Lecture 4 General Phase Equilibrium Minimum Energy Principle

Lecture 4 General Phase Equilibrium Ø Minimum Energy Principle Ø Degrees of freedom Ø Gibbs-Duhem equation Ø Equilibrium in multiphase systems Ø Gibbs phase rule Ø Problems

Minimum Energy Principle Maximum entropy principle: For isolated system the entropy is maximum in equilibrium Minimum energy principle: For a system kept at constant entropy and volume the energy is minimum in equilibrium The two principles are equivalent
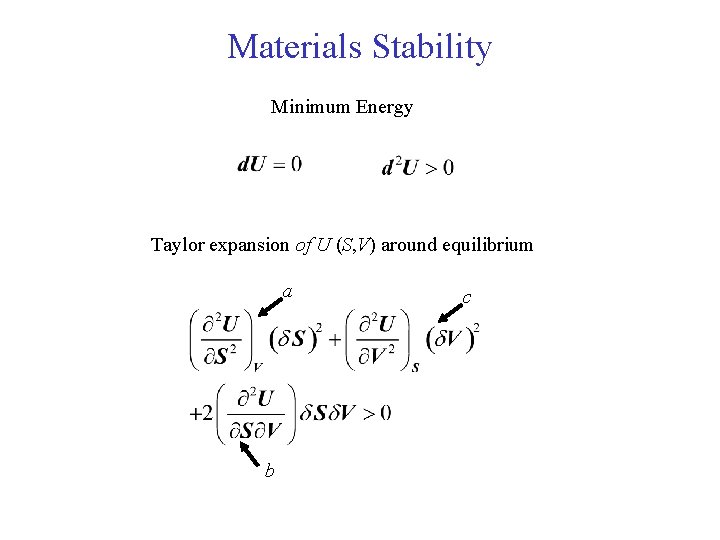
Materials Stability Minimum Energy Taylor expansion of U (S, V) around equilibrium a b c
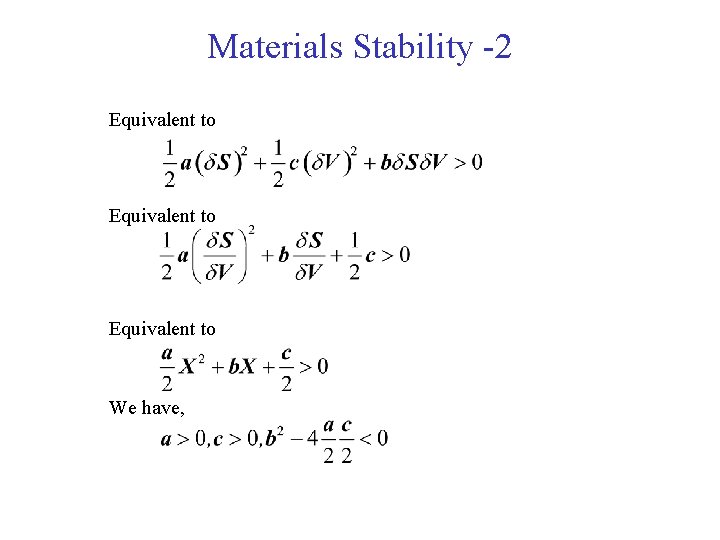
Materials Stability -2 Equivalent to We have,
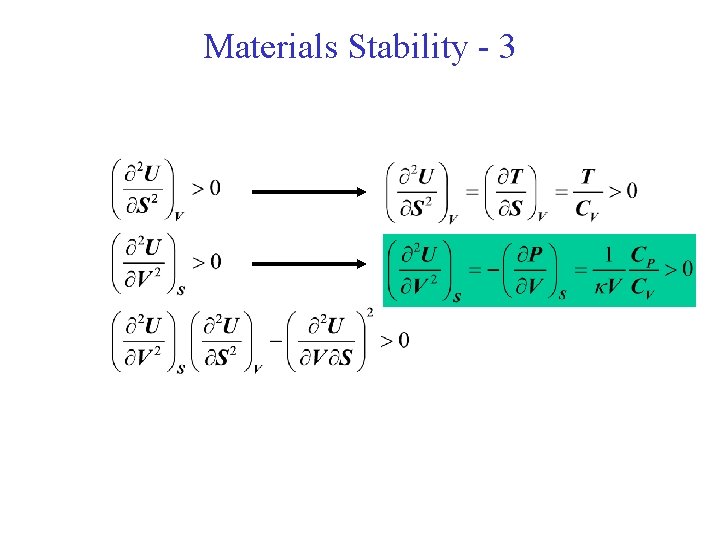
Materials Stability - 3
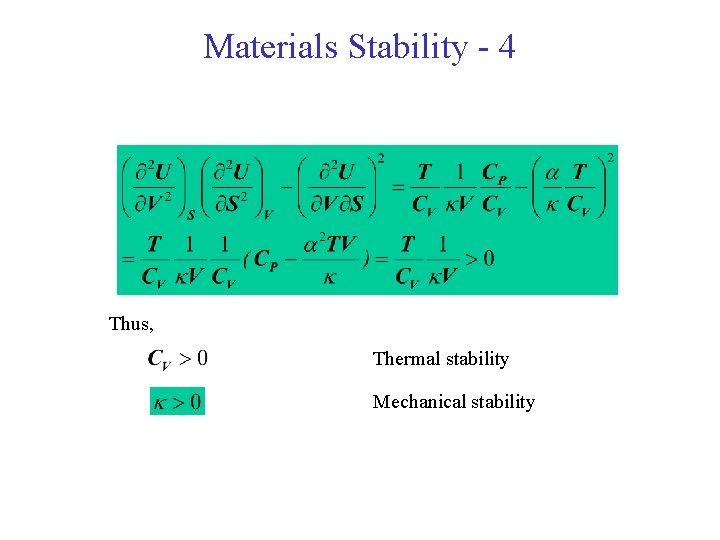
Materials Stability - 4 Thus, Thermal stability Mechanical stability
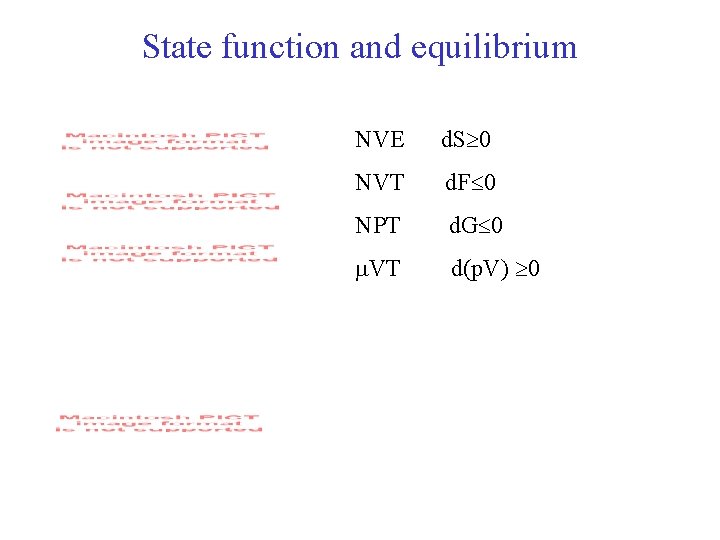
State function and equilibrium NVE d. S 0 NVT d. F 0 NPT d. G 0 VT d(p. V) 0
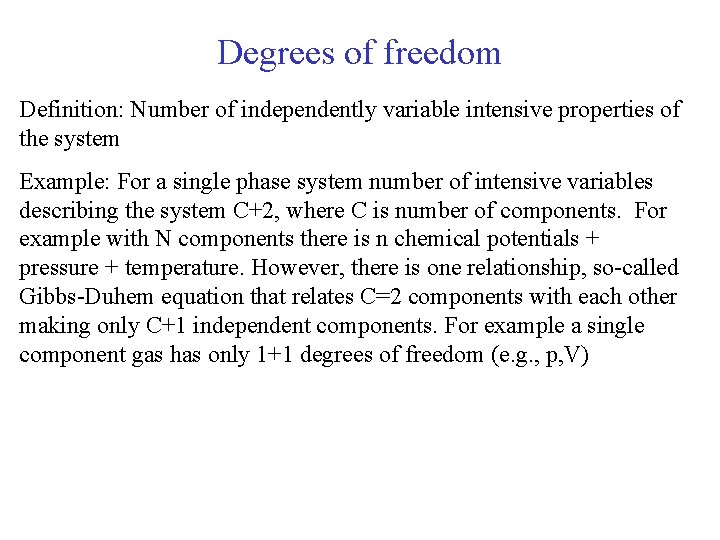
Degrees of freedom Definition: Number of independently variable intensive properties of the system Example: For a single phase system number of intensive variables describing the system C+2, where C is number of components. For example with N components there is n chemical potentials + pressure + temperature. However, there is one relationship, so-called Gibbs-Duhem equation that relates C=2 components with each other making only C+1 independent components. For example a single component gas has only 1+1 degrees of freedom (e. g. , p, V)
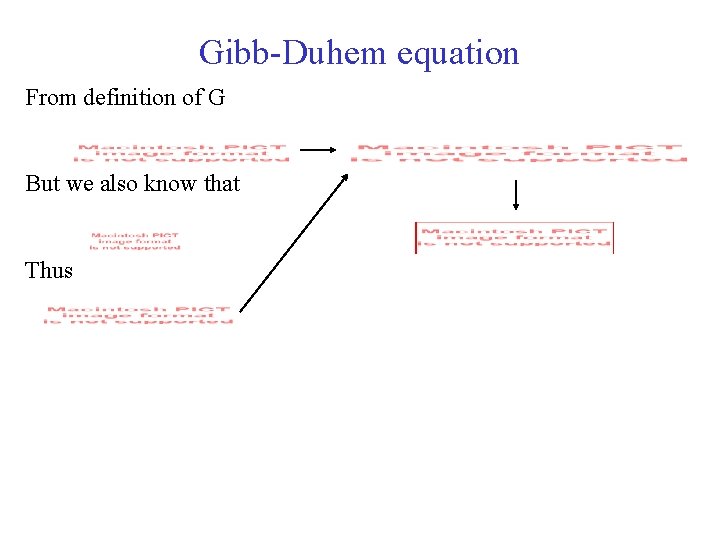
Gibb-Duhem equation From definition of G But we also know that Thus
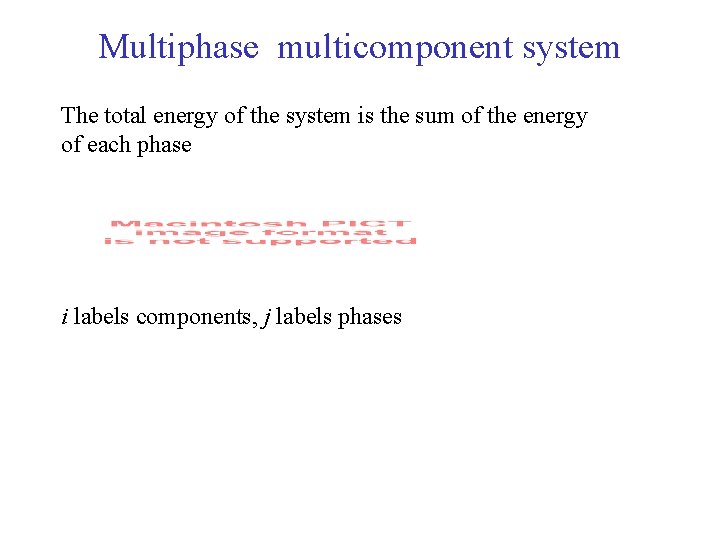
Multiphase multicomponent system The total energy of the system is the sum of the energy of each phase i labels components, j labels phases

Equilibrium in multiphase multicomponent system 0 th law of thermodynamics - each phase has the same T d. F = 0 at const T and leads to hydrostatic equilibrium d. G = 0 at const P and T gives distributive equilibrium - chemical potential of a given component is the same in every phase
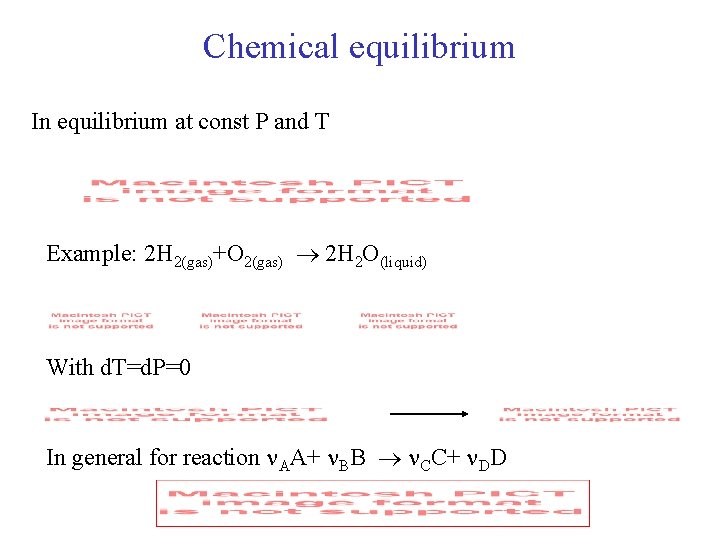
Chemical equilibrium In equilibrium at const P and T Example: 2 H 2(gas)+O 2(gas) 2 H 2 O(liquid) With d. T=d. P=0 In general for reaction AA+ BB CC+ DD
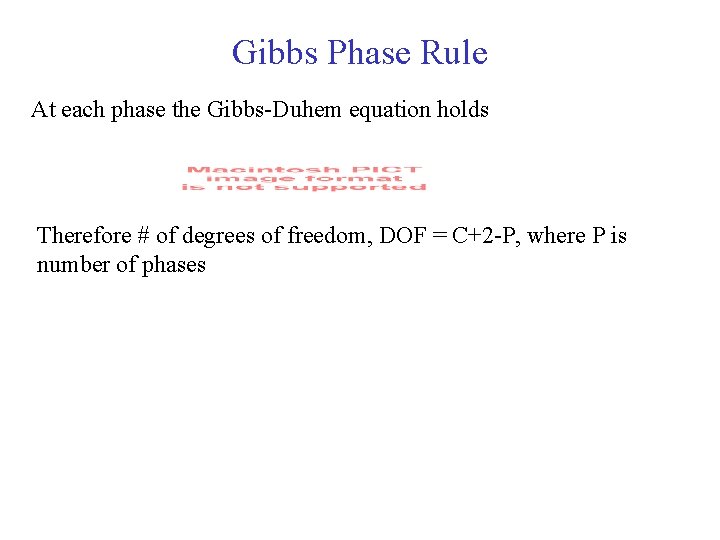
Gibbs Phase Rule At each phase the Gibbs-Duhem equation holds Therefore # of degrees of freedom, DOF = C+2 -P, where P is number of phases
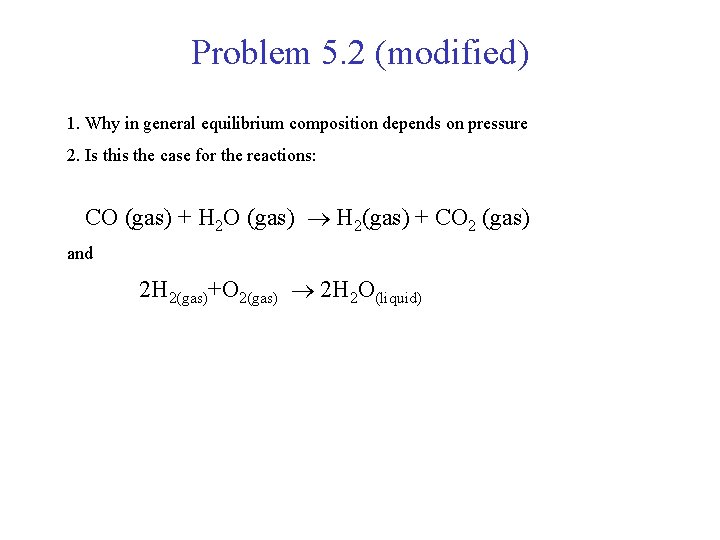
Problem 5. 2 (modified) 1. Why in general equilibrium composition depends on pressure 2. Is this the case for the reactions: CO (gas) + H 2 O (gas) H 2(gas) + CO 2 (gas) and 2 H 2(gas)+O 2(gas) 2 H 2 O(liquid)
- Slides: 14