Lecture 3 Absorption in Plate and Packed Towers

Lecture 3 Absorption in Plate and Packed Towers Part (3)

Design of Packed Towers using Transfer Using transfer-unit concept, the four z-expressions, in general, can be written as: Units Where HG (in m or in ft) is the height of transfer units based on gas phase The integrals are the number of transfer units; NG, NL, NOL and NOG The height of the packed tower is then, MU Department of Chemical Engineering Separation – Ch. E 321 Chapter 4 -2
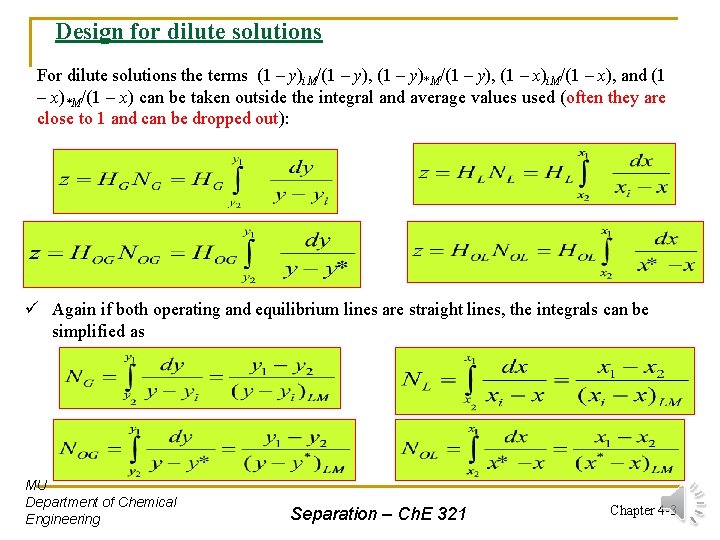
Design for dilute solutions For dilute solutions the terms (1 – y)i. M/(1 – y), (1 – y)*M/(1 – y), (1 – x)i. M/(1 – x), and (1 – x)*M/(1 – x) can be taken outside the integral and average values used (often they are close to 1 and can be dropped out): ü Again if both operating and equilibrium lines are straight lines, the integrals can be simplified as MU Department of Chemical Engineering Separation – Ch. E 321 Chapter 4 -3
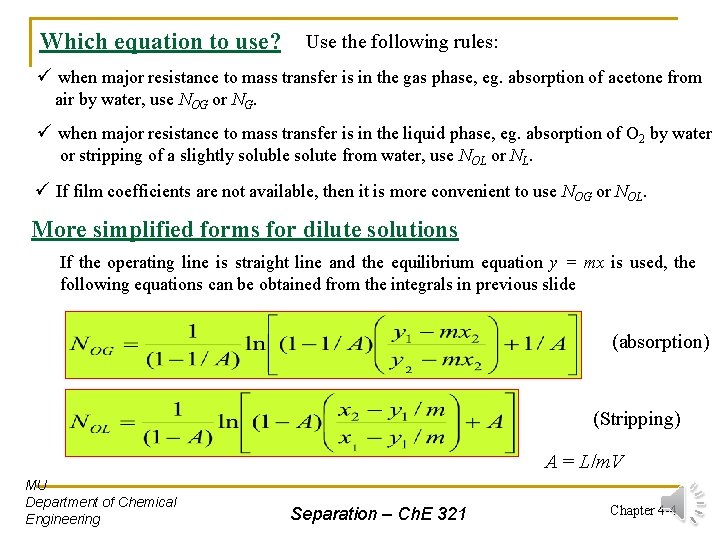
Which equation to use? Use the following rules: ü when major resistance to mass transfer is in the gas phase, eg. absorption of acetone from air by water, use NOG or NG. ü when major resistance to mass transfer is in the liquid phase, eg. absorption of O 2 by water or stripping of a slightly soluble solute from water, use NOL or NL. ü If film coefficients are not available, then it is more convenient to use NOG or NOL. More simplified forms for dilute solutions If the operating line is straight line and the equilibrium equation y = mx is used, the following equations can be obtained from the integrals in previous slide (absorption) (Stripping) A = L/m. V MU Department of Chemical Engineering Separation – Ch. E 321 Chapter 4 -4

ü When the operating line and equilibrium line are straight and not parallel, the number of overall gas-transfer units NOG for absorption is related to the number of theoretical trays or stages N (according to Kremser equation) is give by: ü The height of a theoretical tray or stage, HETP, in m is related to HOG by: üHETP the height of the packed column necessary to give a separation equivalent to one theoretical plate. ü If the operating line and equilibrium line are straight and parallel (A= 1. 0), then (use Kremser equation) MU Department of Chemical Engineering Separation – Ch. E 321 Chapter 4 -5

Example 10. 6 -5: Use of Transfer Units and Analytical Equations for Packed Tower Repeat example 10. 6 -4 using transfer units and height of a transfer unit as follows: 1. Use HG and NG to calculate tower height. 2. Use HOG and NOG to calculate tower height. 3. Use the following equation to calculate NOG and tower height 4. Using the analytical equations, calculate HETP, number of theoretical steps and tower height. MU Department of Chemical Engineering Separation – Ch. E 321 Chapter 4 -6

Solution: From Example 10. 6 -4: k’y a = 3. 78 x 10 -2 kg mol/s. m 3. mol frac; S = 0. 186 m 2 a) Use HG and NG to calculate tower height: From Example 10. 6 -4: MU Department of Chemical Engineering Separation – Ch. E 321 Chapter 4 -7
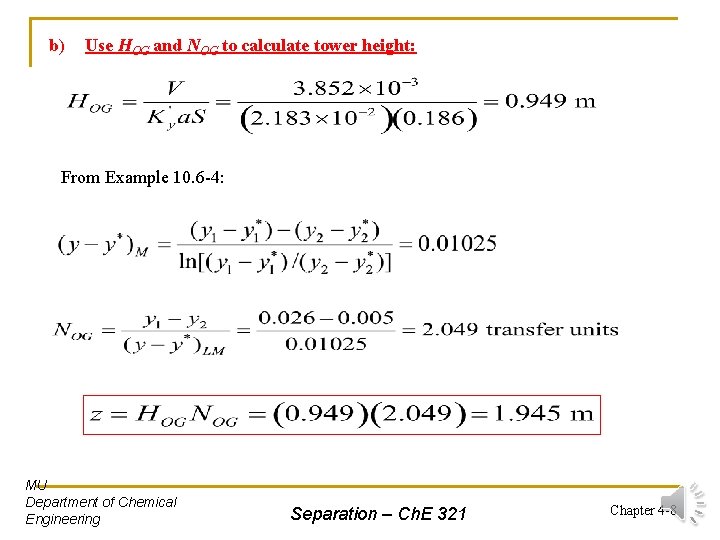
b) Use HOG and NOG to calculate tower height: From Example 10. 6 -4: MU Department of Chemical Engineering Separation – Ch. E 321 Chapter 4 -8
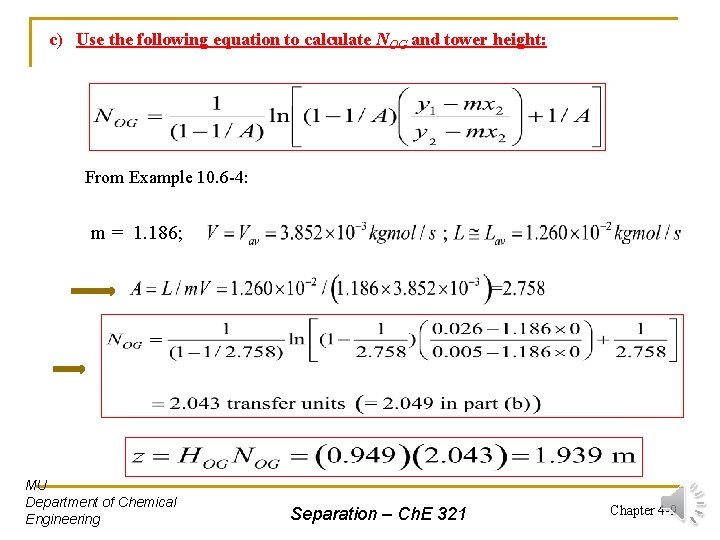
c) Use the following equation to calculate NOG and tower height: From Example 10. 6 -4: m = 1. 186; MU Department of Chemical Engineering Separation – Ch. E 321 Chapter 4 -9

d) Using the analytical equations, calculate HETP, number of theoretical steps and tower height: MU Department of Chemical Engineering Separation – Ch. E 321 Chapter 4 -10
- Slides: 10