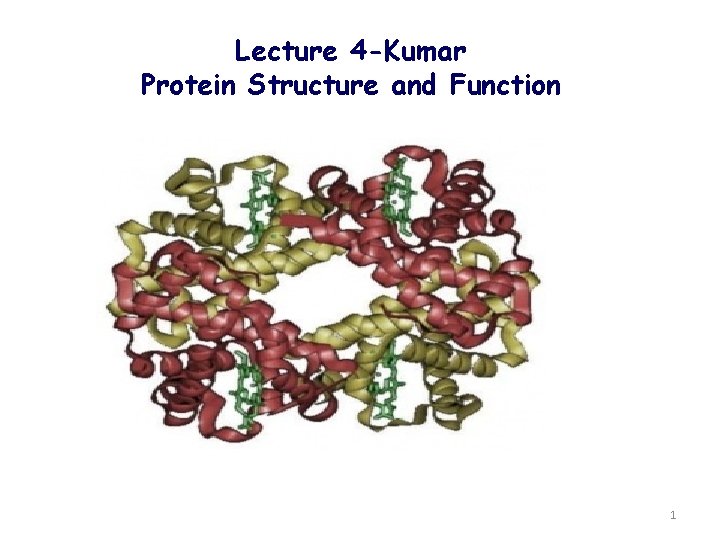
Lecture 4 Kumar Protein Structure and Function 1








- Slides: 25

Lecture 4 -Kumar Protein Structure and Function 1

2

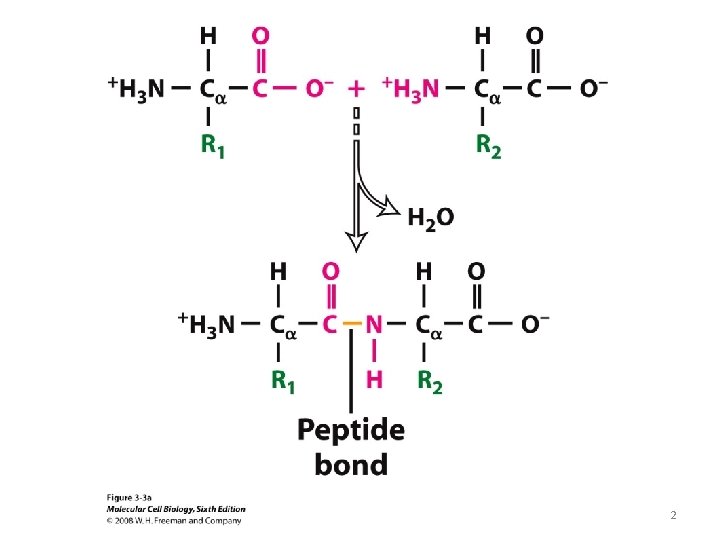
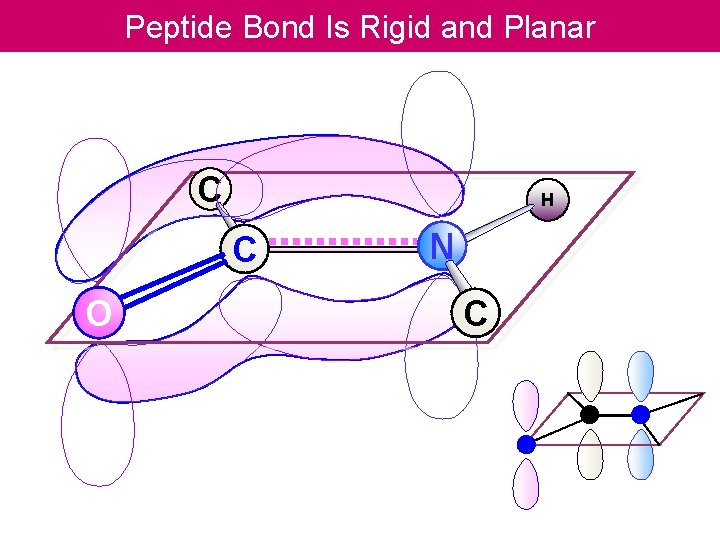
Peptide Bond Is Rigid and Planar C H C O N C

4

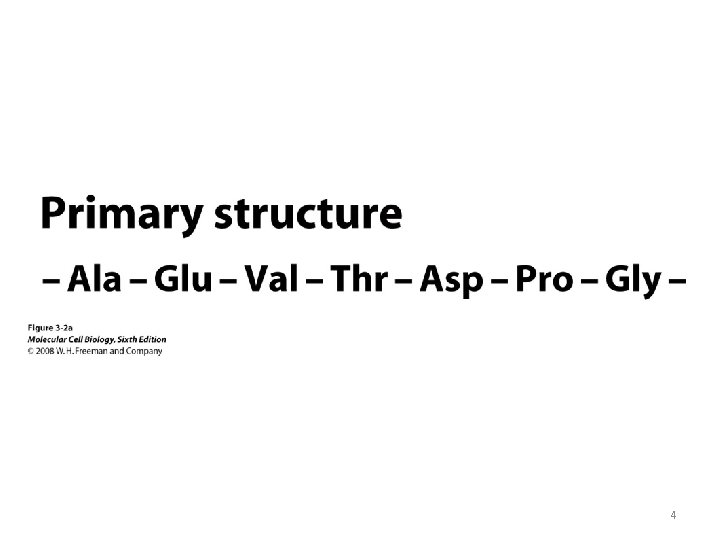
Terminology • Conformation – spatial arrangement of atoms in a protein • Native conformation – conformation of functional protein 5

6

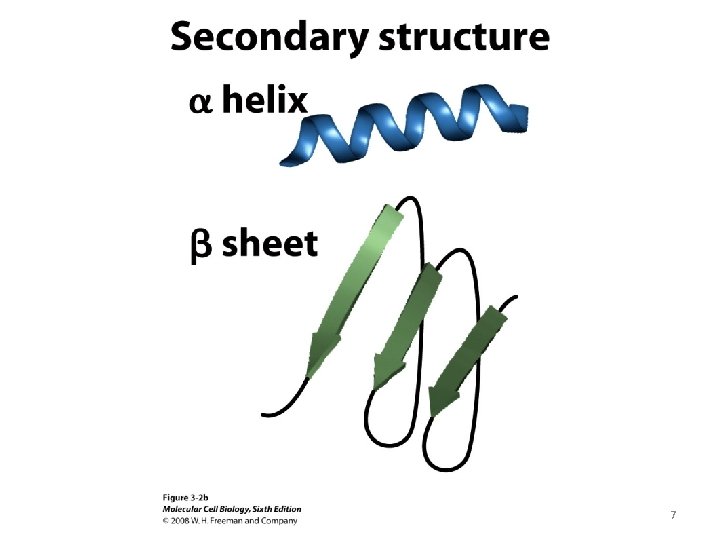
7

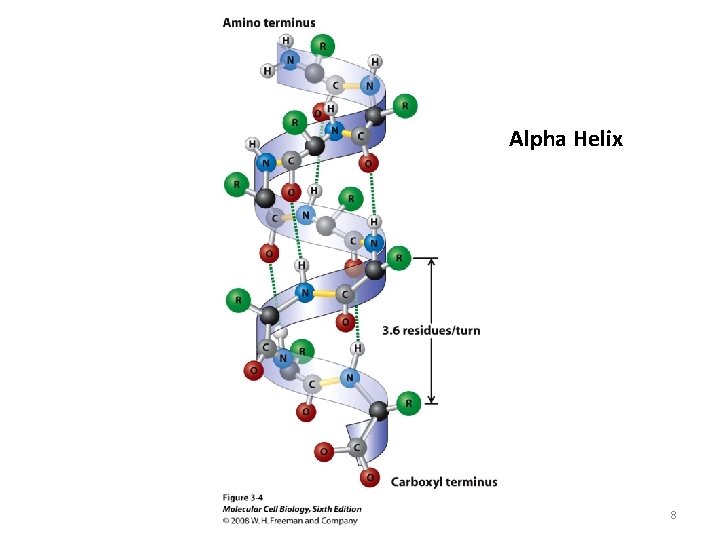
Alpha Helix 8

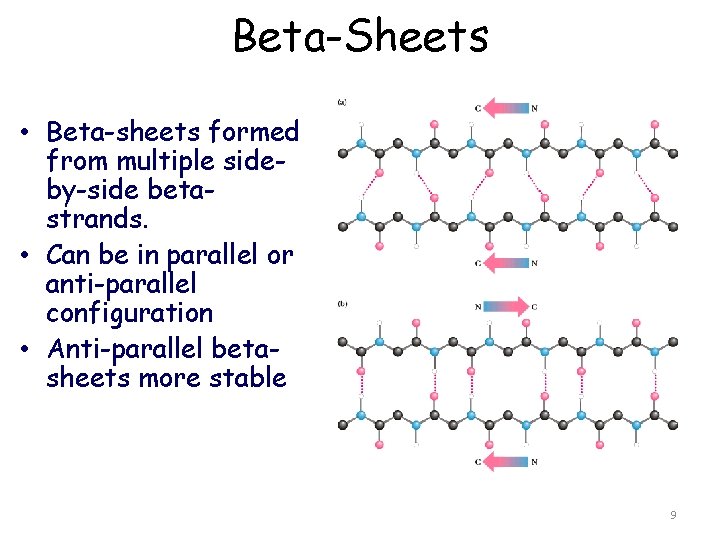
Beta-Sheets • Beta-sheets formed from multiple sideby-side betastrands. • Can be in parallel or anti-parallel configuration • Anti-parallel betasheets more stable 9

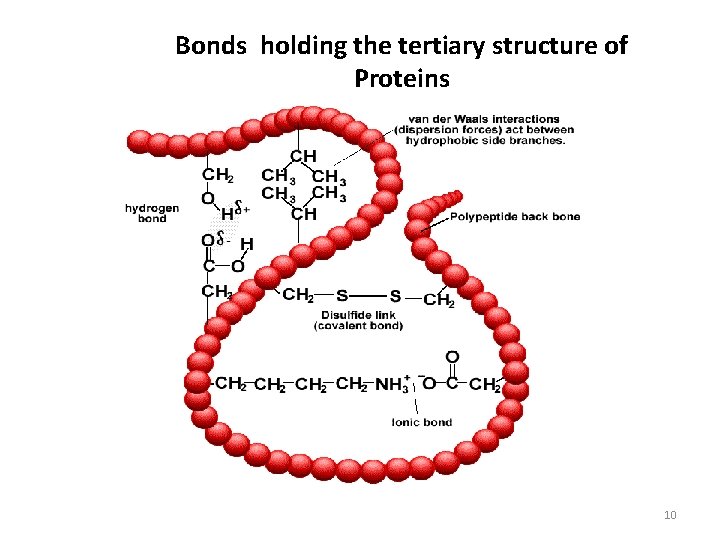
Bonds holding the tertiary structure of Proteins 10

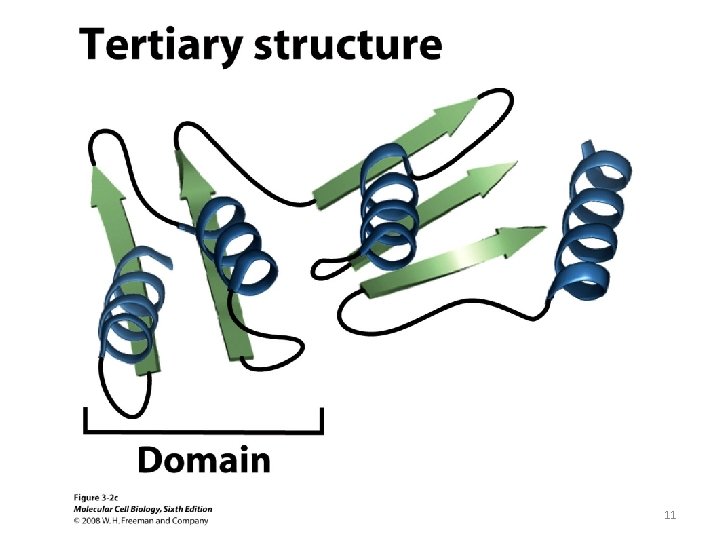
11

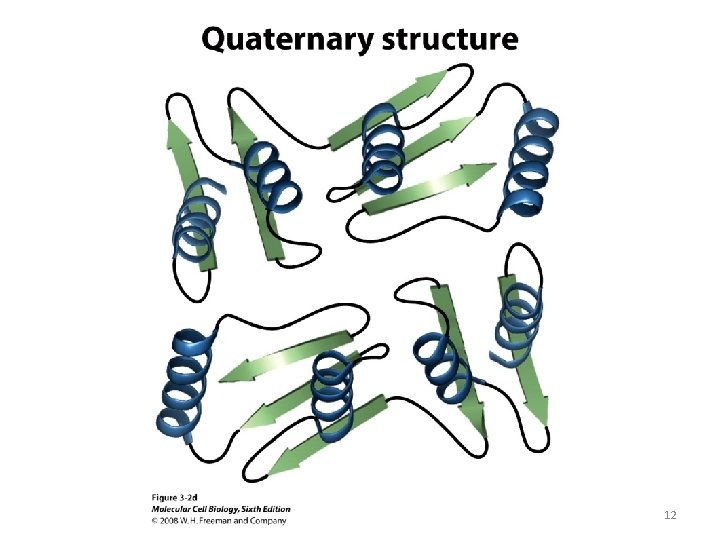
12

13

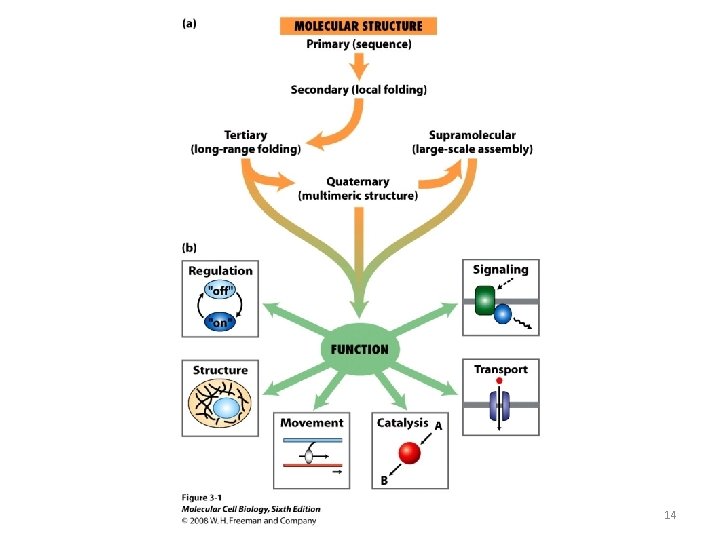
14

Protein Function • • • Catalysis – enzymes Structural – keratin Transport – hemoglobin Trans-membrane transport – Na+/K+ ATPases Toxins – rattle snake venom, ricin Contractile function – actin, myosin Hormones – insulin Storage Proteins – seeds and eggs Defensive proteins – antibodies 15

Protein Classification • One polypeptide chain - monomeric protein • More than one - multimeric protein Homomultimer – all one kind of chain Heteromultimer - two or more different chains (e. g. Hemoglobin is a heterotetramer. It has two alpha chains and two beta chains). 16

Protein Classification Fibrous – 1) polypeptides arranged in long strands or sheets 2) water insoluble (lots of hydrophobic AA’s) 3) strong but flexible 4) Structural (keratin, collagen) Globular 1) 2) 3) 4) – polypeptide chains folded into spherical or globular form water soluble contain several types of secondary structure diverse functions (enzymes, regulatory proteins) 17

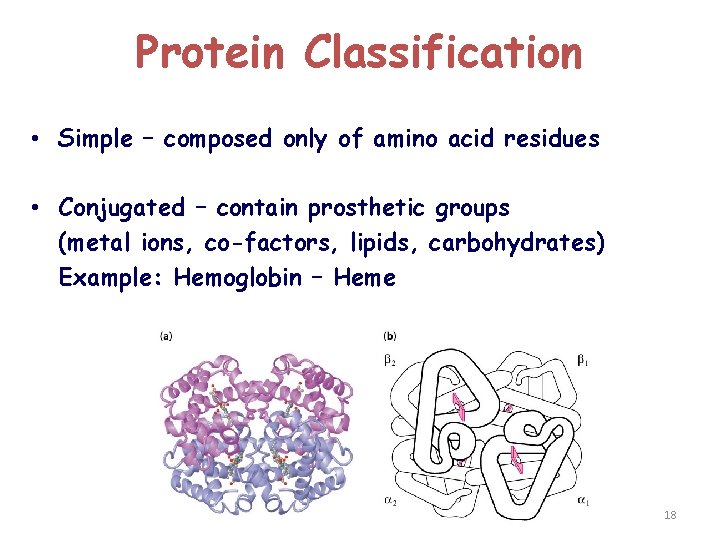
Protein Classification • Simple – composed only of amino acid residues • Conjugated – contain prosthetic groups (metal ions, co-factors, lipids, carbohydrates) Example: Hemoglobin – Heme 18

Diseases caused by changes in protein structure • spongiform encephalopathy (BSE or mad cow disease) seen in cattle and livestock and Creutzfeldt-Jakob disease (CJD) seen in humans. • Sickle Cell Anemia – single amino acid change in hemoglobin related to disease • Osteoarthritis – single amino acid change in collagen protein causes joint damage 19

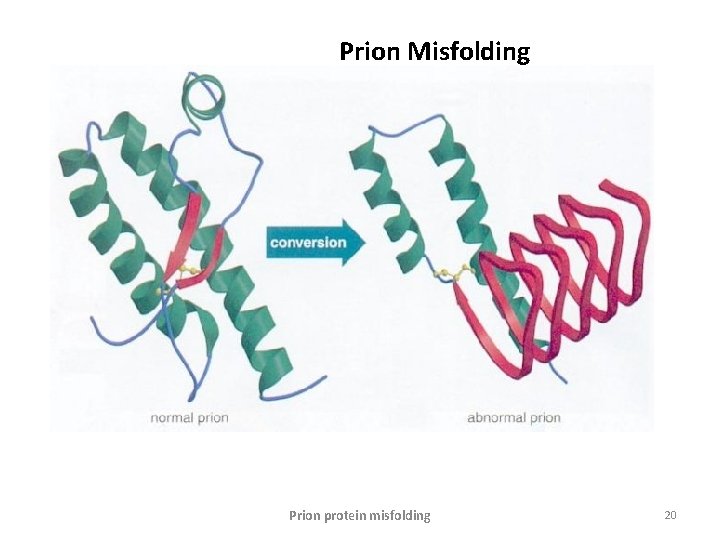
Prion Misfolding Prion protein misfolding 20

21

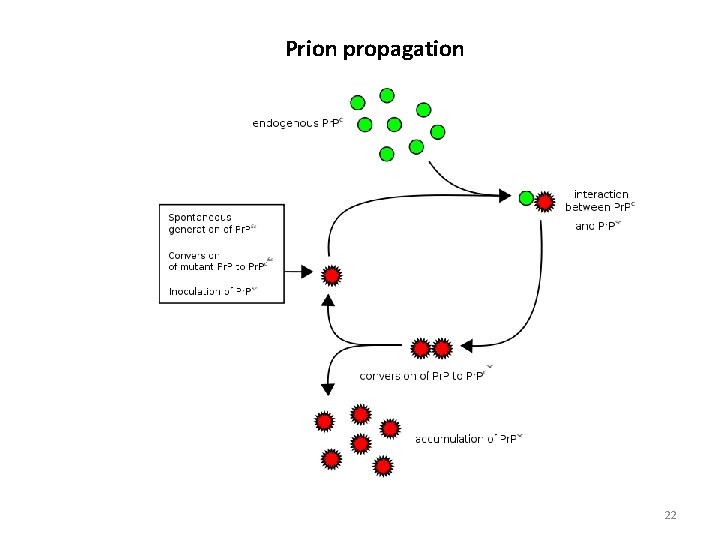
Prion propagation 22

Sickle cell anemia 23

Mutations in a- or b-globin genes can cause disease state • Sickle cell anemia – E 6 to V 6 • Causes V 6 to bind to hydrophobic pocket in deoxy-Hb • Polymerizes to form long filaments • Cause sickling of cells • Sickle cell trait offers advantage against malaria • Fragile sickle cells can not support parasite 24

Learning objectives-protein structure and function • Know the definitions of primary, secondary, tertiary, and quaternary structures or protein • Understand the differences between globular and fibrous proteins • Know the forces that stabilize these structures • Understand the type of bonds that stabilize these structures • Understand that certain changes in protein structure can change the structure and function of protein 25