LECTURE 4 UNITS CHEMICAL ENGINEERING 170 UNITS A









- Slides: 21

LECTURE 4: UNITS CHEMICAL ENGINEERING 170

UNITS A unit of measurement defines a specific magnitude of a physical quantity Units are about communication—between people, over time and over space Units have been around a long time—consider the cubit!

STANDARDIZATION In medieval Europe peasants were taxed in baskets (“bushels”) of grain This lead to “long and bitter controversy”* over: How the basket should be woven How damp the grain should be How the grain should be poured (from shoulder-level? Waist-level? Lesson: Problems arise when your bushel is not the same as my bushel. Therefore, units need to be standardized. *James C. Scott, Seeing Like a State Bronze Exchequer Standard Winchester Bushel measure, 1601

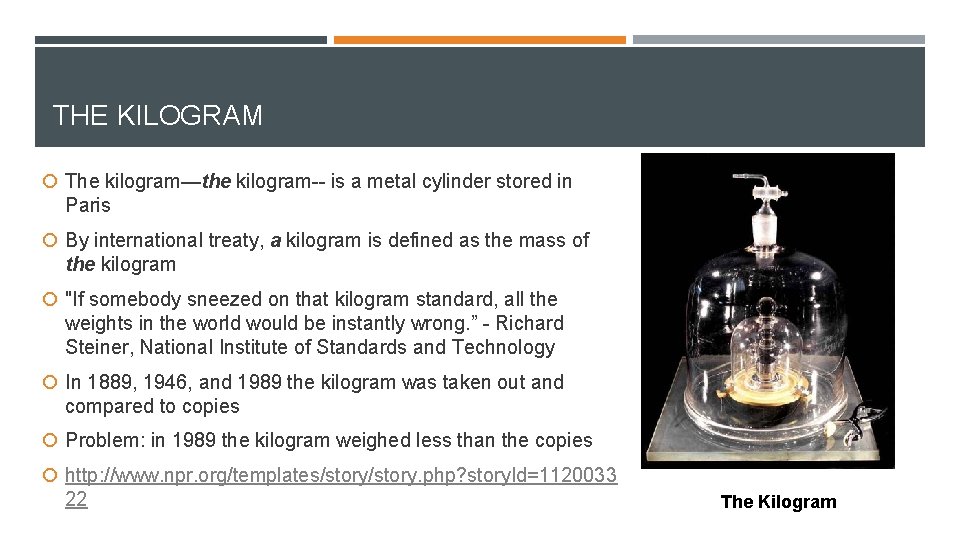
THE KILOGRAM The kilogram—the kilogram-- is a metal cylinder stored in Paris By international treaty, a kilogram is defined as the mass of the kilogram "If somebody sneezed on that kilogram standard, all the weights in the world would be instantly wrong. ” - Richard Steiner, National Institute of Standards and Technology In 1889, 1946, and 1989 the kilogram was taken out and compared to copies Problem: in 1989 the kilogram weighed less than the copies http: //www. npr. org/templates/story. php? story. Id=1120033 22 The Kilogram

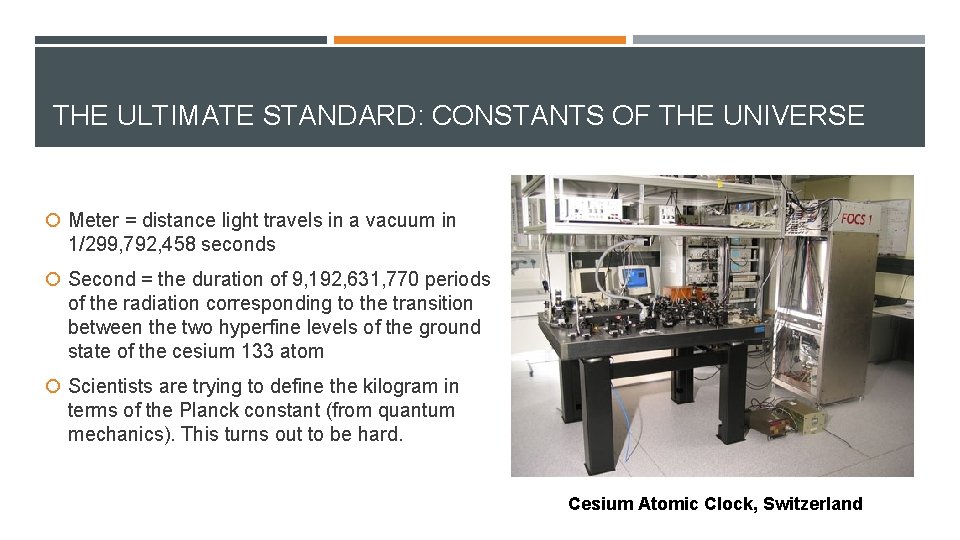
THE ULTIMATE STANDARD: CONSTANTS OF THE UNIVERSE Meter = distance light travels in a vacuum in 1/299, 792, 458 seconds Second = the duration of 9, 192, 631, 770 periods of the radiation corresponding to the transition between the two hyperfine levels of the ground state of the cesium 133 atom Scientists are trying to define the kilogram in terms of the Planck constant (from quantum mechanics). This turns out to be hard. Cesium Atomic Clock, Switzerland

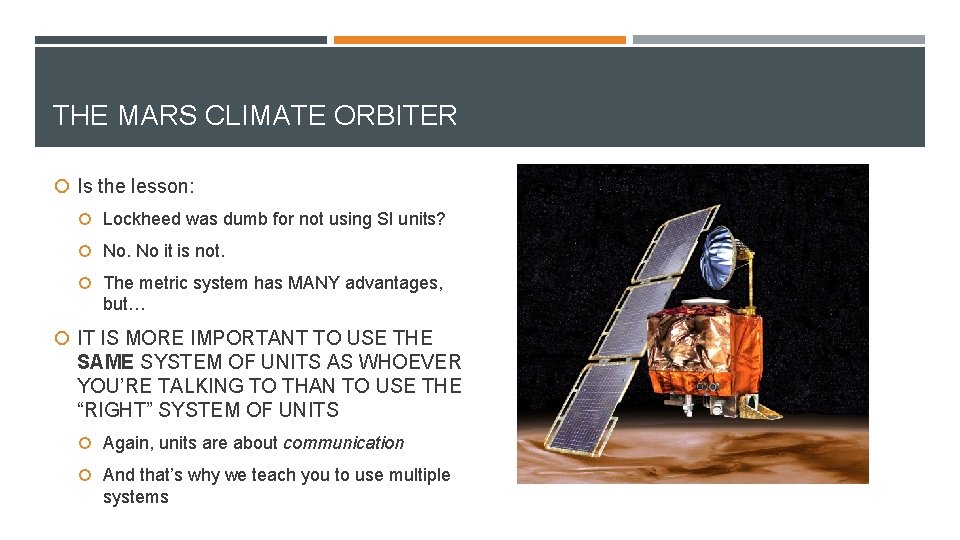
THE MARS CLIMATE ORBITER Robotic probe launched in 1998 with a total mission cost of $327. 6 million Meant to study Martian climate, atmosphere, surface changes Communication with the orbiter was lost; it came too close to the planet and disintegrated in the upper atmosphere Why? One piece of ground software (from Lockheed Martin) produced results for impulse in pound-seconds, while another piece of software (from NASA) expected Newtonseconds

Metric System/Systeme Internationale (SI) “The centuries old dream of the masses of only one just measure has come true! The Revolution has given the people the meter!” – The Estates General American Engineering System

THE MARS CLIMATE ORBITER Is the lesson: Lockheed was dumb for not using SI units? No. No it is not. The metric system has MANY advantages, but… IT IS MORE IMPORTANT TO USE THE SAME SYSTEM OF UNITS AS WHOEVER YOU’RE TALKING TO THAN TO USE THE “RIGHT” SYSTEM OF UNITS Again, units are about communication And that’s why we teach you to use multiple systems

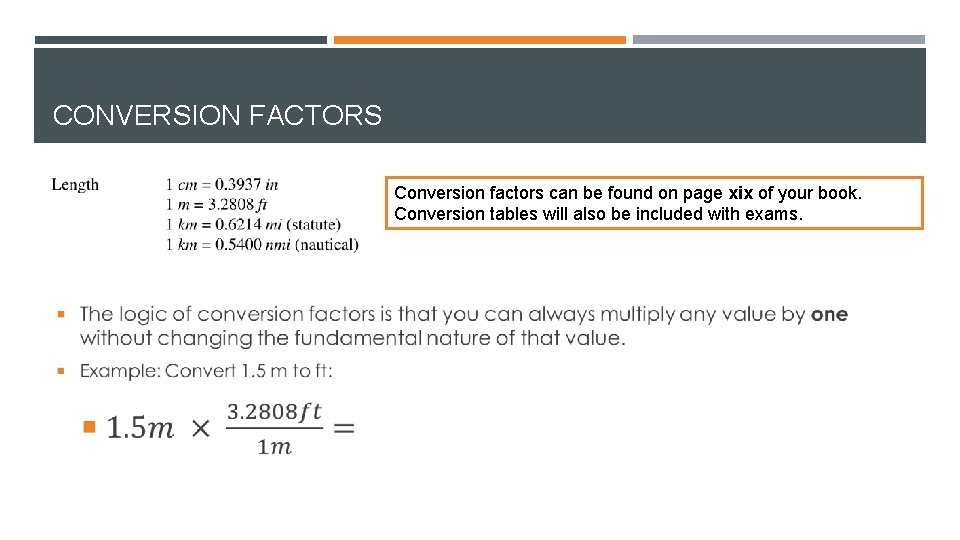
CONVERSION FACTORS Conversion factors can be found on page xix of your book. Conversion tables will also be included with exams.

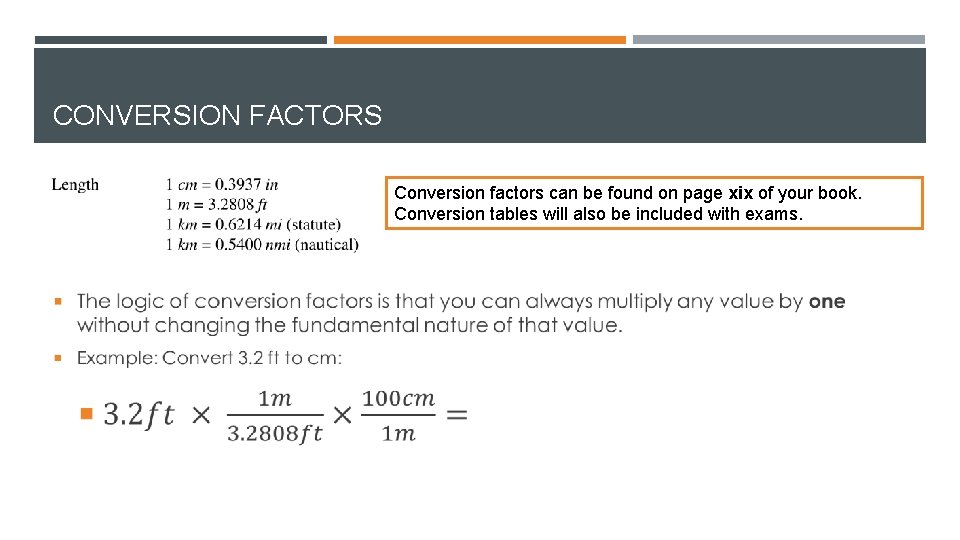
CONVERSION FACTORS Conversion factors can be found on page xix of your book. Conversion tables will also be included with exams.

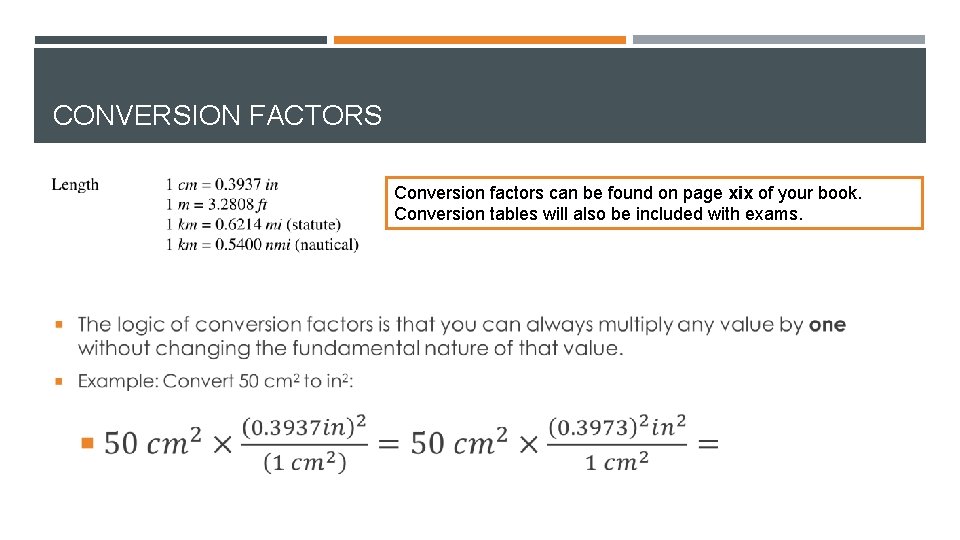
CONVERSION FACTORS Conversion factors can be found on page xix of your book. Conversion tables will also be included with exams.

UNITS DESCRIBE ACTUAL PHYSICAL THINGS It is important to use the correct type of unit to describe a particular physical quantity. The relationship between physical quantities can tell us something about the relationship between units and vice versa.

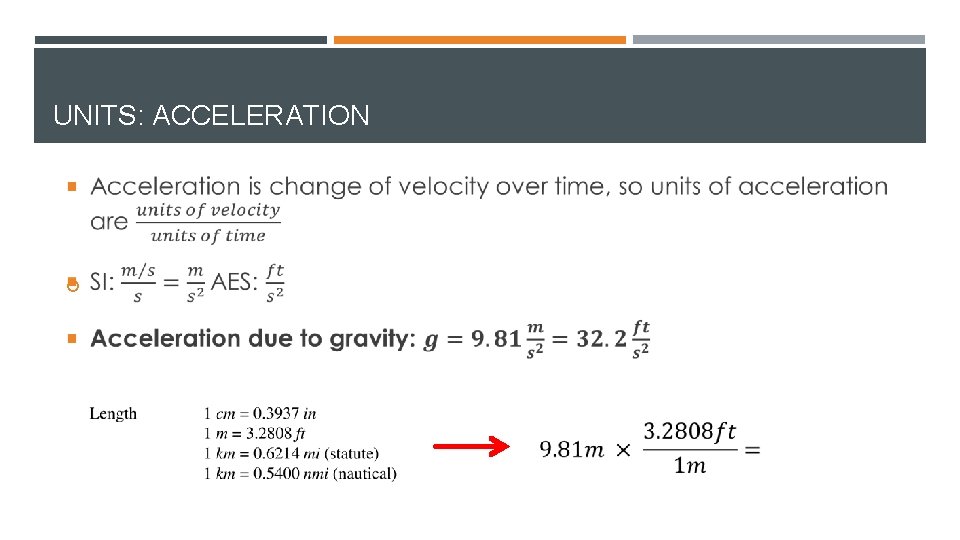
UNITS: ACCELERATION

UNITS: MASS AND FORCE

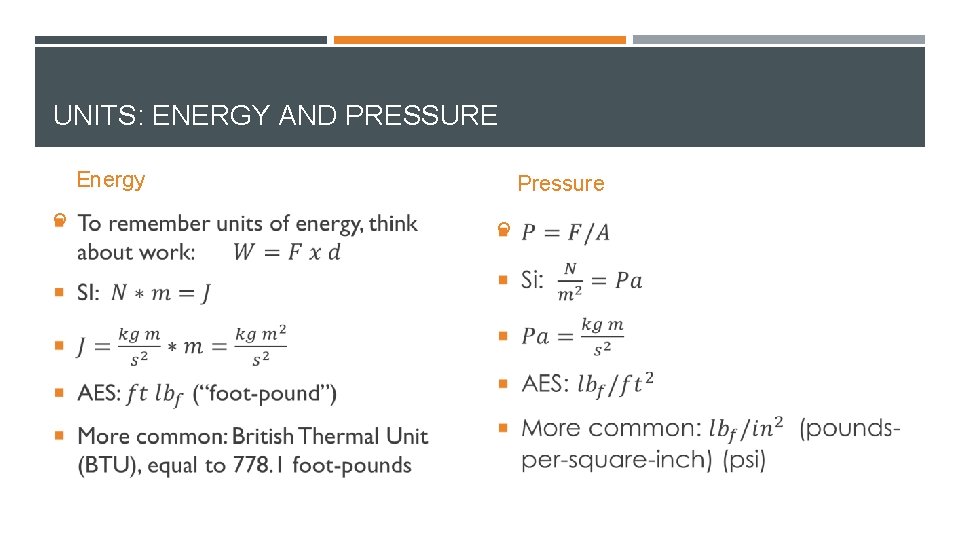
UNITS: ENERGY AND PRESSURE Energy Pressure

UNITS: MOLES A mole is a measure of amount of stuff. In your chemistry class you learned that one mole = Avogadro’s number of particles (6. 02 x 1023). Engineers often deal in VERY LARGE QUANTITIES of stuff, so it is convenient to define different types of moles.

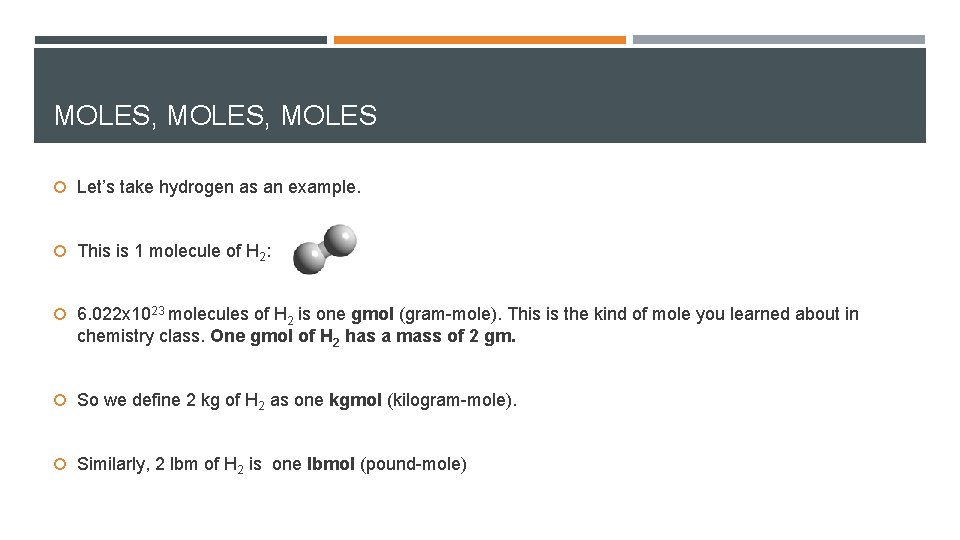
MOLES, MOLES Let’s take hydrogen as an example. This is 1 molecule of H 2: 6. 022 x 1023 molecules of H 2 is one gmol (gram-mole). This is the kind of mole you learned about in chemistry class. One gmol of H 2 has a mass of 2 gm. So we define 2 kg of H 2 as one kgmol (kilogram-mole). Similarly, 2 lbm of H 2 is one lbmol (pound-mole)

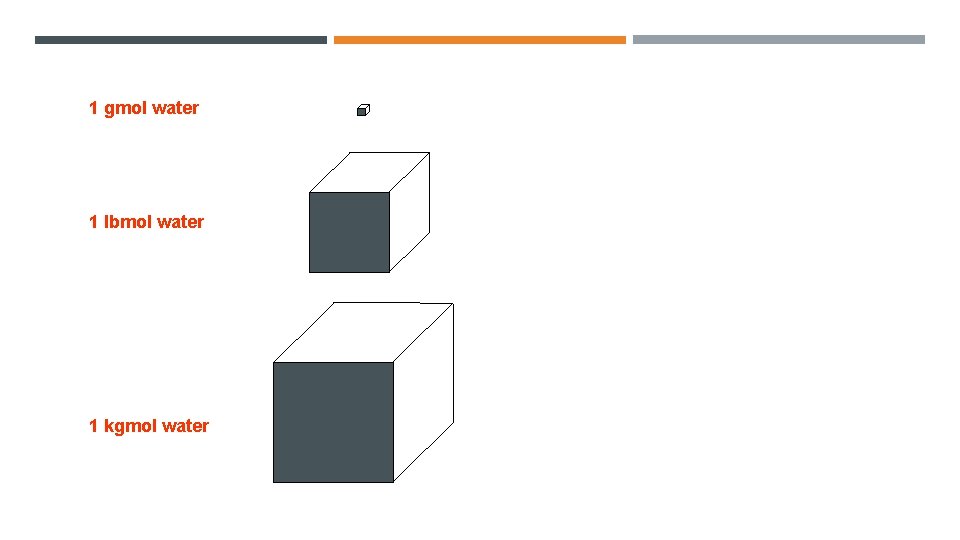
1 gmol water 1 lbmol water 1 kgmol water

EXAMPLE: CONVERTING BETWEEN MOLES AND LBMOLS One of your process streams is 10 gmol/min of molecule X. How many lbmol/min is that? (Think: Do you need to know the molecular weight of molecule X? )

DIMENSIONAL CONSISTENCY

What’s important is: Using the correct units to communicate effectively Using an appropriate unit for the physical property you are describing Keeping track of units and converting them as necessary I’m an engineer— I keep track of units!