Lecture 3 Ch 1 Review Temperature Heat Transfer























- Slides: 29

Lecture 3 Ch. 1 Review Temperature & Heat Transfer

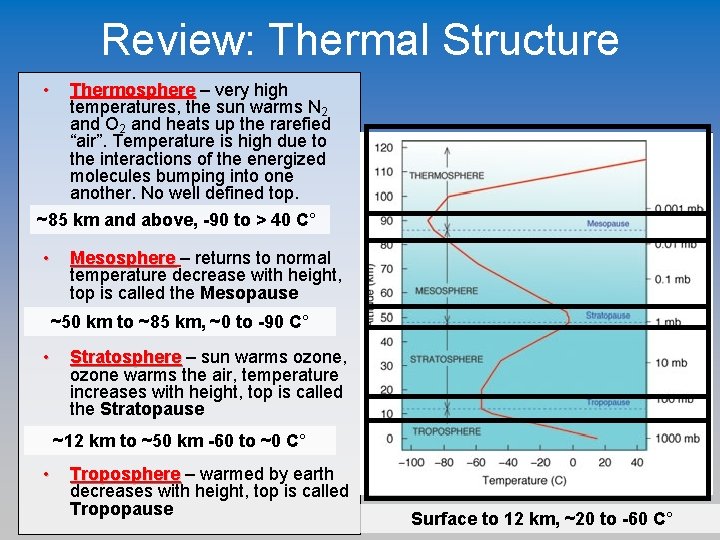
Review: Thermal Structure • Thermosphere – very high temperatures, the sun warms N 2 and O 2 and heats up the rarefied “air”. Temperature is high due to the interactions of the energized molecules bumping into one another. No well defined top. ~85 km and above, -90 to > 40 C° • Mesosphere – returns to normal temperature decrease with height, top is called the Mesopause ~50 km to ~85 km, ~0 to -90 C° • Stratosphere – sun warms ozone, ozone warms the air, temperature increases with height, top is called the Stratopause ~12 km to ~50 km -60 to ~0 C° • Troposphere – warmed by earth decreases with height, top is called Tropopause Surface to 12 km, ~20 to -60 C°

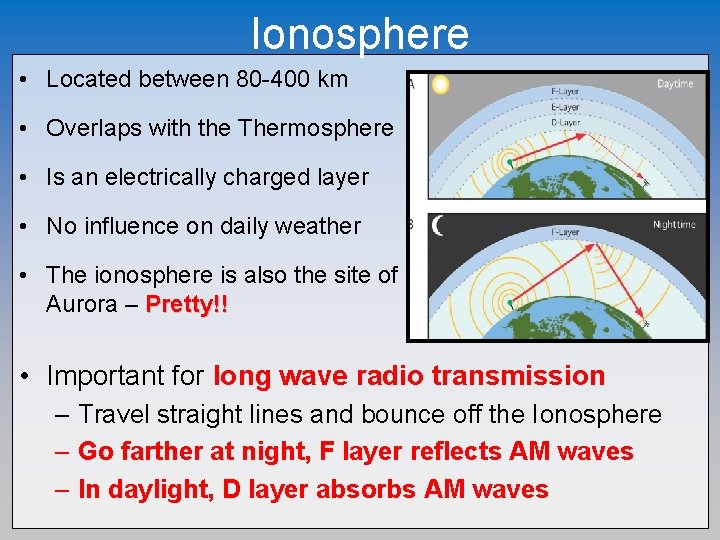
Ionosphere • Located between 80 -400 km • Overlaps with the Thermosphere • Is an electrically charged layer • No influence on daily weather • The ionosphere is also the site of Aurora – Pretty!! • Important for long wave radio transmission – Travel straight lines and bounce off the Ionosphere – Go farther at night, F layer reflects AM waves – In daylight, D layer absorbs AM waves

Auroras • Aurora borealis (northern lights) • Aurora australis (southern lights) • Closely correlated with solar-flares • Geographic location is important – (Earth’s magnetic poles) • Appear in the night sky as overlapping curtains – Bottom at 100 km (62 miles) – Tops at 400 km (248 miles or higher)

Aurora Borealis from the International Space Station

Reminder from First Class • Weather – The state of the atmosphere at any given time. • Climate – A description of aggregate weather conditions. – The sum of all statistical weather information that helps describe a place or region. “Climate is what you expect, but weather is what you get”

Weather • Weather is the condition of the atmosphere at any particular time – Air Temperature – Air Pressure – Humidity – Clouds – Precipitation – Visibility – Wind

Climate • Average range of “weather elements” elements over a long period of time. – How hot, cold, wet, dry…. • Includes the “extreme” extreme weather event too. – Droughts, heat wave, cold snaps…. • Climate changes on geological time scales – think ice ages and non ice ages

Kinetic Energy and Temperature • The energy within a body that is a result of its motion! motion • Temperature (i. e. of air) is a measure of its average kinetic energy. – describes how warm or cold an object is.

Heat • The TRANSFER of energy into or out of an object because of TEMPERATURE DIFFERENCES • It is the FLOW of energy! • After heat is transferred it is stored as internal energy

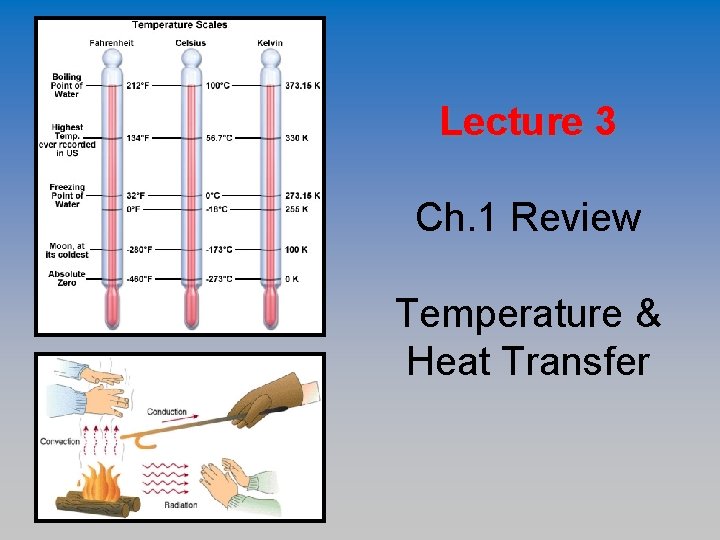
Temperature Scales 1. Fahrenheit 2. Celsius 3. Kelvin

Fahrenheit • Based on “Fixed Points” Points – coldest temperature he could measure and assumed human body temp. • Freezing is at 32 and Boiling is a 212 • 180 Divisions between Freezing and Boiling • This temperature scale is used in the USA.

Celsius • Decimal Scale – powers of 10 • 0 degrees = Freezing • 100 degrees = Boiling • 100 between Freezing and Boiling • Scientists use this. • This temperature scale is used in Saudi Arabia.

Kelvin • Called the “Absolute Scale” Scale • Same Spacing as Celsius – 100 divisions between boiling and freezing • 0 K = the temperature at which all molecular motion is presumed to cease – Absolute Zero = molecules stop moving, no thermal motion.

Latent Heat • The heat energy required to change a substance from one state to another. • Example: – Water from solid to liquid – temperature stays constant. – Heat is used to MELT the ice does not produce a temperature change

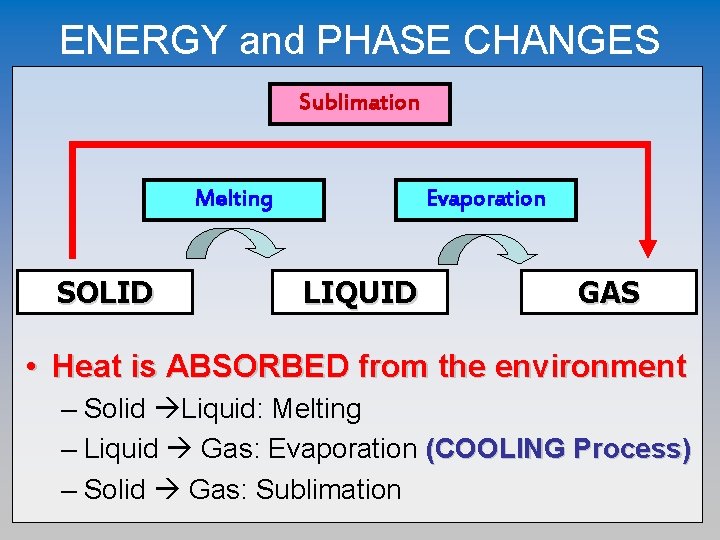
ENERGY and PHASE CHANGES Sublimation Melting SOLID Evaporation LIQUID GAS • Heat is ABSORBED from the environment – Solid Liquid: Melting – Liquid Gas: Evaporation (COOLING Process) – Solid Gas: Sublimation

ENERGY and PHASE CHANGES • Heat is RELEASED into the environment – Gas Liquid: Condensation – Liquid Solid: Freezing (WARMING Process) – Gas Solid: Deposition SOLID LIQUID Freezing GAS Condensation Deposition

PHASE CHANGES Sublimation Melting SOLID Evaporation LIQUID Freezing GAS Condensation Deposition

Mechanisms of Heat Transfer

Conduction • The TRANSFER of heat through election and molecular collisions from one molecule to another. • Ability to conduct varies: – Metals are better – Air doesn’t conduct well, called an INSULATOR Only important for heating the air in DIRECT contact with the surface of the Earth

Convection • Heat transfer that involves the actual movement or circulation of substance – Air and water – Most common form of transfer in the atmosphere • Advection = horizontal movement.

Radiation • Travels through the vacuum of space! • How solar energy reaches the planet!

CONDUCTION CONVECTION RADIATION

Solar Radiation • Electromagnetic Radiation! • Wavelengths – the distance from one crest to the next • All types travel at 300, 000 km/sec or 186, 000 miles/sec

Radiation emitted by the Earth • Earth emits radiation at longer wavelengths than the sun. – Emits considerably less radiant energy than the sun – Over 95% of the Earth’s radiation has wavelengths between 2. 5 and 30 micrometers (Infrared)

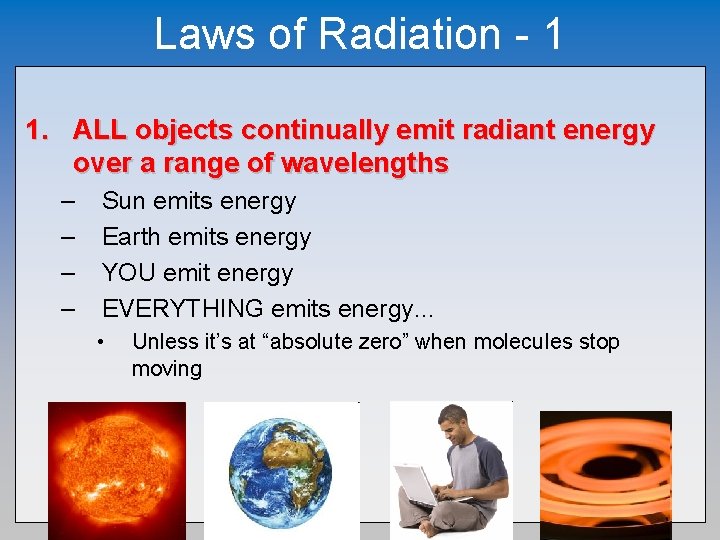
Laws of Radiation - 1 1. ALL objects continually emit radiant energy over a range of wavelengths – – Sun emits energy Earth emits energy YOU emit energy EVERYTHING emits energy. . . • Unless it’s at “absolute zero” when molecules stop moving

Laws of Radiation - 2 2. Hotter objects radiate more energy in the form of short wavelength radiation than cooler objects l Hot burner on a stove glows Red l Cool burner on a stove doesn’t glow at all but could still FEEL hot

Laws of Radiation - 3 3. Hotter objects radiate more total energy per unit area than do cooler objects l l Sun is 6000 K (10, 000 F) Earth is 289 K (59 F) Sun 160, 000 times more energy than the Earth This concept is called the Stephan-Boltzman Law Don’t worry, you don’t need to memorize this!

Next class Absorption, Emission, Equilibrium & SEASONS