowly, on air as well) SO 2 + oxidant + H+ = SO 42(Mn. O 4 -, Cr 2 O 7 -, Cl. O 3 -, Cl 2, Br 2, I 2, H 2 O 2) • reduction SO 2 + 2 H 2 S = 3 S + 2 H 2 O

O-containing compounds of Se 4+ and Te 4+ Oxidation of Se or Te by O 2 results in EO 2 Se. O 2 + H 2 O H 2 Se. O 3, can act as oxidant: H 2 Se. O 3 + 2 SO 2 + H 2 O Se + 2 H 2 SO 4 Te. O 2 is poorly soluble in water, but: Te. O 2 + 2 Na. OH Na 2 Te. O 3 + H 2 O Na 2 Te. O 3 + 2 HCl 2 Na. Cl + H 2 Te. O 3 (actually Te. O 2·x. H 2 O) Te. O 2 is amphoteric: Te. O 2 + 6 HCl H 2[Te. Cl 6] + 2 H 2 O

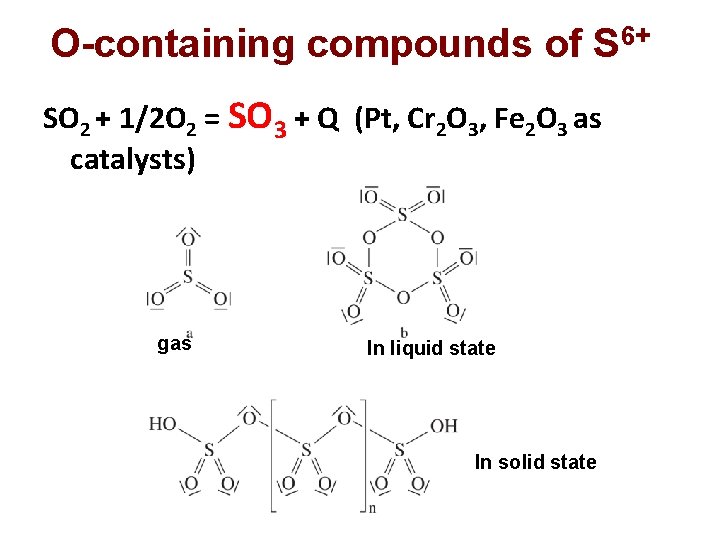
O-containing compounds of S 6+ SO 2 + 1/2 O 2 = SO 3 + Q (Pt, Cr 2 O 3, Fe 2 O 3 as catalysts) gas In liquid state In solid state

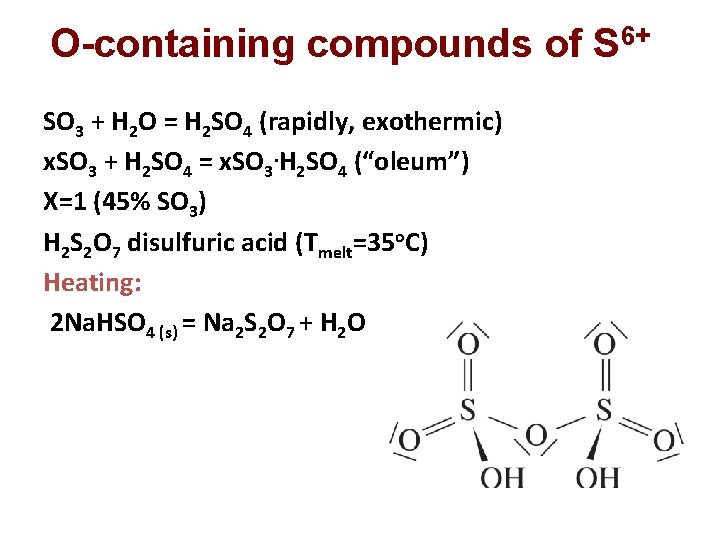
O-containing compounds of S 6+ SO 3 + H 2 O = H 2 SO 4 (rapidly, exothermic) x. SO 3 + H 2 SO 4 = x. SO 3. H 2 SO 4 (“oleum”) X=1 (45% SO 3) H 2 S 2 O 7 disulfuric acid (Тmelt=35 о. С) Heating: 2 Na. HSO 4 (s) = Na 2 S 2 O 7 + H 2 O

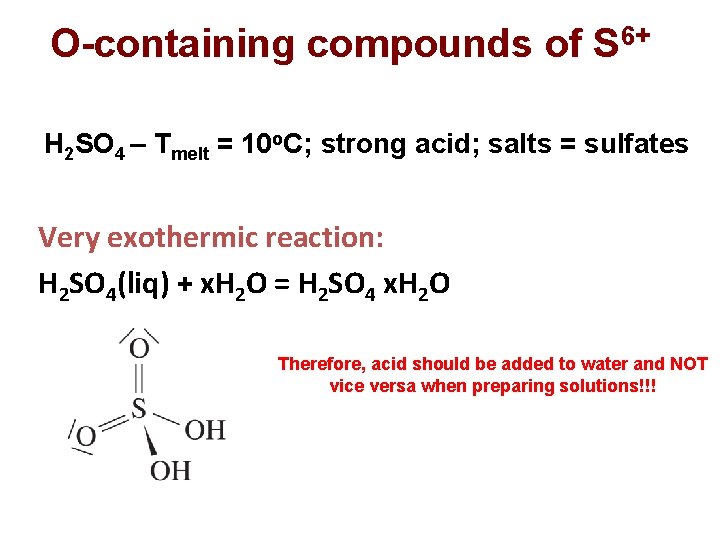
O-containing compounds of S 6+ H 2 SO 4 – Тmelt = 10 о. С; strong acid; salts = sulfates Very exothermic reaction: H 2 SO 4(liq) + x. H 2 O = H 2 SO 4 x. H 2 O Therefore, acid should be added to water and NOT vice versa when preparing solutions!!!

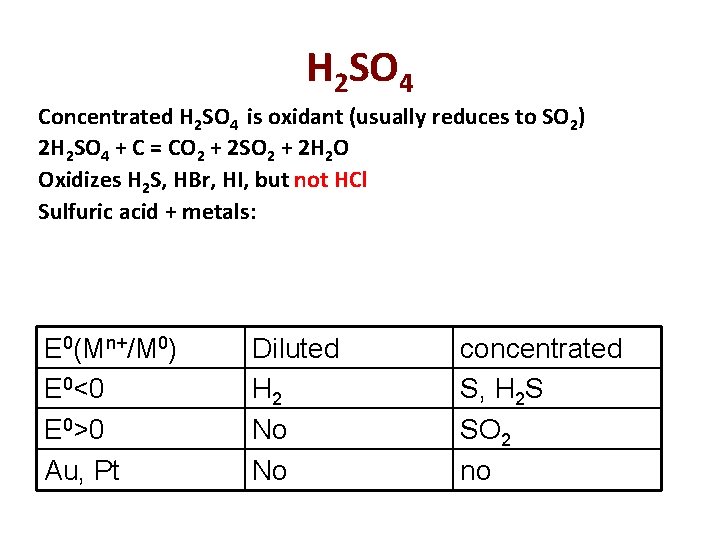
H 2 SO 4 Concentrated H 2 SO 4 is oxidant (usually reduces to SO 2) 2 H 2 SO 4 + C = CO 2 + 2 SO 2 + 2 H 2 O Oxidizes H 2 S, HBr, HI, but not HCl Sulfuric acid + metals: E 0(Mn+/M 0) E 0<0 E 0>0 Au, Pt Diluted H 2 No No concentrated S, H 2 S SO 2 no

Se. O 3 and selenic acid Se. O 3: very toxic, very strong oxidant H 2 Se. O 4: strong acid Preparation: Se + 3 Cl 2 + 4 H 2 O H 2 Se. O 4 or Se. O 2 + H 2 O 2 H 2 Se. O 4 For Se. O 3: H 2 Se. O 4 (heating) Se. O 3 + H 2 O, further heating: 2 Se. O 3 2 Se. O 2 + O 2 Oxidizes HCl: H 2 Se. O 4 + 2 HCl H 2 Se. O 3 + Cl 2 + H 2 O Dissolves metals and even gold(!) 2 Au + 6 H 2 Se. O 4 Au 2(Se. O 4)3 + 2 H 2 Se. O 3 + 3 H 2 O

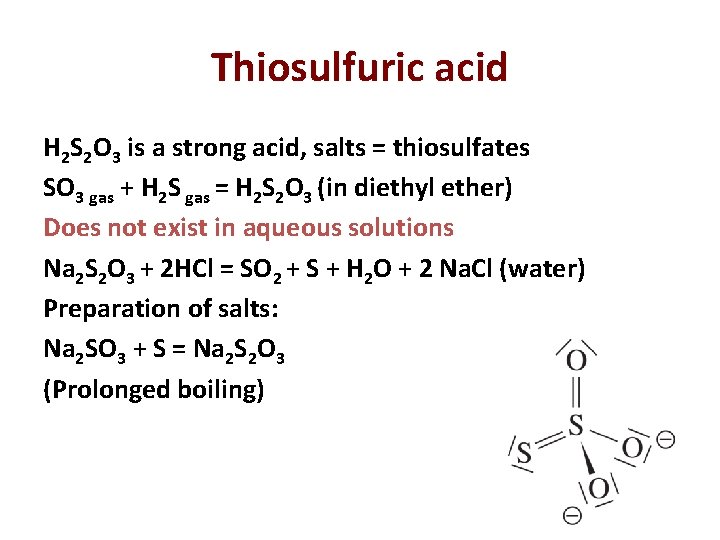
Thiosulfuric acid H 2 S 2 O 3 is a strong acid, salts = thiosulfates SO 3 gas + H 2 S gas = H 2 S 2 O 3 (in diethyl ether) Does not exist in aqueous solutions Na 2 S 2 O 3 + 2 HCl = SO 2 + S + H 2 O + 2 Na. Cl (water) Preparation of salts: Na 2 SO 3 + S = Na 2 S 2 O 3 (Prolonged boiling) 30

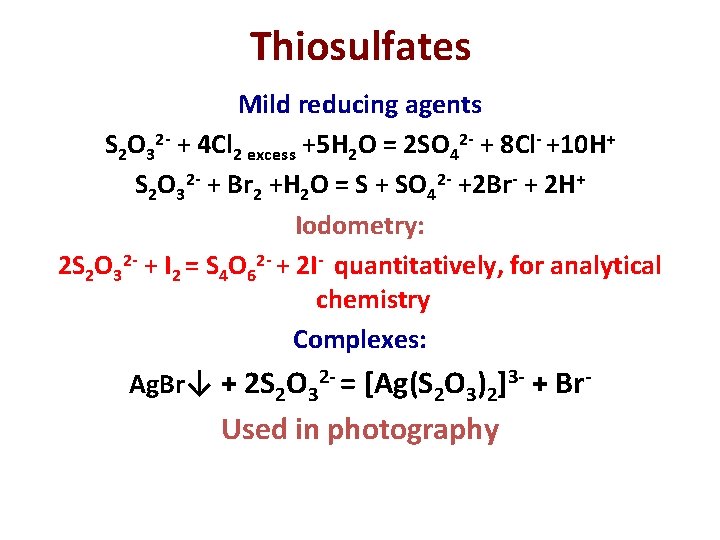
Thiosulfates Mild reducing agents S 2 O 32 - + 4 Cl 2 excess +5 H 2 O = 2 SO 42 - + 8 Cl- +10 H+ S 2 O 32 - + Br 2 +H 2 O = S + SO 42 - +2 Br- + 2 H+ Iodometry: 2 S 2 O 32 - + I 2 = S 4 O 62 - + 2 I- quantitatively, for analytical chemistry Complexes: Ag. Br↓ + 2 S 2 O 32 - = [Ag(S 2 O 3)2]3 - + Br- Used in photography

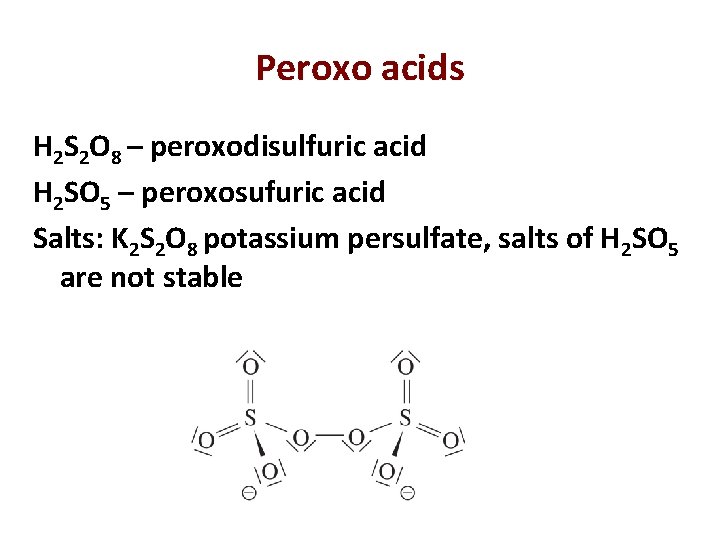
Peroxo acids H 2 S 2 O 8 – peroxodisulfuric acid H 2 SO 5 – peroxosufuric acid Salts: K 2 S 2 O 8 potassium persulfate, salts of H 2 SO 5 are not stable

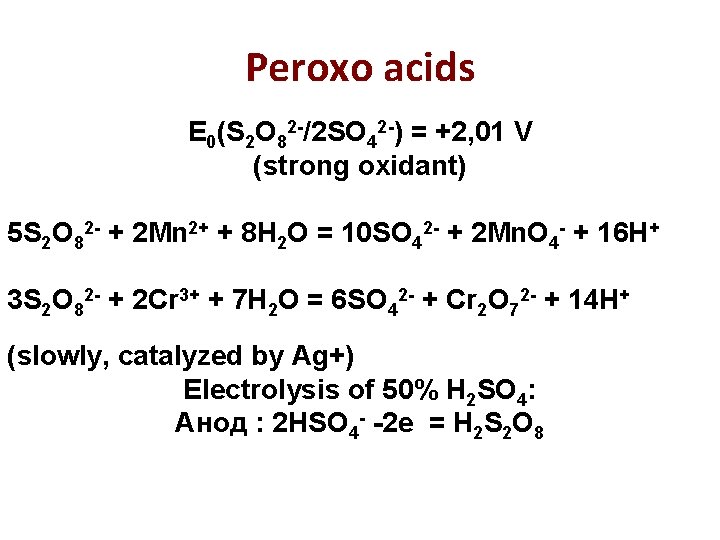
Peroxo acids E 0(S 2 O 82 -/2 SO 42 -) = +2, 01 V (strong oxidant) 5 S 2 O 82 - + 2 Mn 2+ + 8 H 2 O = 10 SO 42 - + 2 Mn. O 4 - + 16 H+ 3 S 2 O 82 - + 2 Cr 3+ + 7 H 2 O = 6 SO 42 - + Cr 2 O 72 - + 14 H+ (slowly, catalyzed by Ag+) Electrolysis of 50% H 2 SO 4: Анод : 2 HSO 4 - -2 e = H 2 S 2 O 8

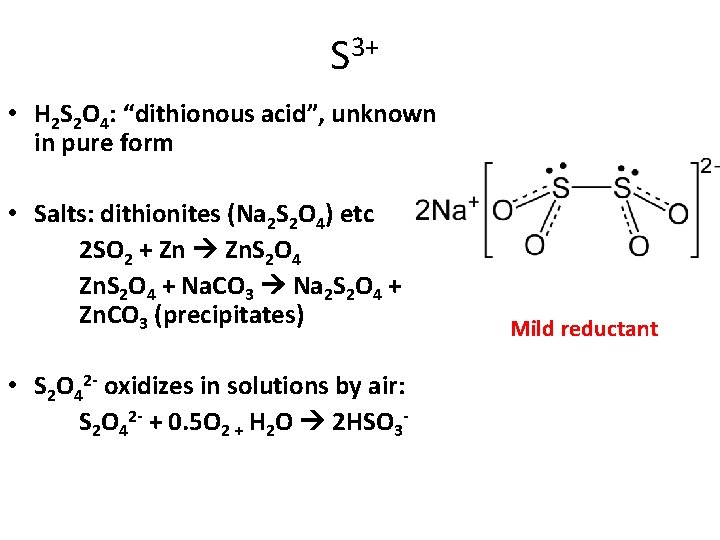
3+ S • H 2 S 2 O 4: “dithionous acid”, unknown in pure form • Salts: dithionites (Na 2 S 2 O 4) etc 2 SO 2 + Zn Zn. S 2 O 4 + Na. CO 3 Na 2 S 2 O 4 + Zn. CO 3 (precipitates) • S 2 O 42 - oxidizes in solutions by air: S 2 O 42 - + 0. 5 O 2 + H 2 O 2 HSO 3 - Mild reductant

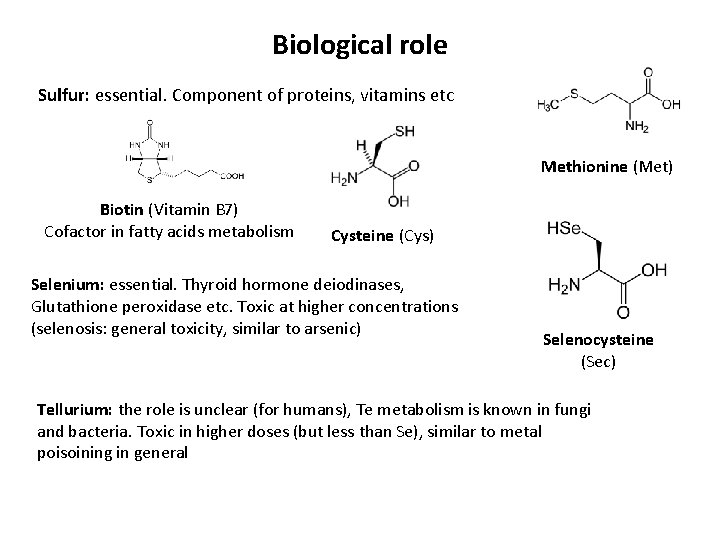
Biological role Sulfur: essential. Component of proteins, vitamins etc Methionine (Met) Biotin (Vitamin B 7) Cofactor in fatty acids metabolism Cysteine (Cys) Selenium: essential. Thyroid hormone deiodinases, Glutathione peroxidase etc. Toxic at higher concentrations (selenosis: general toxicity, similar to arsenic) Selenocysteine (Sec) Tellurium: the role is unclear (for humans), Te metabolism is known in fungi and bacteria. Toxic in higher doses (but less than Se), similar to metal poisoining in general