Lecture 3 Kumar Amino acids and AA Transport
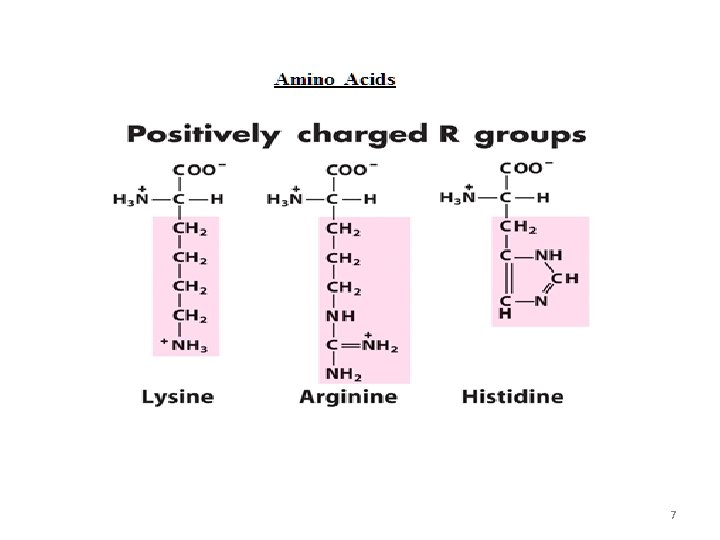
- Slides: 17

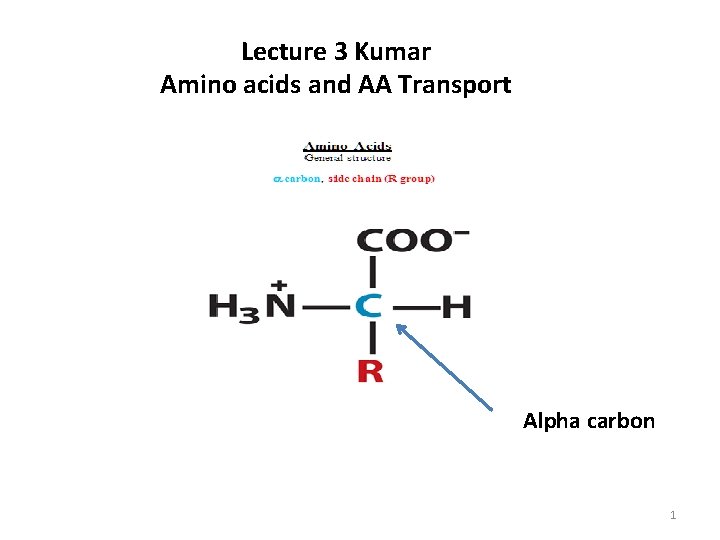
Lecture 3 Kumar Amino acids and AA Transport Alpha carbon 1

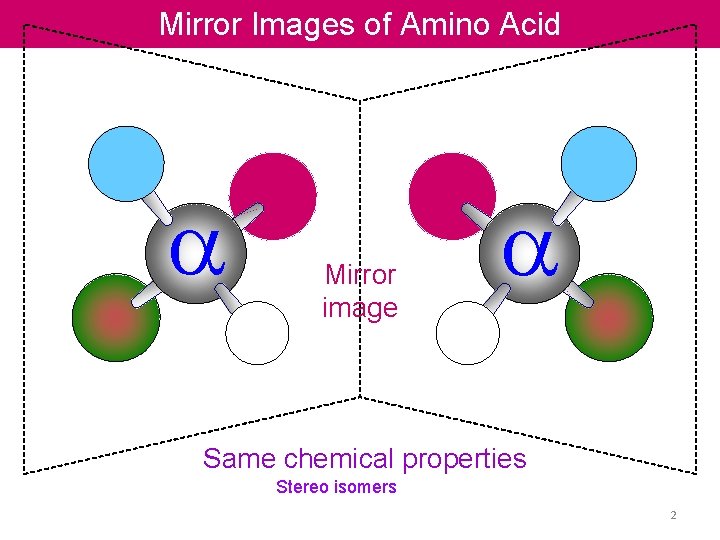
Mirror Images of Amino Acid a Mirror image a Same chemical properties Stereo isomers 2

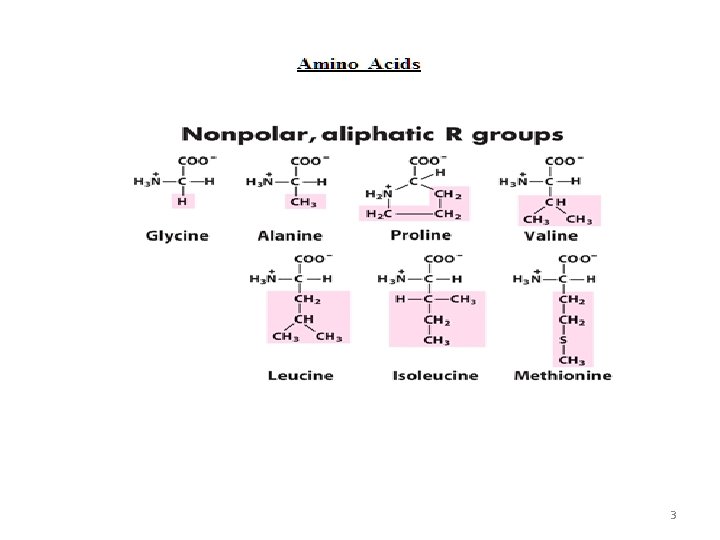
3

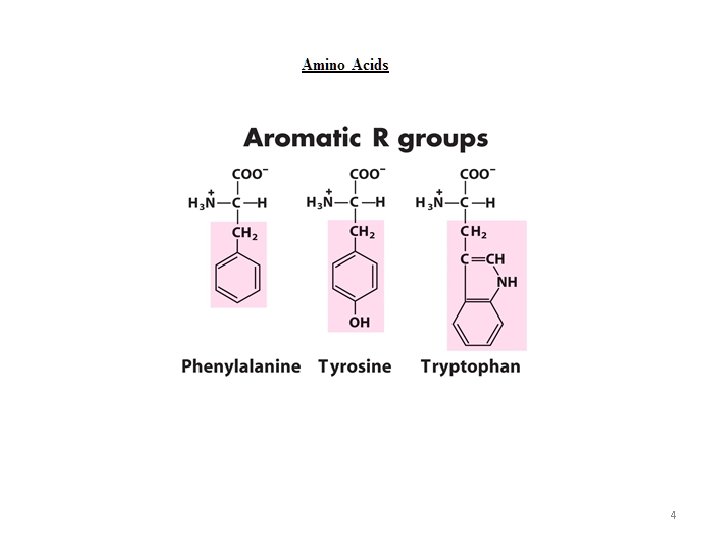
4

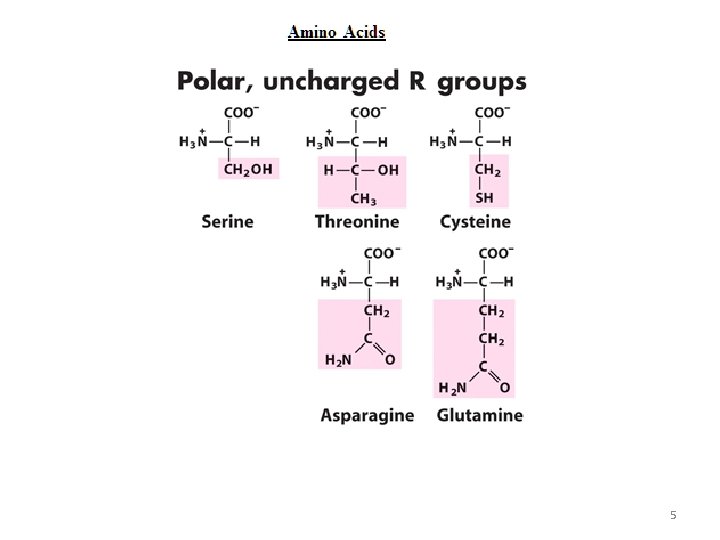
5

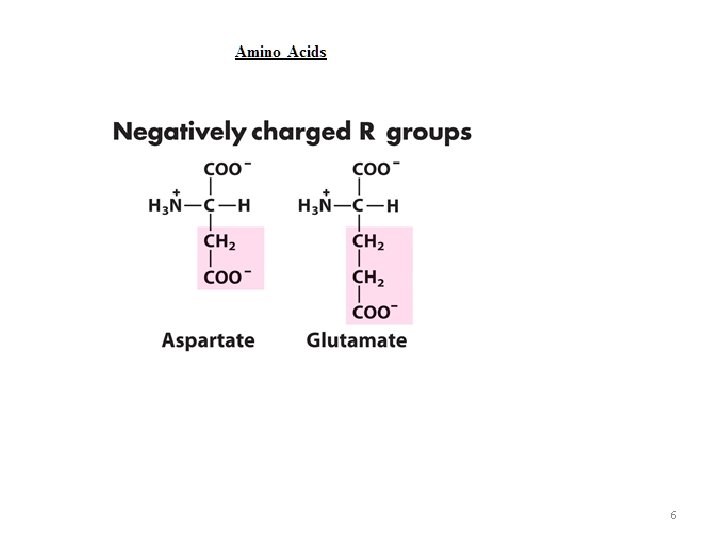
6
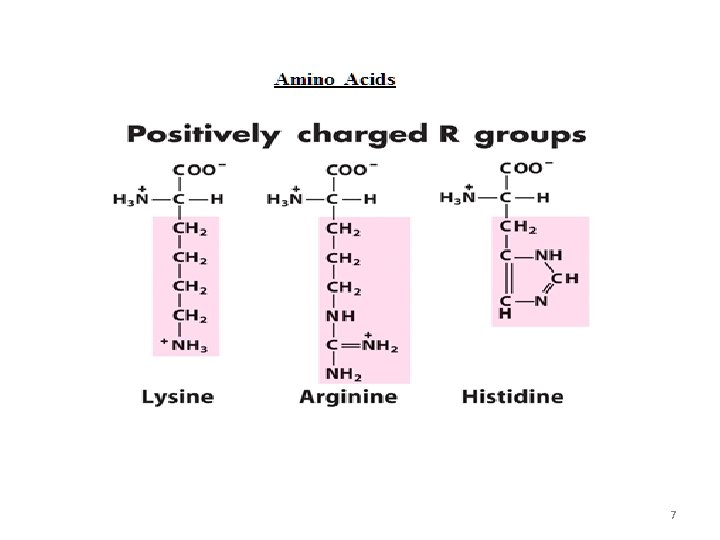
7

8

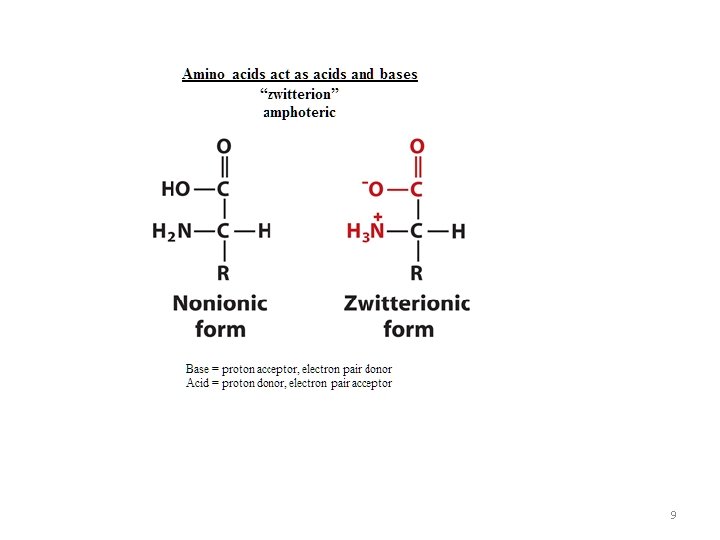
9

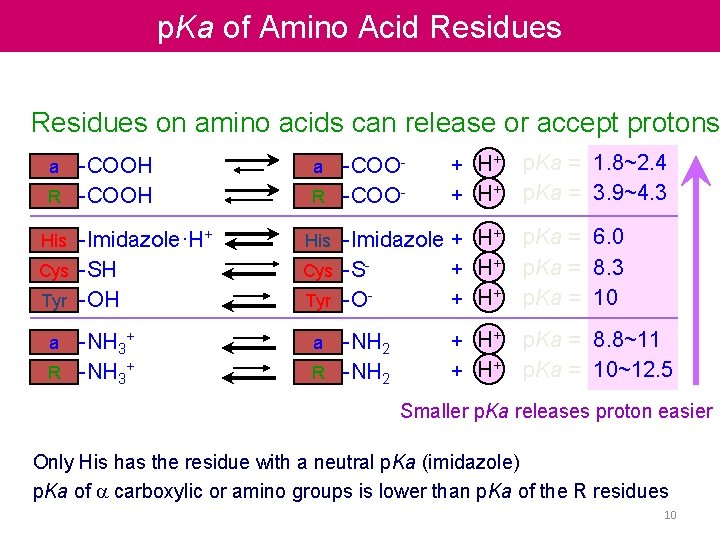
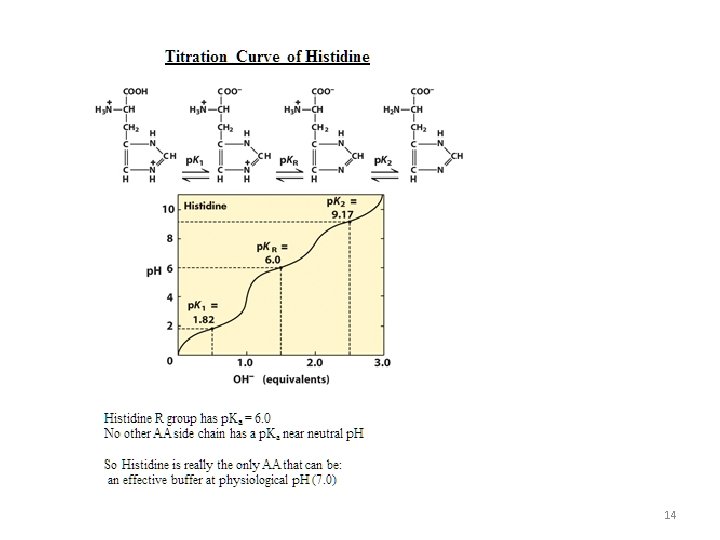
p. Ka of Amino Acid Residues on amino acids can release or accept protons -COOH R -COOH a -Imidazole·H+ Cys -SH Tyr -OH His -NH 3+ R -NH 3+ a -COOR -COOa + H+ -Imidazole + H+ Cys -S+ H+ Tyr -O+ H+ His -NH 2 R -NH 2 a + H+ p. Ka = 1. 8~2. 4 p. Ka = 3. 9~4. 3 p. Ka = 6. 0 p. Ka = 8. 3 p. Ka = 10 p. Ka = 8. 8~11 p. Ka = 10~12. 5 Smaller p. Ka releases proton easier Only His has the residue with a neutral p. Ka (imidazole) p. Ka of a carboxylic or amino groups is lower than p. Ka of the R residues 10

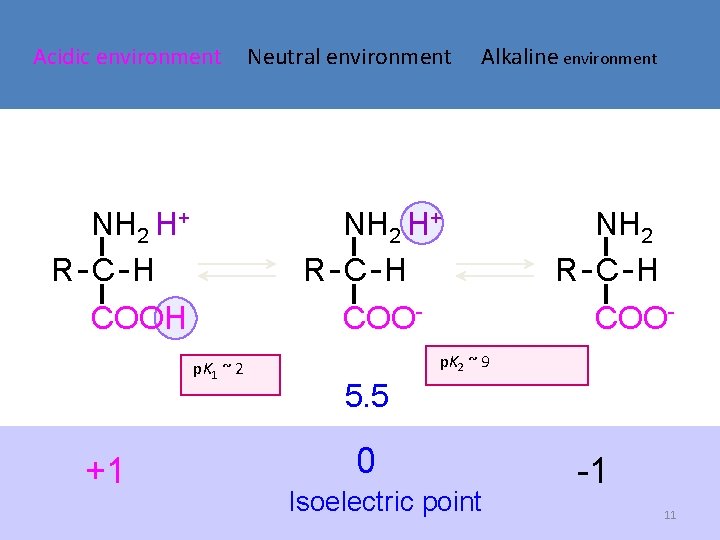
Acidic environment NH 2 H+ R-C-H COOH Alkaline environment NH 2 H+ R-C-H COOp. K 1 ~ 2 +1 Neutral environment NH 2 R-C-H COO- p. K 2 ~ 9 5. 5 0 Isoelectric point -1 11

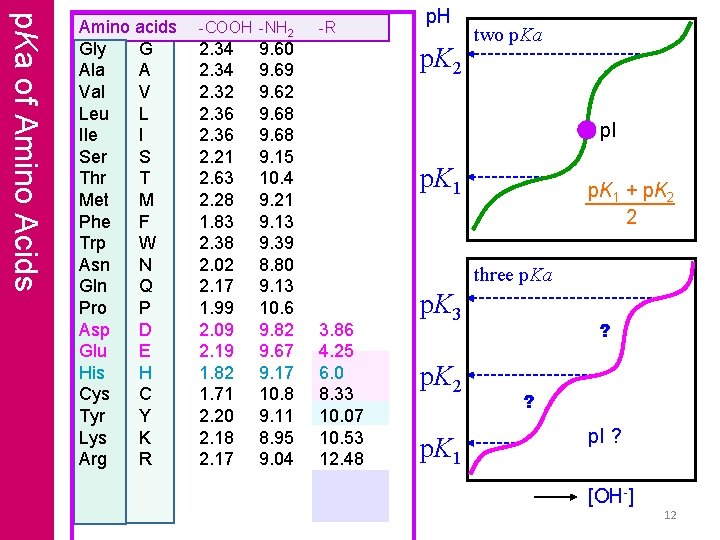
p. Ka of Amino Acids Amino acids Gly G Ala A Val V Leu L Ile I Ser S Thr T Met M Phe F Trp W Asn N Gln Q Pro P Asp D Glu E His H Cys C Tyr Y Lys K Arg R -COOH -NH 2 2. 34 2. 32 2. 36 2. 21 2. 63 2. 28 1. 83 2. 38 2. 02 2. 17 1. 99 2. 09 2. 19 1. 82 1. 71 2. 20 2. 18 2. 17 9. 60 9. 69 9. 62 9. 68 9. 15 10. 4 9. 21 9. 13 9. 39 8. 80 9. 13 10. 6 9. 82 9. 67 9. 17 10. 8 9. 11 8. 95 9. 04 -R p. H p. K 2 two p. Ka p. I p. K 1 + p. K 2 2 three p. Ka 3. 86 4. 25 6. 0 8. 33 10. 07 10. 53 12. 48 p. K 3 p. K 2 p. K 1 ? ? p. I ? [OH-] 12

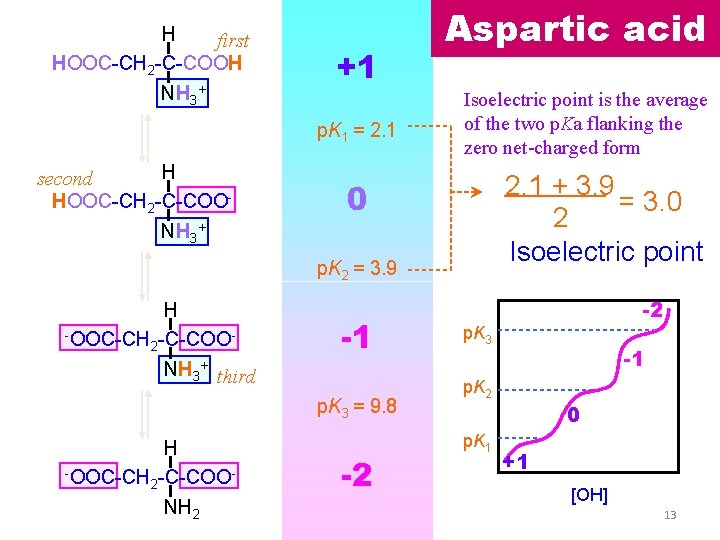
H first HOOC-CH 2 -C-COOH NH 3+ +1 p. K 1 = 2. 1 H second HOOC-CH 2 -C-COO- Aspartic acid Isoelectric point is the average of the two p. Ka flanking the zero net-charged form 2. 1 + 3. 9 = 3. 0 2 Isoelectric point 0 NH 3+ p. K 2 = 3. 9 H -OOC-CH -C-COO 2 -1 NH 3+ third p. K 3 = 9. 8 H -OOC-CH -C-COO 2 NH 2 -2 -2 p. K 3 -1 p. K 2 p. K 1 0 +1 [OH] 13

14

15

16

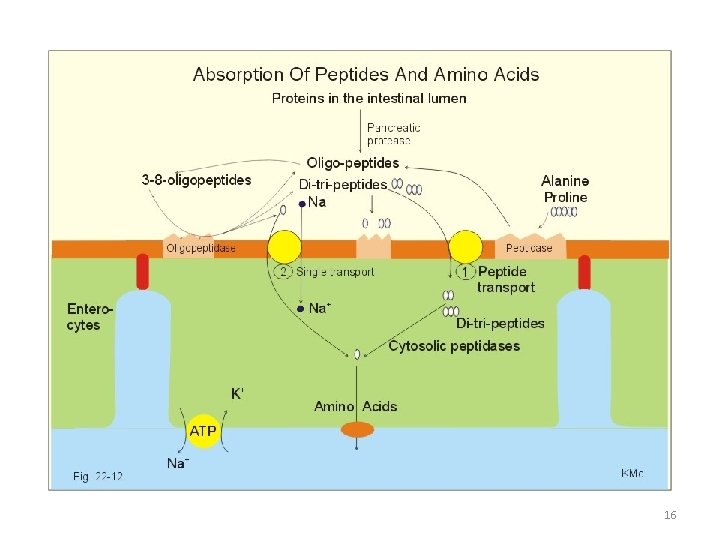
Learning objectives for lecture 3 • Know the structures of simple sugars • Understand the difference between D and L forms • Know the structural differences between isomers and enantiomers • Know the basis for denoting amino acids as neutral, acid or basic • Understand how p. Ka of amino acids are defined and how are they used to calculate isoelectric points • Know what makes amino acids good buffers at many p. H values • Understand how amino acids are generally transported from intestinal lumen. 17