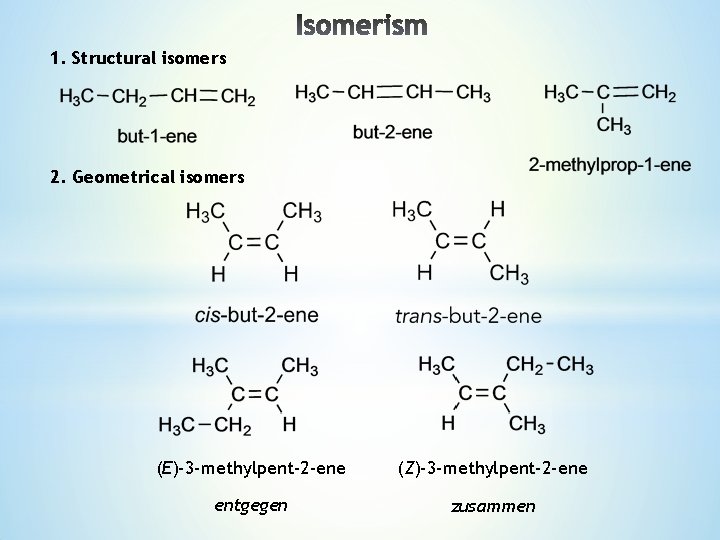
Lecture 4 1 Structural isomers 2 Geometrical isomers


- Slides: 26

Lecture 4

1. Structural isomers 2. Geometrical isomers (E)-3 -methylpent-2 -ene (Z)-3 -methylpent-2 -ene entgegen zusammen

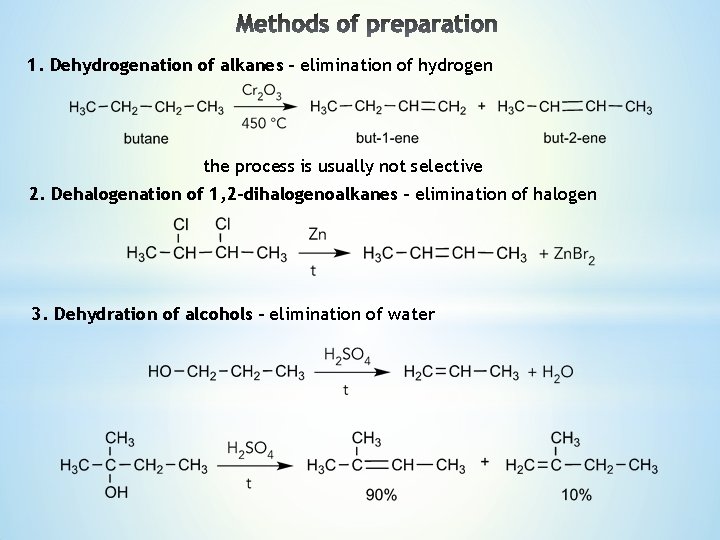
1. Dehydrogenation of alkanes – elimination of hydrogen the process is usually not selective 2. Dehalogenation of 1, 2 -dihalogenoalkanes – elimination of halogen 3. Dehydration of alcohols – elimination of water

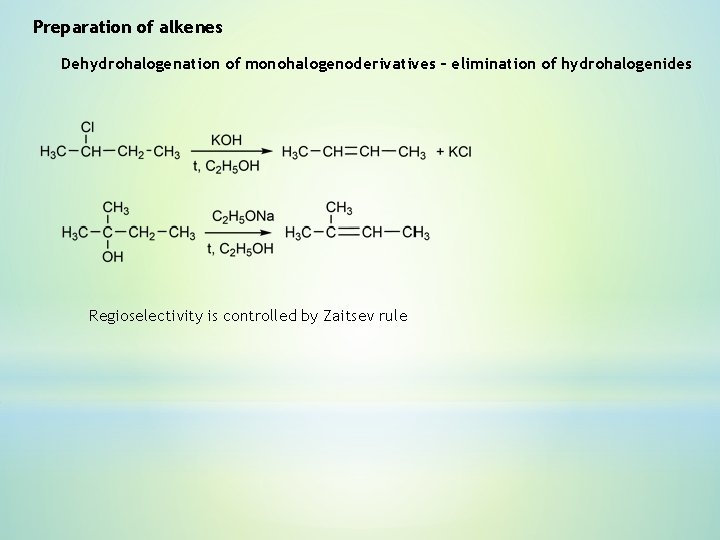
Zaitsev rule (1875) - elimination predominates in the direction that leads to the more highly substituted alkene. Aleksander Zaitsev 1841 -1910

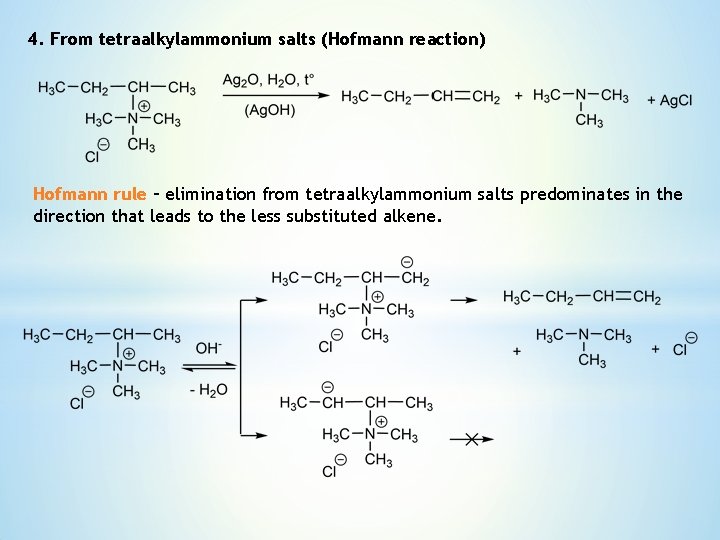
4. From tetraalkylammonium salts (Hofmann reaction) Hofmann rule – elimination from tetraalkylammonium salts predominates in the direction that leads to the less substituted alkene.

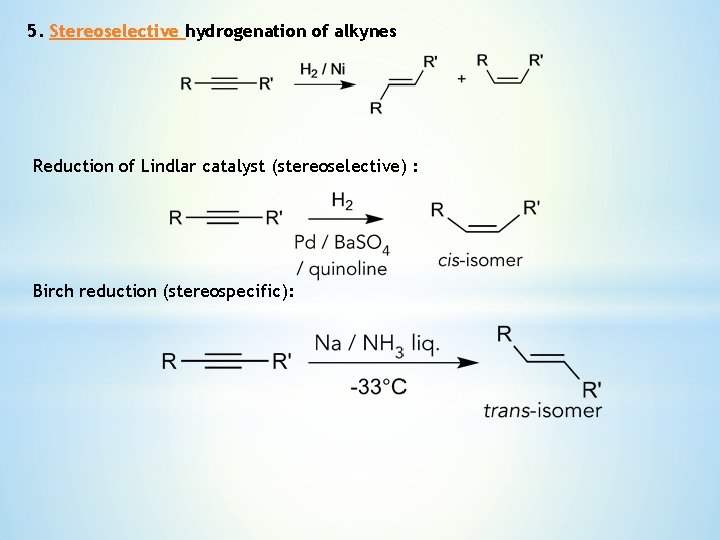
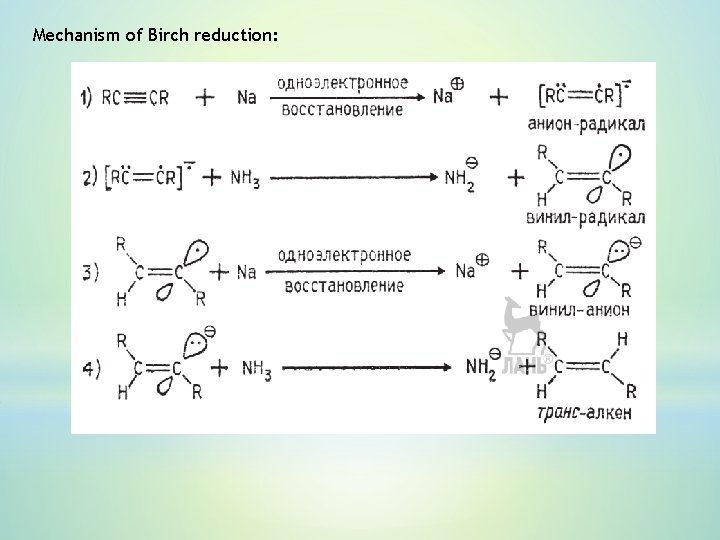
5. Stereoselective hydrogenation of alkynes Reduction of Lindlar catalyst (stereoselective) : Birch reduction (stereospecific):

Mechanism of Birch reduction:

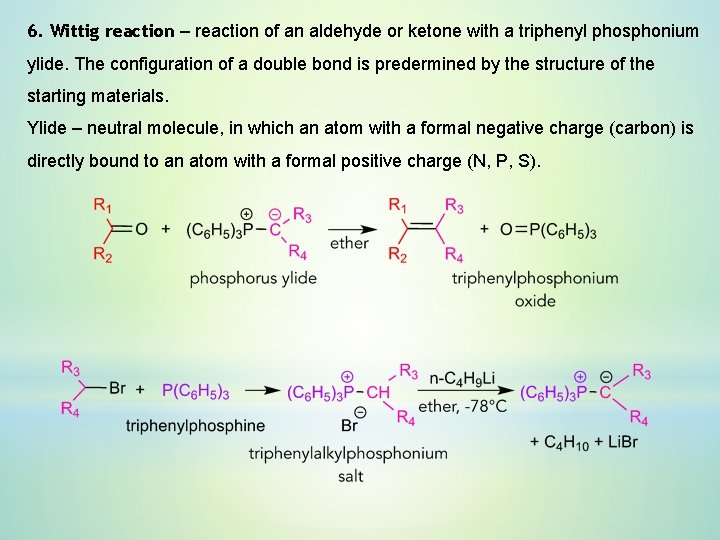
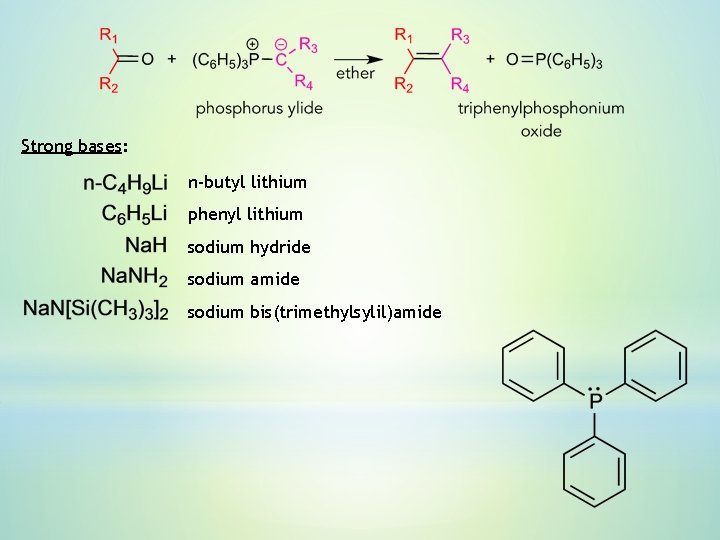
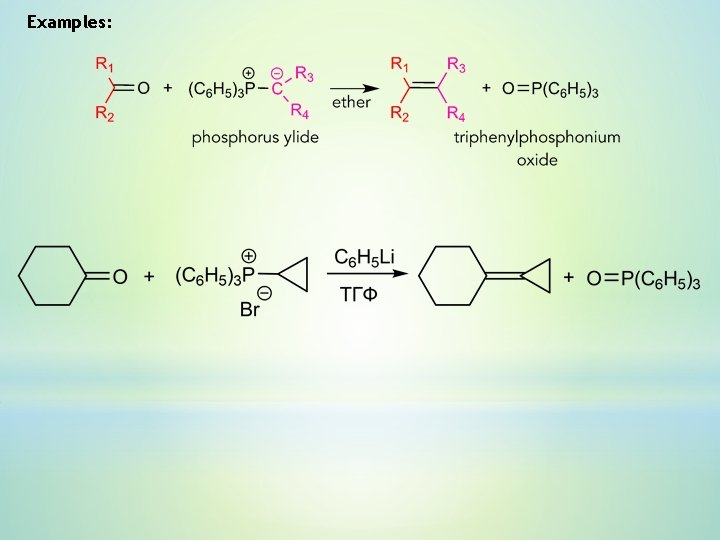
6. Wittig reaction – reaction of an aldehyde or ketone with a triphenyl phosphonium ylide. The configuration of a double bond is predermined by the structure of the starting materials. Ylide – neutral molecule, in which an atom with a formal negative charge (carbon) is directly bound to an atom with a formal positive charge (N, P, S).

Strong bases: n-butyl lithium phenyl lithium sodium hydride sodium amide sodium bis(trimethylsylil)amide

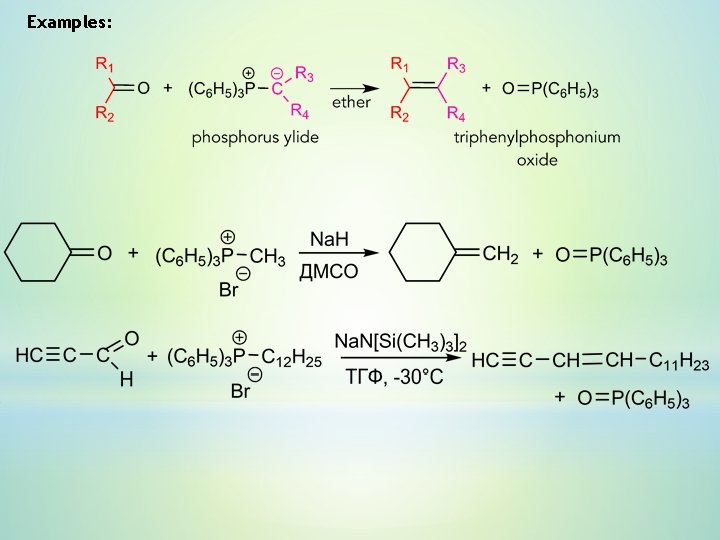
Examples:

Examples:

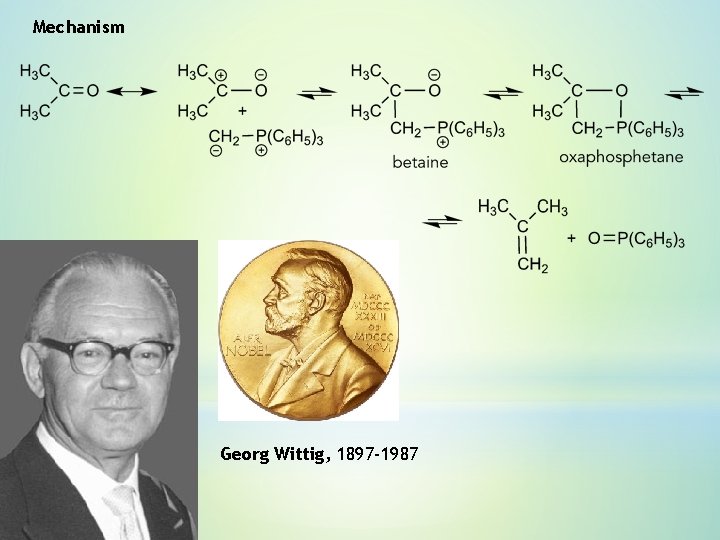
Mechanism Georg Wittig, 1897 -1987

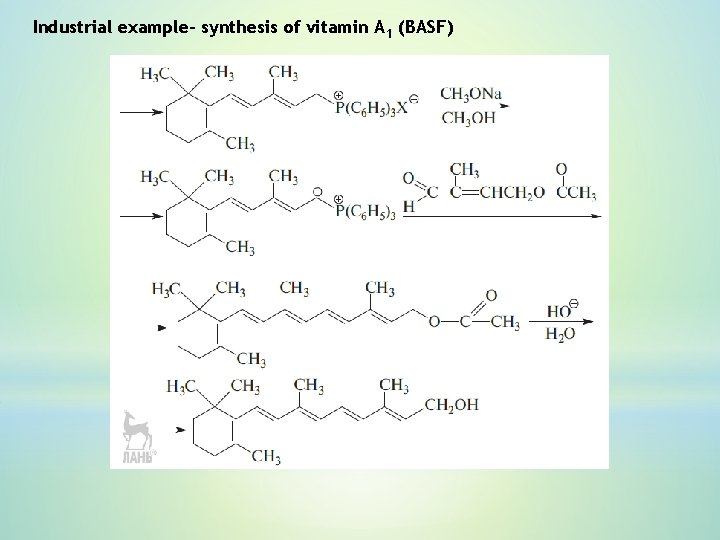
Industrial example– synthesis of vitamin А 1 (BASF)

Preparation of alkenes Dehydrohalogenation of monohalogenoderivatives – elimination of hydrohalogenides Regioselectivity is controlled by Zaitsev rule

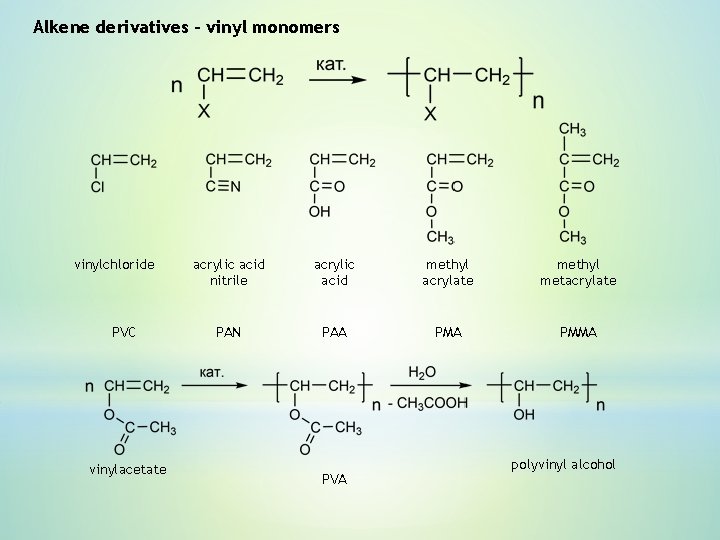
Alkene derivatives – vinyl monomers vinylchloride PVC vinylacetate acrylic acid nitrile acrylic acid methyl acrylate methyl metacrylate PAN PAA PMMA PVA polyvinyl alcohol

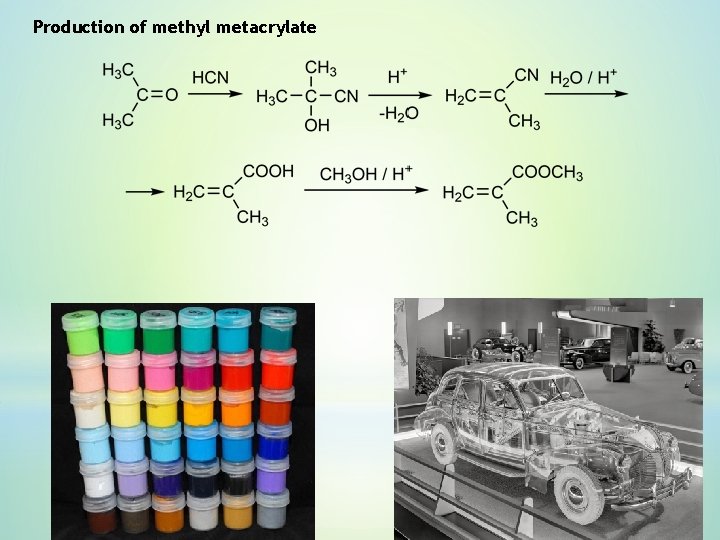
Production of methyl metacrylate

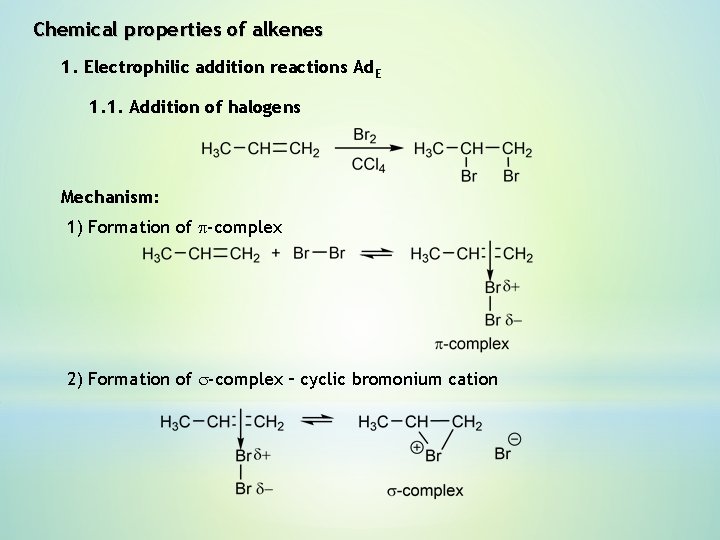
Chemical properties of alkenes 1. Electrophilic addition reactions Ad. E 1. 1. Addition of halogens Mechanism: 1) Formation of p-complex 2) Formation of s-complex – cyclic bromonium cation

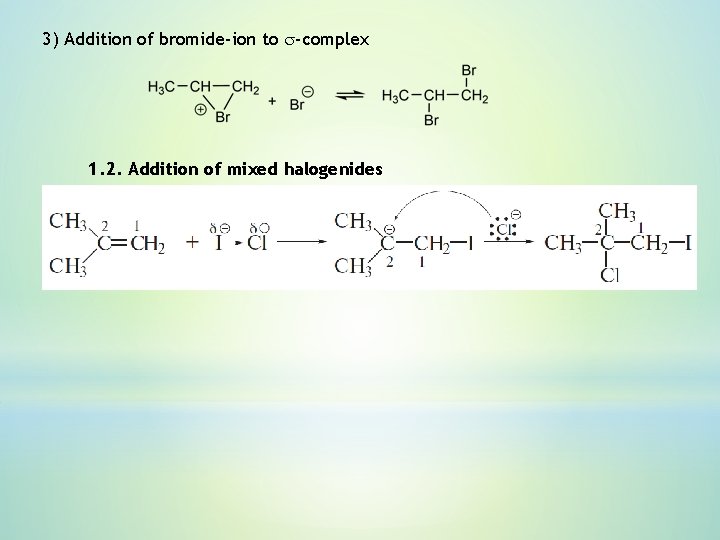
3) Addition of bromide-ion to s-complex 1. 2. Addition of mixed halogenides

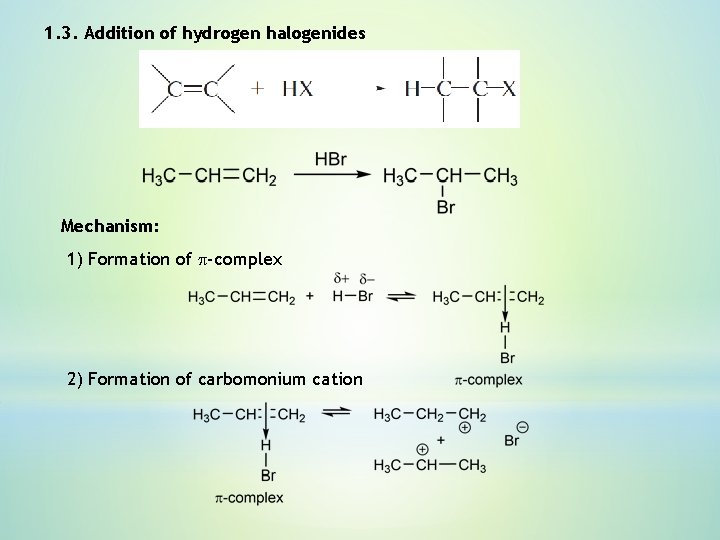
1. 3. Addition of hydrogen halogenides Mechanism: 1) Formation of p-complex 2) Formation of carbomonium cation

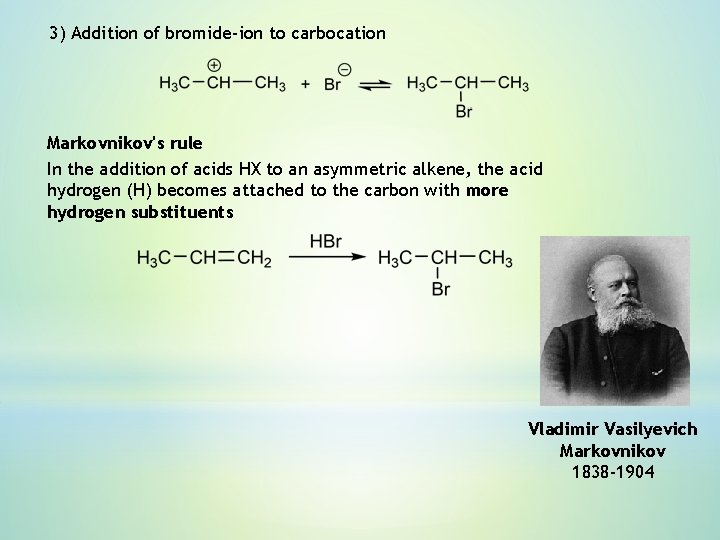
3) Addition of bromide-ion to carbocation Markovnikov's rule In the addition of acids HX to an asymmetric alkene, the acid hydrogen (H) becomes attached to the carbon with more hydrogen substituents Vladimir Vasilyevich Markovnikov 1838 -1904

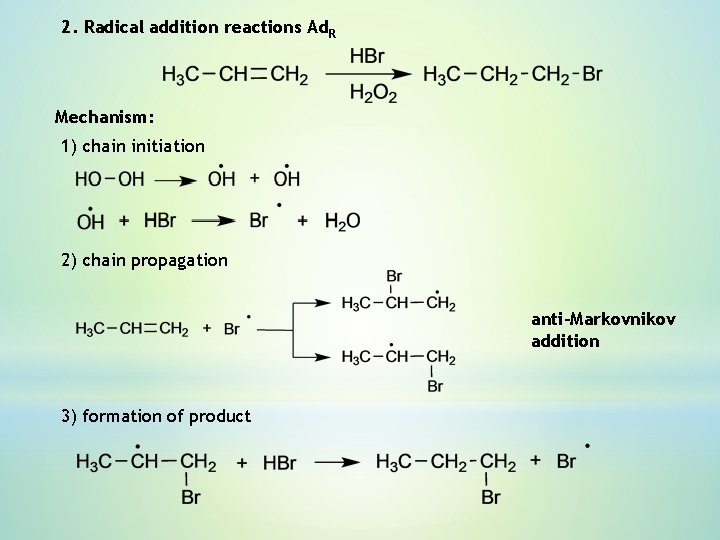
2. Radical addition reactions Ad. R Mechanism: 1) chain initiation 2) chain propagation anti-Markovnikov addition 3) formation of product

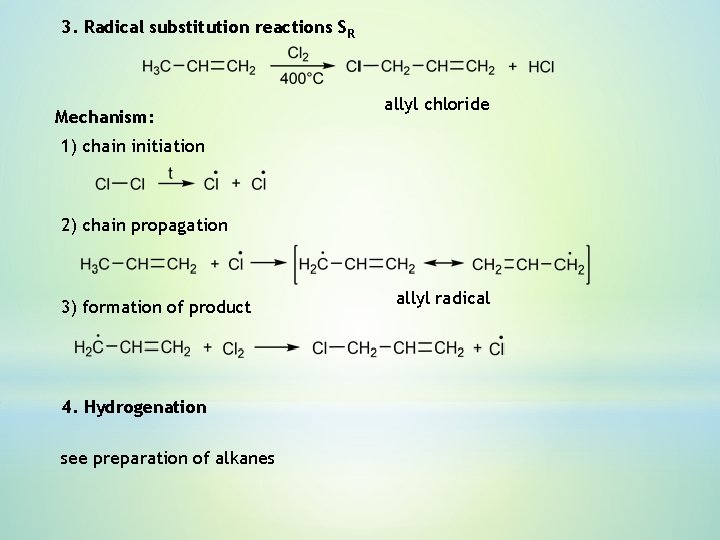
3. Radical substitution reactions SR Mechanism: allyl chloride 1) chain initiation 2) chain propagation 3) formation of product 4. Hydrogenation see preparation of alkanes allyl radical

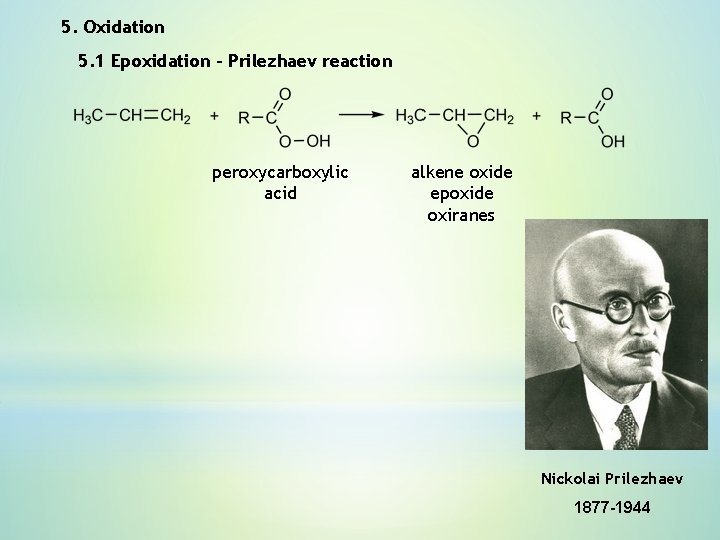
5. Oxidation 5. 1 Epoxidation – Prilezhaev reaction peroxycarboxylic acid alkene oxide epoxide oxiranes Nickolai Prilezhaev 1877 -1944

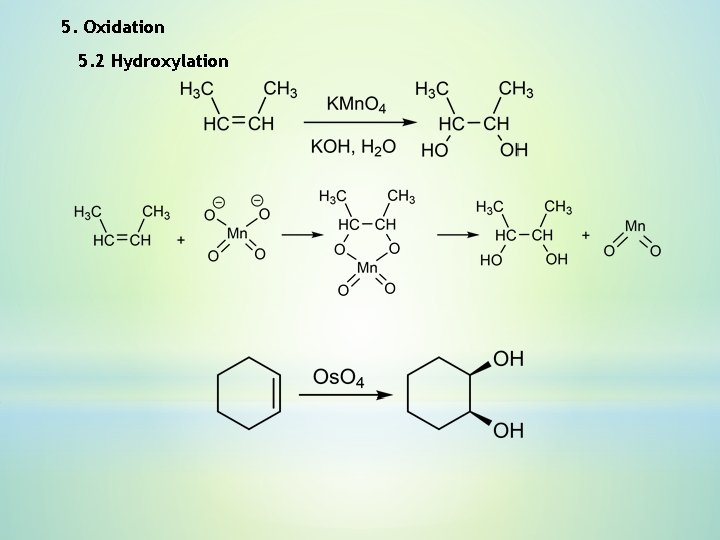
5. Oxidation 5. 2 Hydroxylation

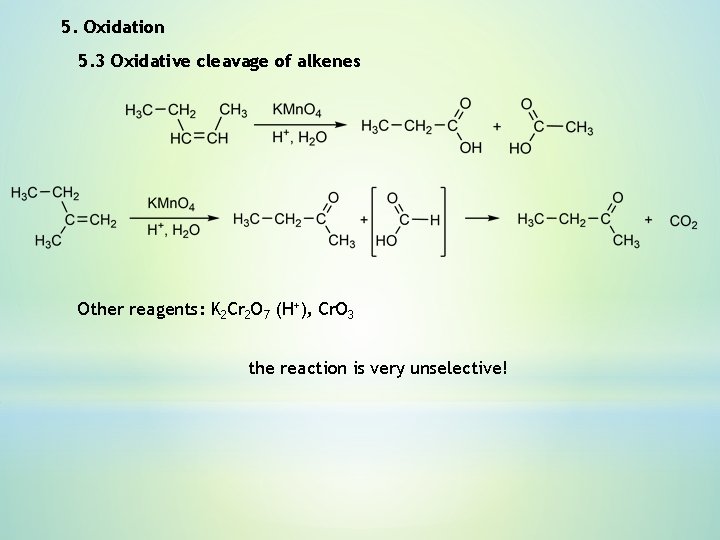
5. Oxidation 5. 3 Oxidative cleavage of alkenes Other reagents: K 2 Cr 2 O 7 (H+), Cr. O 3 the reaction is very unselective!

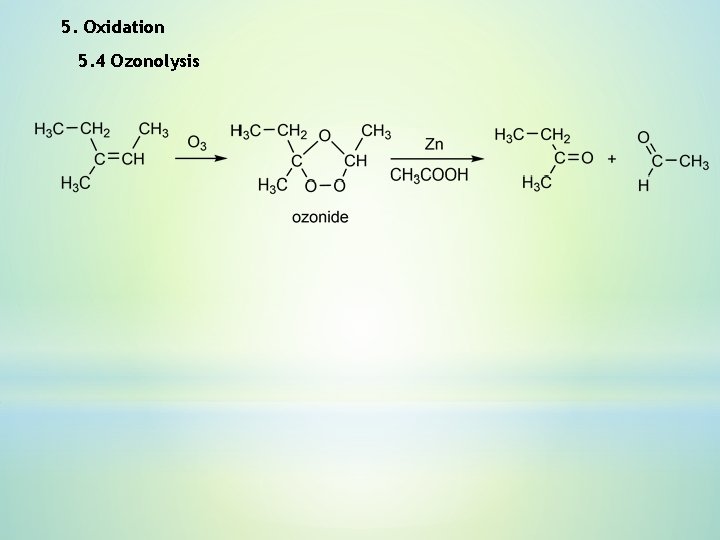
5. Oxidation 5. 4 Ozonolysis