Lecture 3 The Kinetic Molecular Theory of Gases










- Slides: 39

Lecture 3. The Kinetic Molecular Theory of Gases

THE IDEAL GAS LAW: • A purely empirical law – solely the consequence of experimental observations • Explains the behavior of gases over a limited range of conditions • A macroscopic explanation. Say nothing about the microscopic behavior of the atoms or molecules that make up the gas.

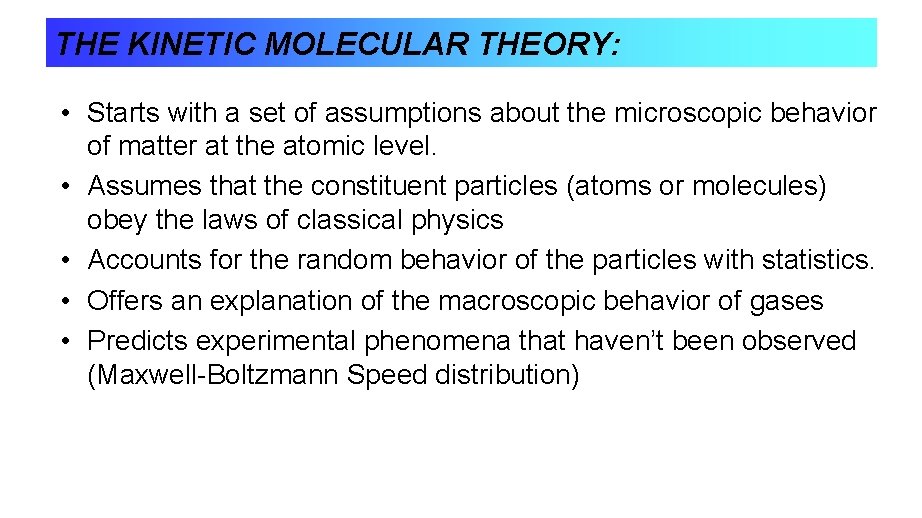
THE KINETIC MOLECULAR THEORY: • Starts with a set of assumptions about the microscopic behavior of matter at the atomic level. • Assumes that the constituent particles (atoms or molecules) obey the laws of classical physics • Accounts for the random behavior of the particles with statistics. • Offers an explanation of the macroscopic behavior of gases • Predicts experimental phenomena that haven’t been observed (Maxwell-Boltzmann Speed distribution)

The Four Postulates of the Kinetic Theory • A gas consists of large number of identical molecules separated by distances that are so large compared to their size. • The gas molecules are constantly moving in random directions with a distribution of speeds. • The molecules exert no forces on one another between collisions, so they move in straight lines with constant velocities. • The collision of molecules with the walls of the container are elastic: no energy is lost during a collision. Collisions with the walls of the container are the source of pressure.

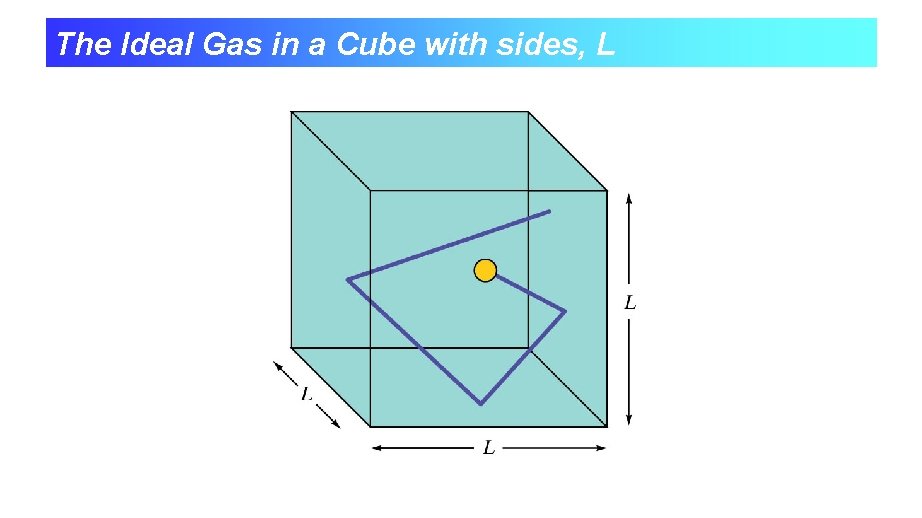
The Ideal Gas in a Cube with sides, L

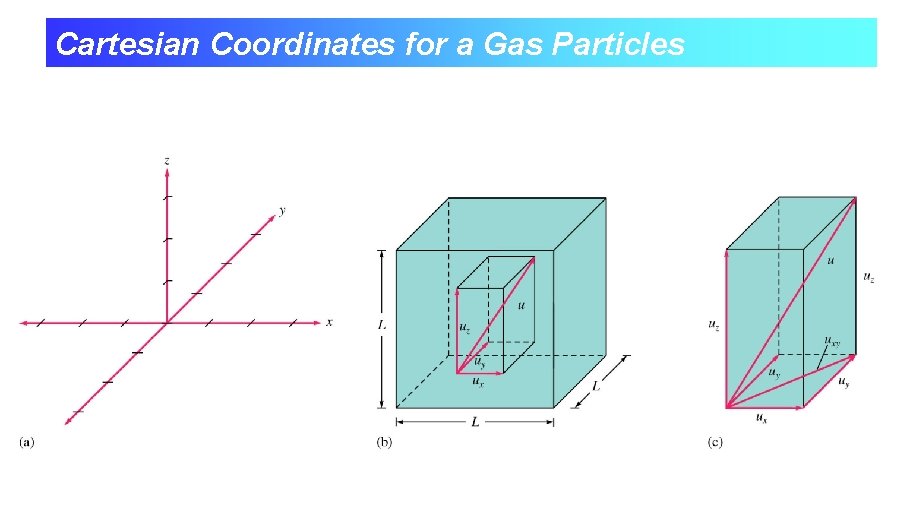
Cartesian Coordinates for a Gas Particles

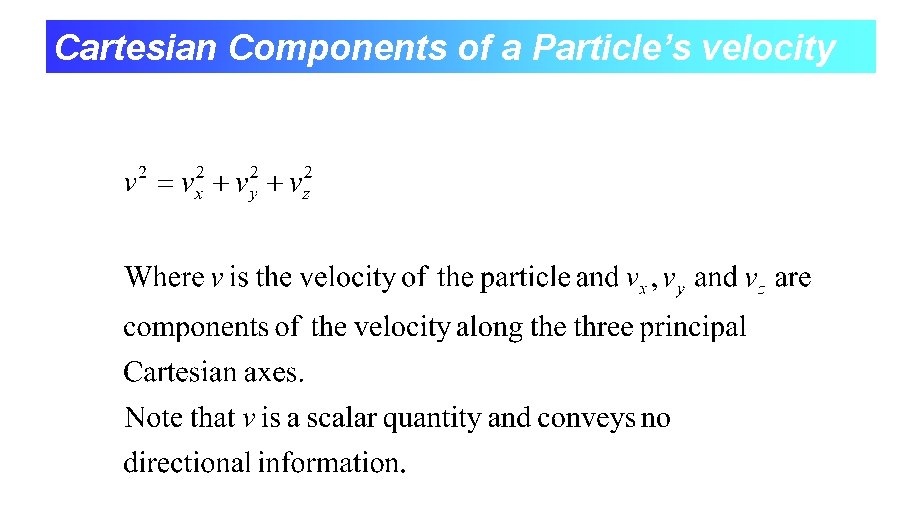
Cartesian Components of a Particle’s velocity

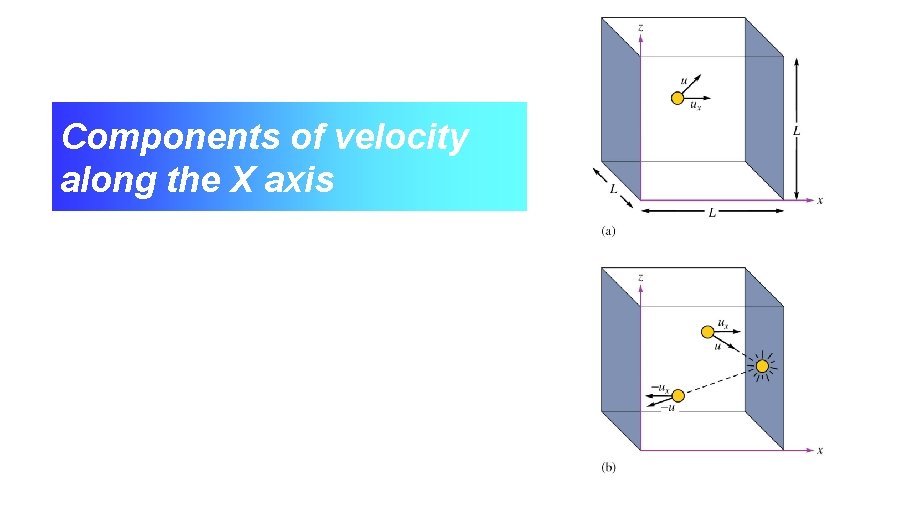
Components of velocity along the X axis

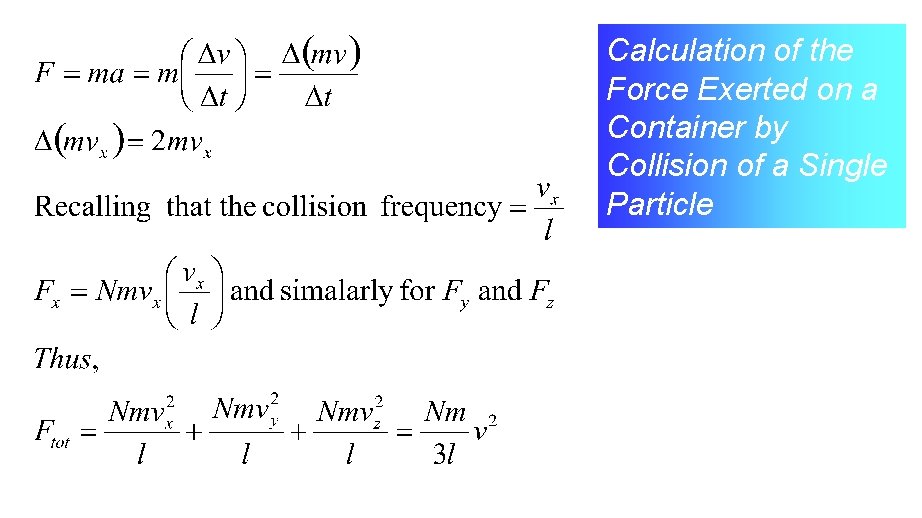
Calculation of the Force Exerted on a Container by Collision of a Single Particle

Calculation of the Pressure in Terms of Microscopic Properties of the Gas Particles

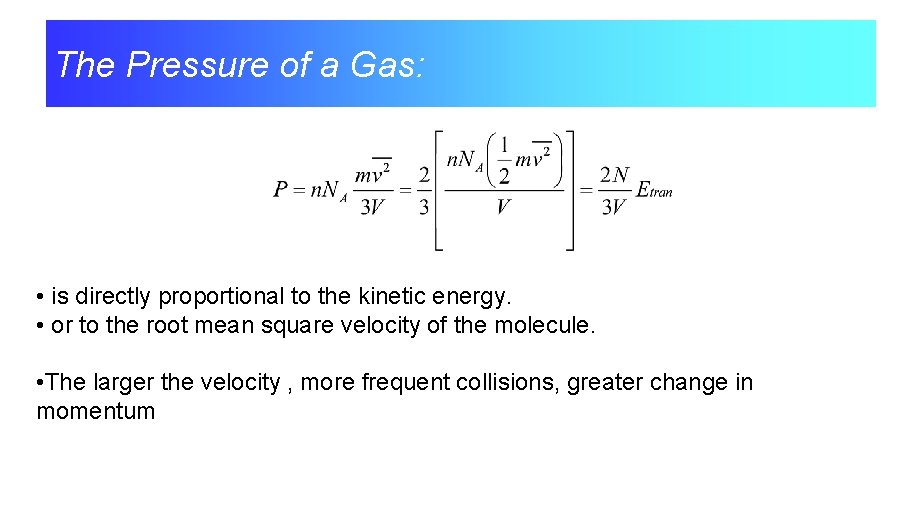
The Pressure of a Gas: • is directly proportional to the kinetic energy. • or to the root mean square velocity of the molecule. • The larger the velocity , more frequent collisions, greater change in momentum

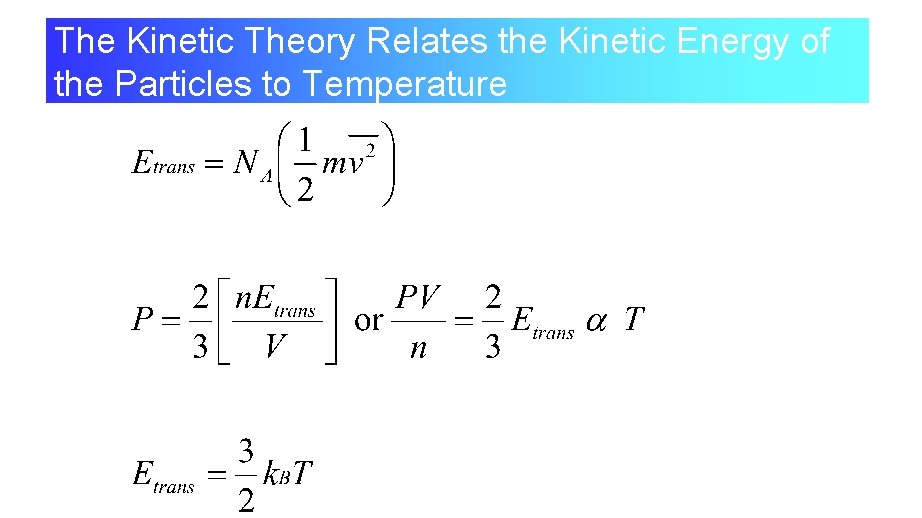
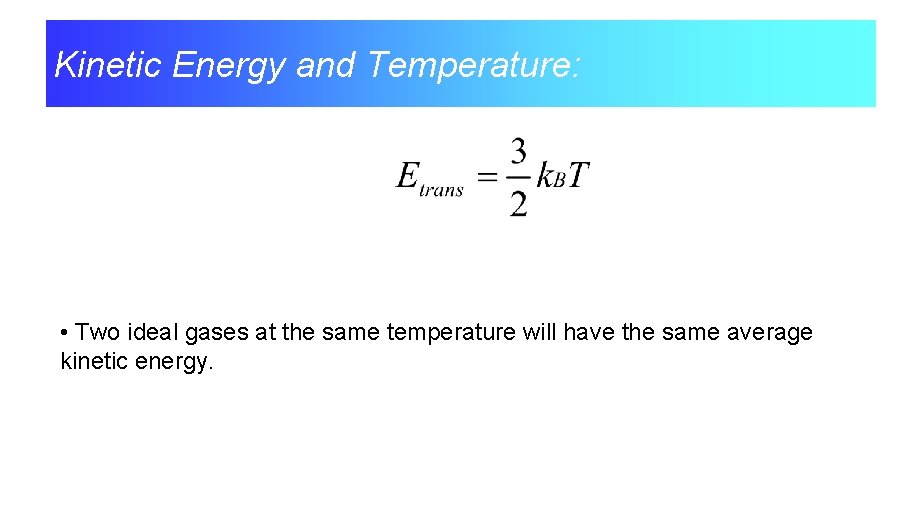
The Kinetic Theory Relates the Kinetic Energy of the Particles to Temperature

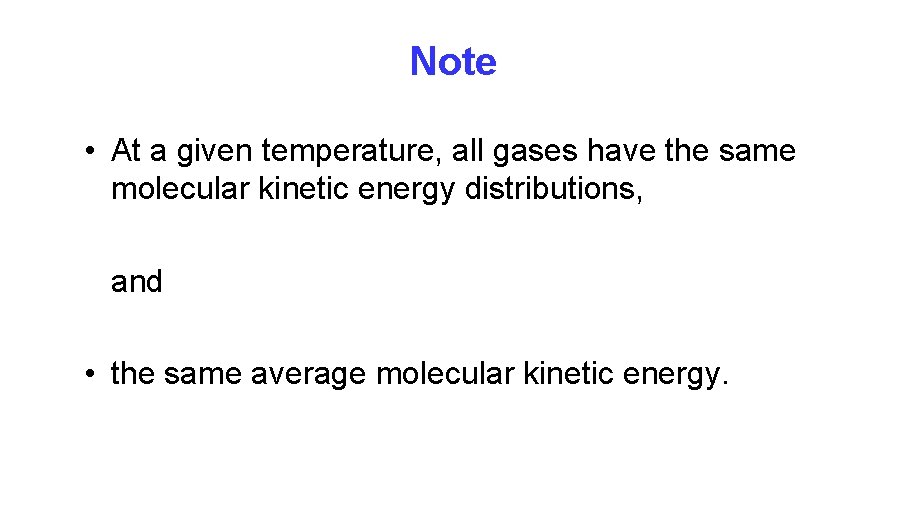
Kinetic Energy and Temperature: • Two ideal gases at the same temperature will have the same average kinetic energy.

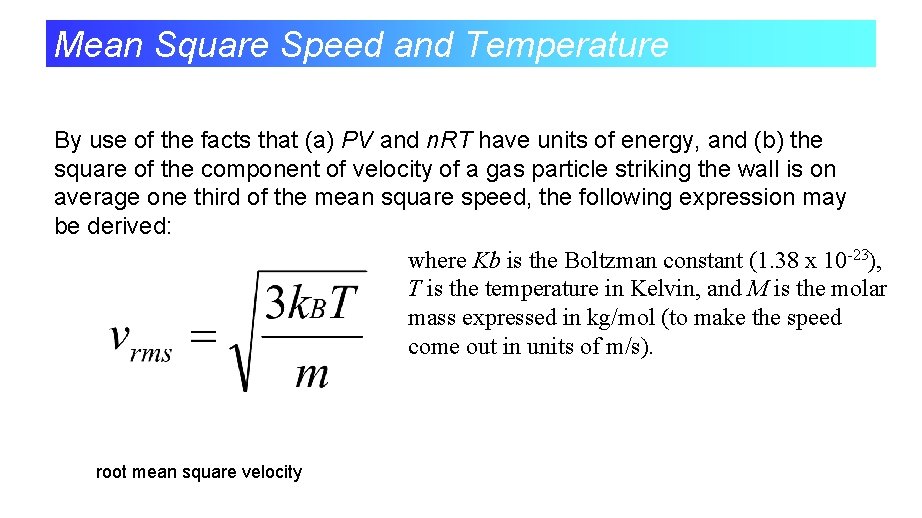
Mean Square Speed and Temperature By use of the facts that (a) PV and n. RT have units of energy, and (b) the square of the component of velocity of a gas particle striking the wall is on average one third of the mean square speed, the following expression may be derived: where Kb is the Boltzman constant (1. 38 x 10 -23), T is the temperature in Kelvin, and M is the molar mass expressed in kg/mol (to make the speed come out in units of m/s). root mean square velocity

Sample Problem Calculate the Kinetic Energy of (a) a Hydrogen Molecule traveling at 1. 57 x 103 m/sec, at 300 K. Mass = KE =

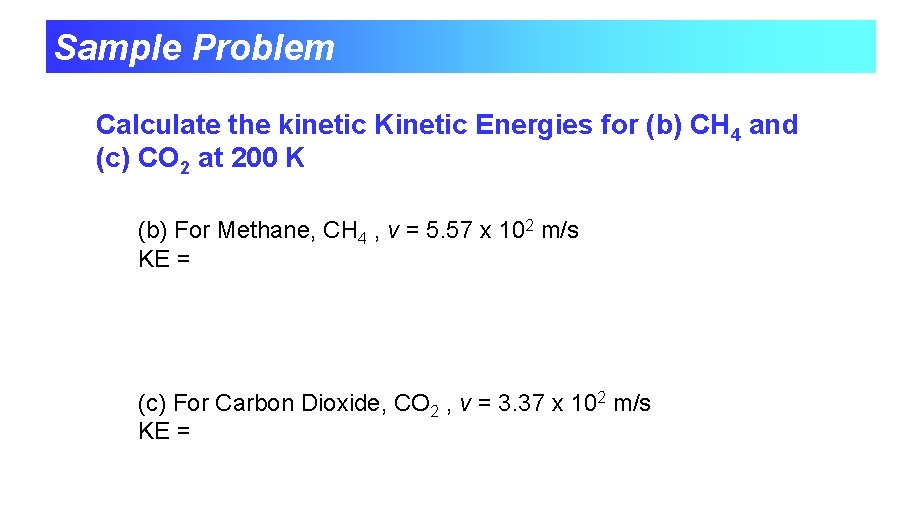
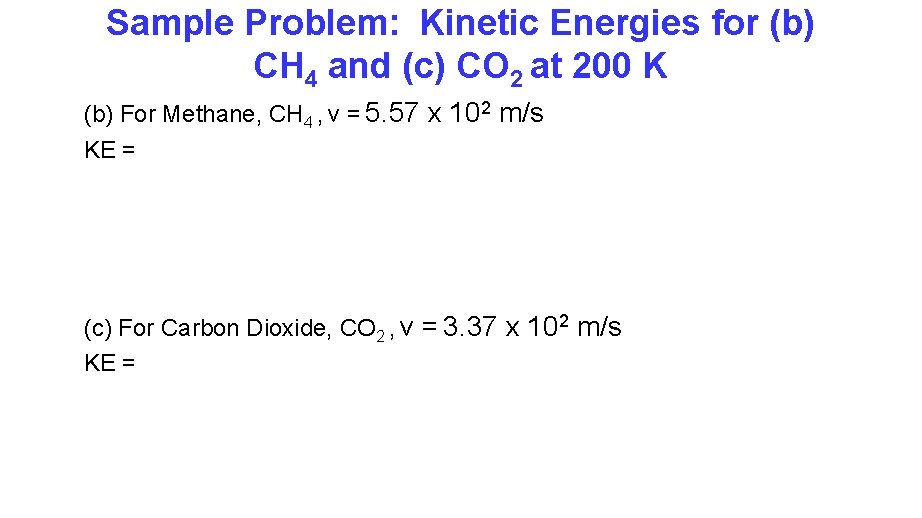
Sample Problem Calculate the kinetic Kinetic Energies for (b) CH 4 and (c) CO 2 at 200 K (b) For Methane, CH 4 , v = 5. 57 x 102 m/s KE = (c) For Carbon Dioxide, CO 2 , v = 3. 37 x 102 m/s KE =

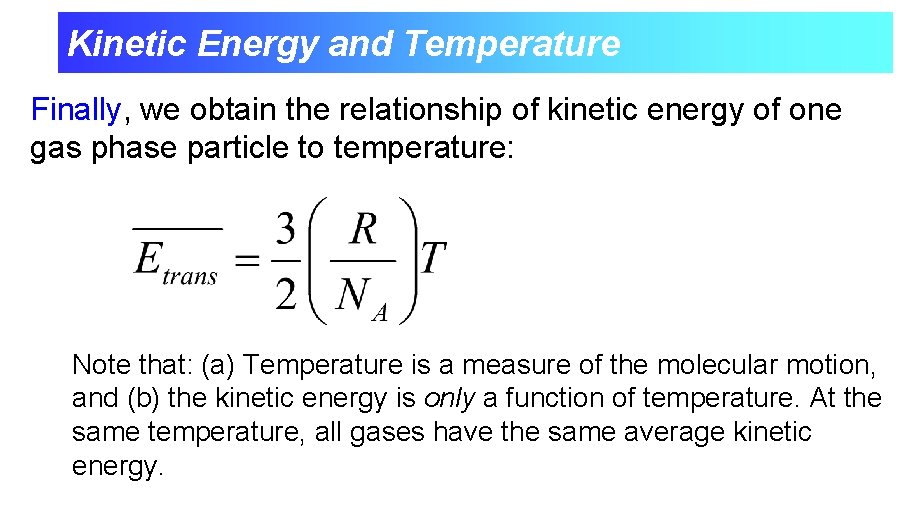
Kinetic Energy and Temperature Finally, we obtain the relationship of kinetic energy of one gas phase particle to temperature: Note that: (a) Temperature is a measure of the molecular motion, and (b) the kinetic energy is only a function of temperature. At the same temperature, all gases have the same average kinetic energy.

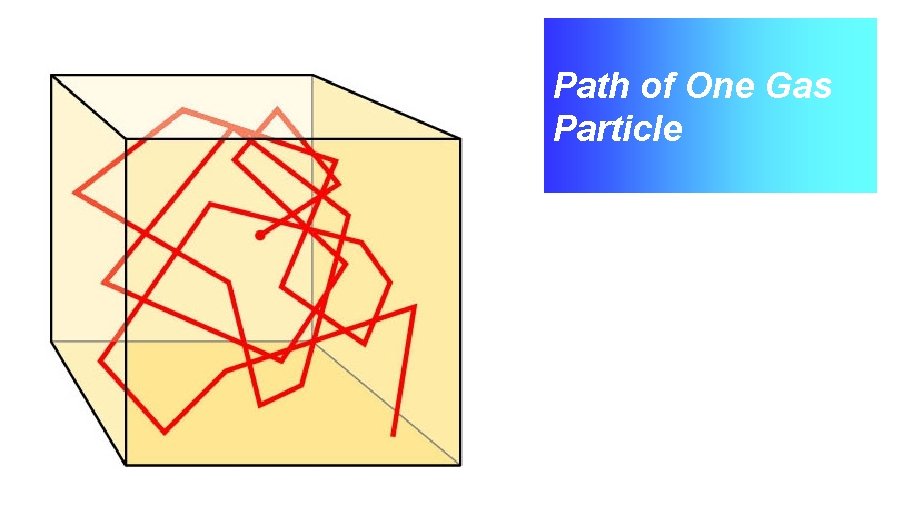
Path of One Gas Particle

Molecular Speed Distribution Thus far we have discussed the random nature of molecular motion in terms of the average (root mean square) speed. But how is this speed distributed? The kinetic theory predicts the distribution function for the molecular speeds. Below we show the distribution of molecular speeds for N 2 gas at three temperatures.


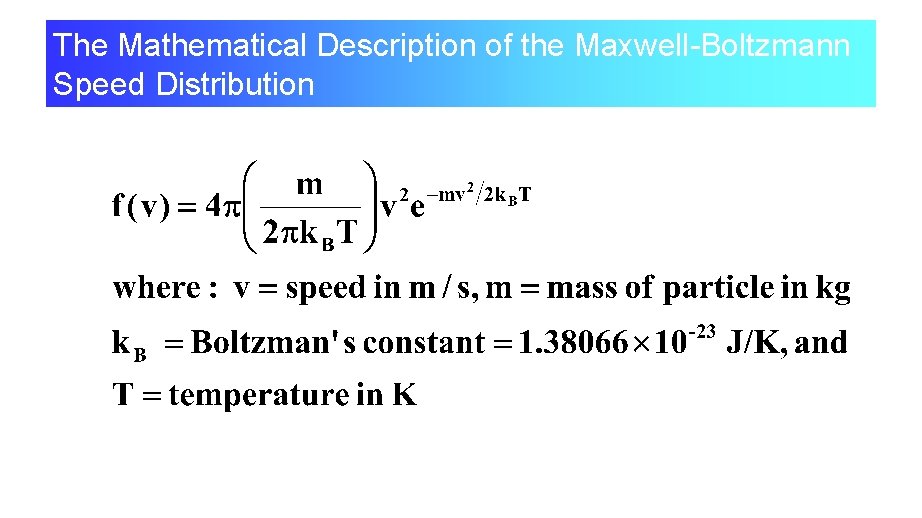
The Mathematical Description of the Maxwell-Boltzmann Speed Distribution

Features of Speed Distribution • The most probable speed is at the peak of the curve. • The most probable speed increases as the temperature increases. • The distribution broadens as the temperature increases.

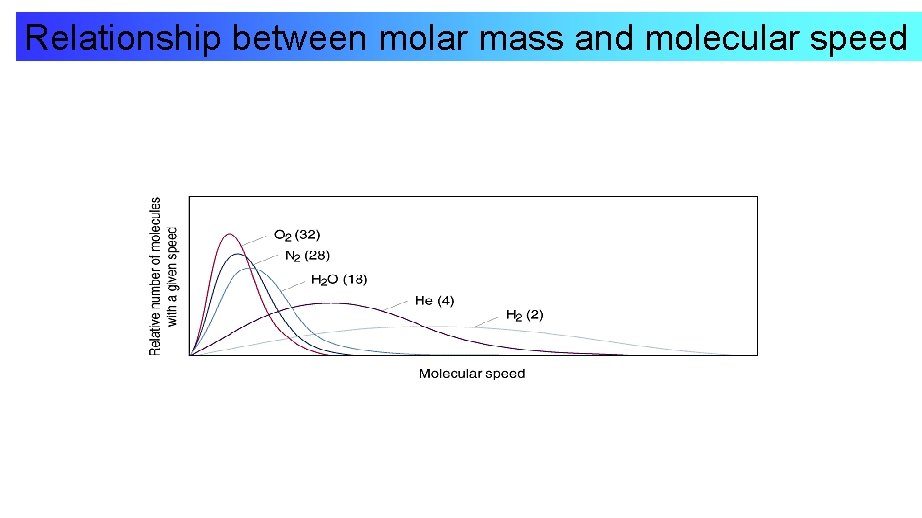
Relationship between molar mass and molecular speed

Features of Speed Distribution • The most probable speed increases as the molecular mass decreases • The distribution broadens as the molecular mass decreases.

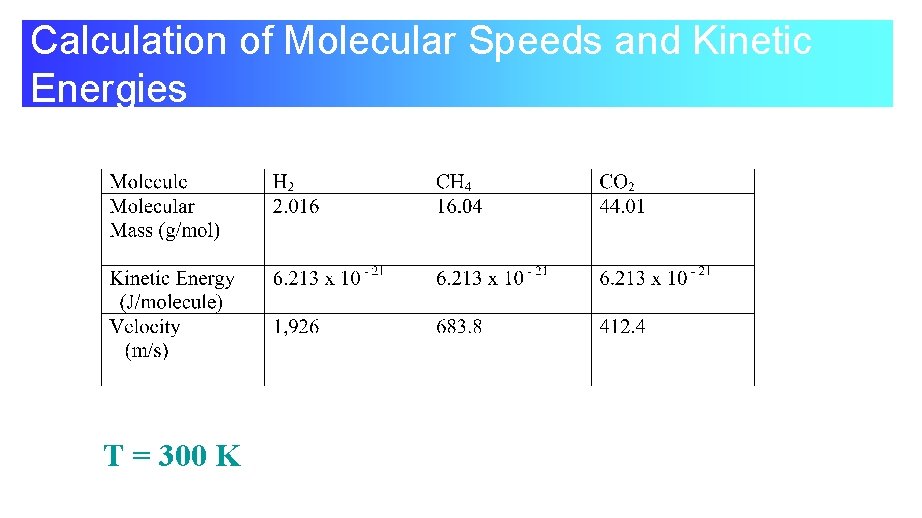
Calculation of Molecular Speeds and Kinetic Energies T = 300 K

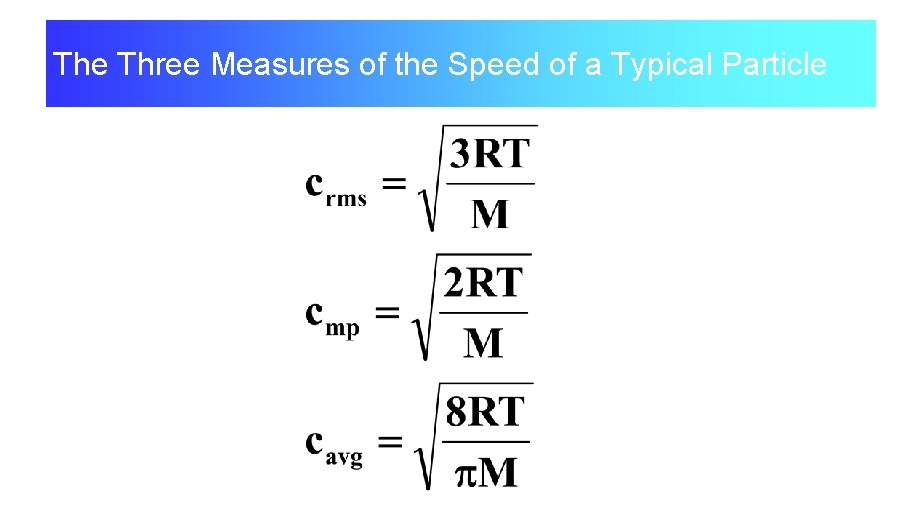
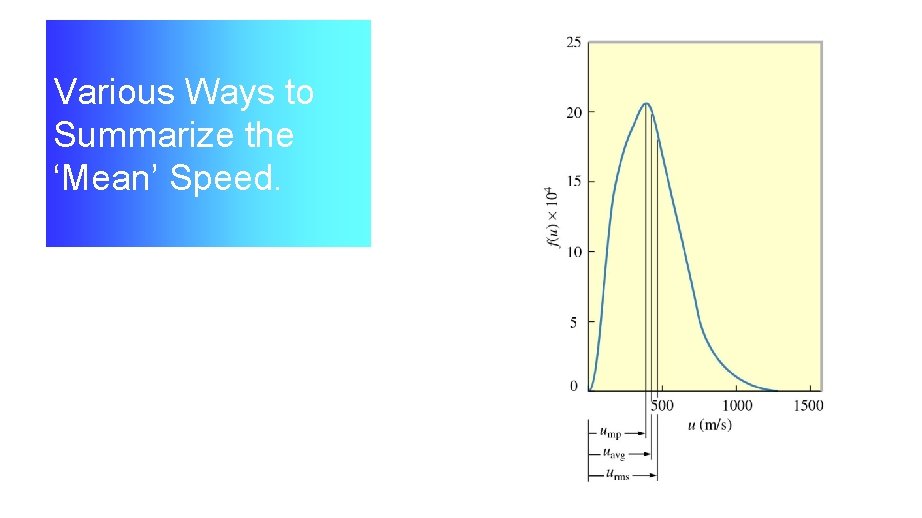
The Three Measures of the Speed of a Typical Particle

Various Ways to Summarize the ‘Mean’ Speed.

Sample Problem: Calculate the Kinetic Energy of (a) a Hydrogen Molecule traveling at 1. 57 x 103 m/sec, at 300 K. Mass = KE =

Sample Problem: Kinetic Energies for (b) CH 4 and (c) CO 2 at 200 K (b) For Methane, CH 4 , v = 5. 57 x 102 m/s KE = (c) For Carbon Dioxide, CO 2 , v = 3. 37 x 102 m/s KE =

Note • At a given temperature, all gases have the same molecular kinetic energy distributions, and • the same average molecular kinetic energy.

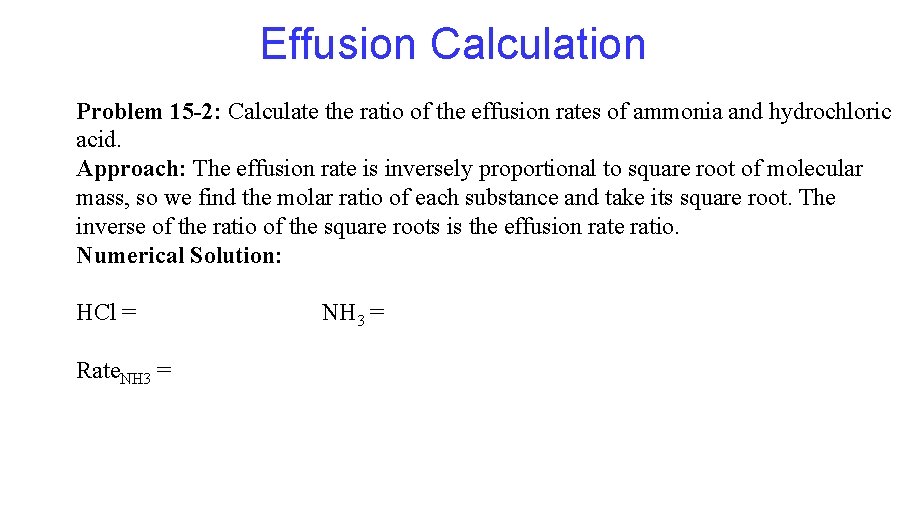
Effusion is the process whereby a gas escapes from its container through a tiny hole into an evacuated space. According to the kinetic theory a lighter gas effuses faster because the most probable speed of its molecules is higher. Therefore molecules escape through the tiny hole in unit time. This is made quantitative in Graham's Law of effusion: The rate of effusion of a gas is inversely proportional to the square root of its molar mass.

The Process of Effusion

Effusion Calculation Problem 15 -2: Calculate the ratio of the effusion rates of ammonia and hydrochloric acid. Approach: The effusion rate is inversely proportional to square root of molecular mass, so we find the molar ratio of each substance and take its square root. The inverse of the ratio of the square roots is the effusion rate ratio. Numerical Solution: HCl = Rate. NH 3 =

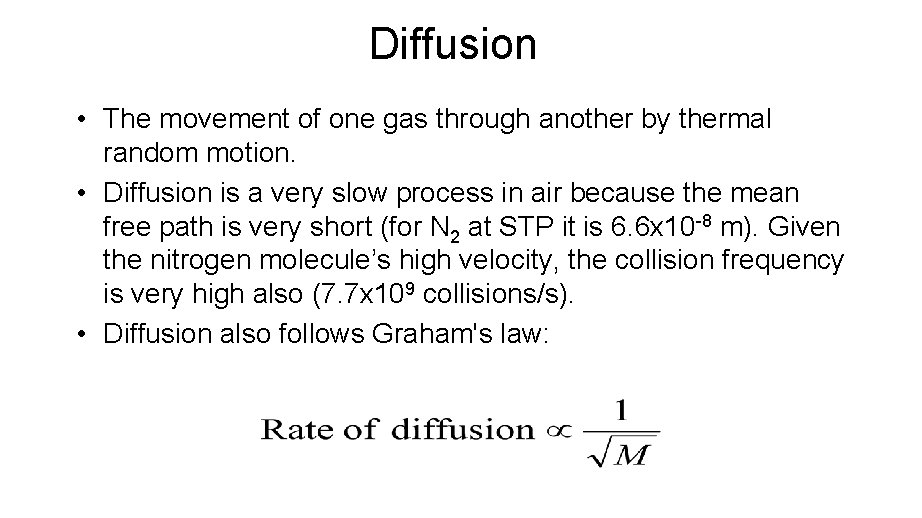
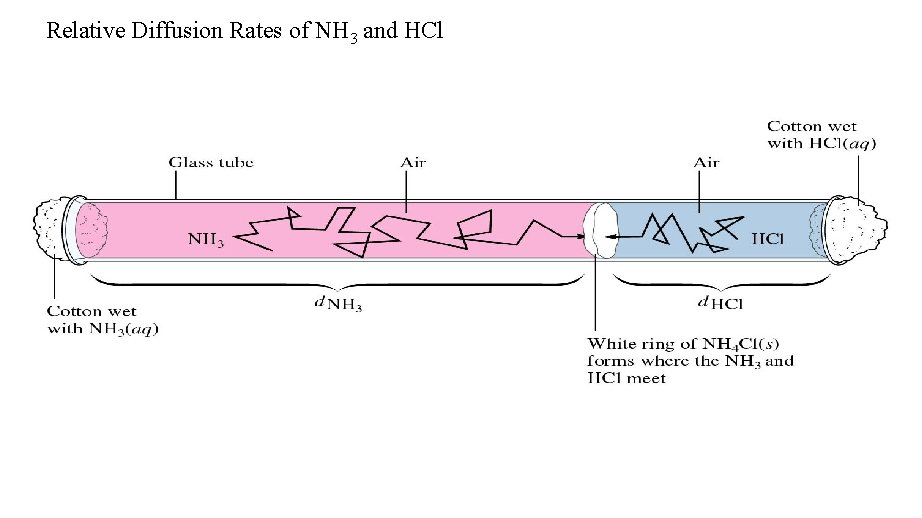
Diffusion • The movement of one gas through another by thermal random motion. • Diffusion is a very slow process in air because the mean free path is very short (for N 2 at STP it is 6. 6 x 10 -8 m). Given the nitrogen molecule’s high velocity, the collision frequency is very high also (7. 7 x 109 collisions/s). • Diffusion also follows Graham's law:

Diffusion of a gas particle through a space filled with other particles

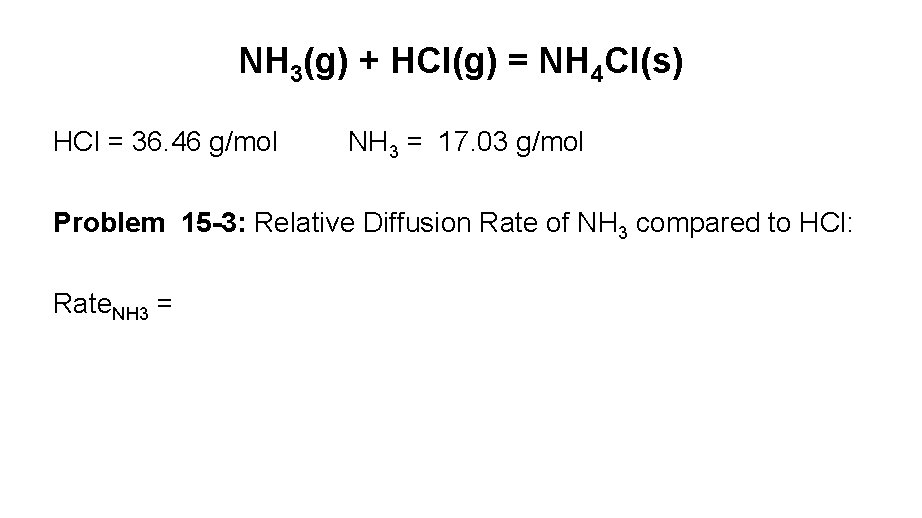
NH 3(g) + HCl(g) = NH 4 Cl(s) HCl = 36. 46 g/mol NH 3 = 17. 03 g/mol Problem 15 -3: Relative Diffusion Rate of NH 3 compared to HCl: Rate. NH 3 =

The inverse relation between diffusion rate and molar mass. NH 3(g) + HCl(g) Due to it’s light mass, ammonia travels 1. 46 times as fast as hydrogen chloride NH 4 Cl(s)

Relative Diffusion Rates of NH 3 and HCl

Gaseous Diffusion Separation of Uranium 235 / 238 235 UF 238 UF vs 6 6 Separation Factor = after Two runs after approximately 2000 runs 235 UF is > 99% Purity ! 6 Y - 12 Plant at Oak Ridge National Lab
 Kinetic molecular theory
Kinetic molecular theory Particle theory freezing
Particle theory freezing Kinetic theory of gases
Kinetic theory of gases Postulates for kinetic theory of gases
Postulates for kinetic theory of gases Kinetic theory
Kinetic theory Write the postulates of kinetic theory of gases
Write the postulates of kinetic theory of gases Postulates of kinetic theory of gases
Postulates of kinetic theory of gases General gas equation is
General gas equation is Molecular theory of gases and liquids
Molecular theory of gases and liquids Kinetic molecular theory of solids
Kinetic molecular theory of solids The kinetic molecular theory
The kinetic molecular theory Adhesive force
Adhesive force Kinetic molecular theory of liquids
Kinetic molecular theory of liquids Postulates of kinetic molecular theory
Postulates of kinetic molecular theory Kinetic molecular theory def
Kinetic molecular theory def Timeline of kinetic molecular theory
Timeline of kinetic molecular theory Charles law in terms of kinetic molecular theory
Charles law in terms of kinetic molecular theory Ideal gas law examples
Ideal gas law examples Kinetic molecular theory formula
Kinetic molecular theory formula Kinetic molecular theory
Kinetic molecular theory Tenets of kinetic molecular theory
Tenets of kinetic molecular theory Reacciones de orden cero
Reacciones de orden cero Mot for o2
Mot for o2 Valence bond vs molecular orbital theory
Valence bond vs molecular orbital theory Valence bond theory and molecular orbital theory
Valence bond theory and molecular orbital theory Valence bond theory and molecular orbital theory
Valence bond theory and molecular orbital theory Sof4 lewis structure
Sof4 lewis structure 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Cell and molecular biology lectures
Cell and molecular biology lectures Fibroblast
Fibroblast Cleavage
Cleavage What is a covalent bond simple definition
What is a covalent bond simple definition Ionic covalent metallic
Ionic covalent metallic Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure The kinetic theory of matter states that
The kinetic theory of matter states that Kinetic theory of matter definition
Kinetic theory of matter definition Kinetic theory of matter
Kinetic theory of matter An explanation of how particles in matter behave
An explanation of how particles in matter behave The attraction between particles gives solids a definite
The attraction between particles gives solids a definite What is a kinetic theory of matter
What is a kinetic theory of matter