Lecture 25 Electrolysis Define electrolysis Some examples What










- Slides: 48

Lecture 25 Electrolysis èDefine electrolysis? èSome examples. èWhat are the values of DG and Ecell? èElectrolysis of water. èSome industrial applications. èCorrosion Houghton Mifflin Company and G. Hall. All rights reserved.

Two Types of Cells èCell 1: does work by releasing free energy from a spontaneous reaction to produce electricity such as a battery. èCell 2: does work by absorbing free energy from a source of electricity to drive a non-spontaneous reaction such as electroplating. Houghton Mifflin Company and G. Hall. All rights reserved.

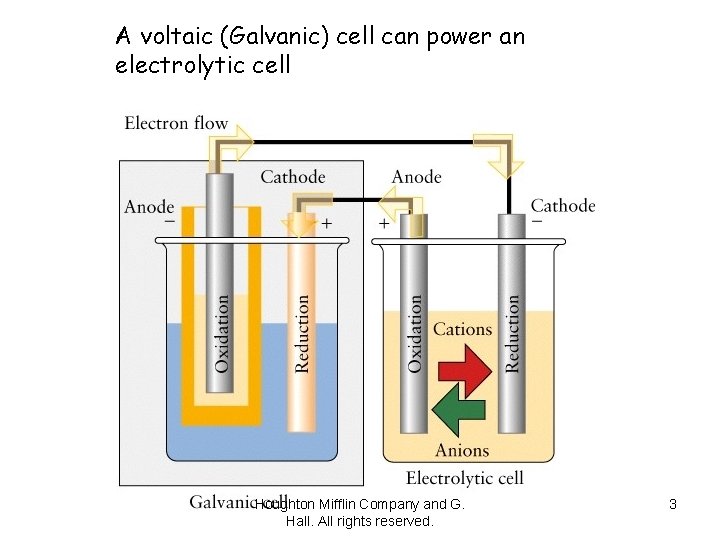
A voltaic (Galvanic) cell can power an electrolytic cell Houghton Mifflin Company and G. Hall. All rights reserved. 3

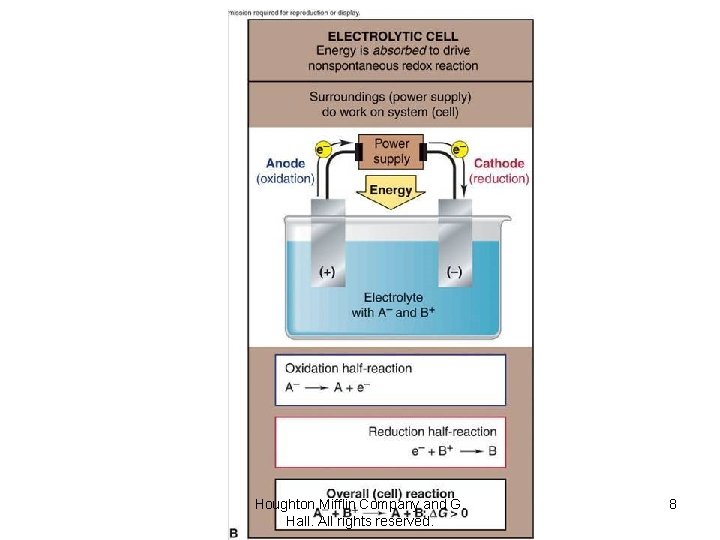
Electrolysis è The splitting (lysing) of a substance or decomposing by forcing a current through a cell to produce a chemical change for which the cell potential is negative. Houghton Mifflin Company and G. Hall. All rights reserved.

Electrolysis èPreviously our lectures on electrochemistry were involved with voltaic cells i. e. cells with Ecell > 0 and DG < 0 that were spontaneous reactions. èToday we discuss electrochemical cells where Ecell < 0 and DG > 0 that are nonspontaneous reactions and require electricity for the reactions to take place. We can take a voltaic cell and reverse the electrodes to make an electrochemical cell. Houghton Mifflin Company and G. Hall. All rights reserved.

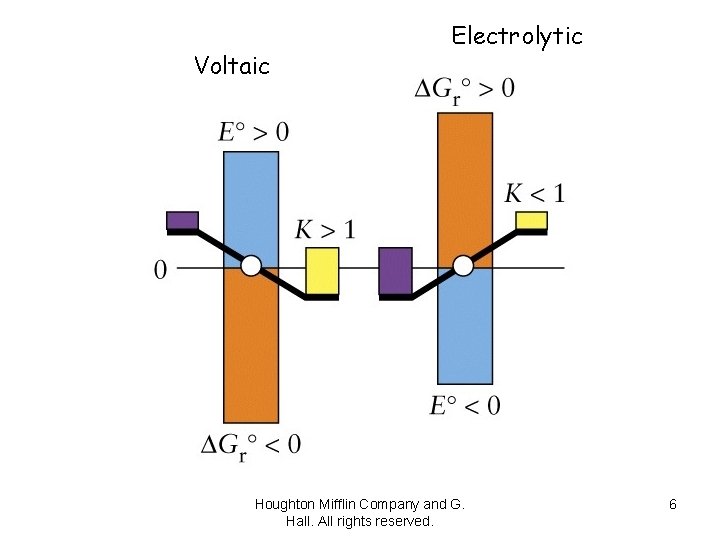
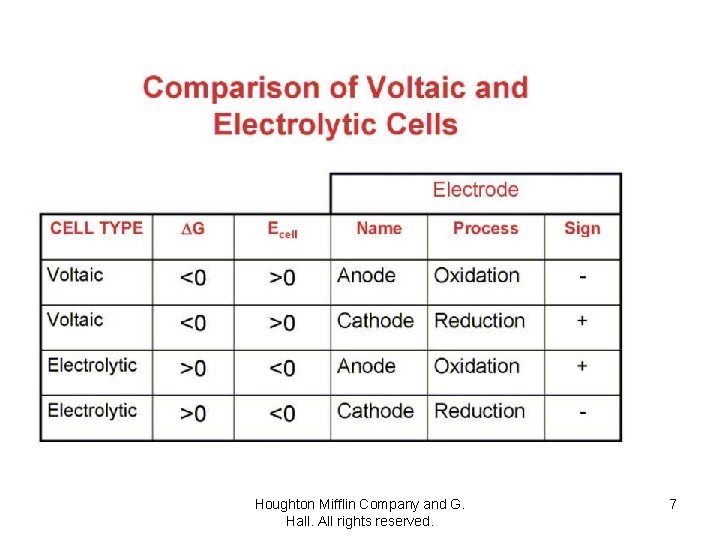
Voltaic Electrolytic Houghton Mifflin Company and G. Hall. All rights reserved. 6

Houghton Mifflin Company and G. Hall. All rights reserved. 7

Houghton Mifflin Company and G. Hall. All rights reserved. 8

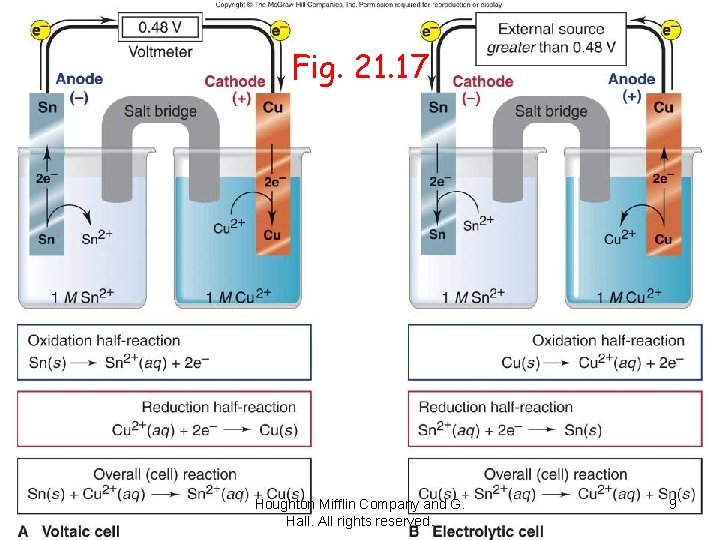
Fig. 21. 17 Houghton Mifflin Company and G. Hall. All rights reserved. 9

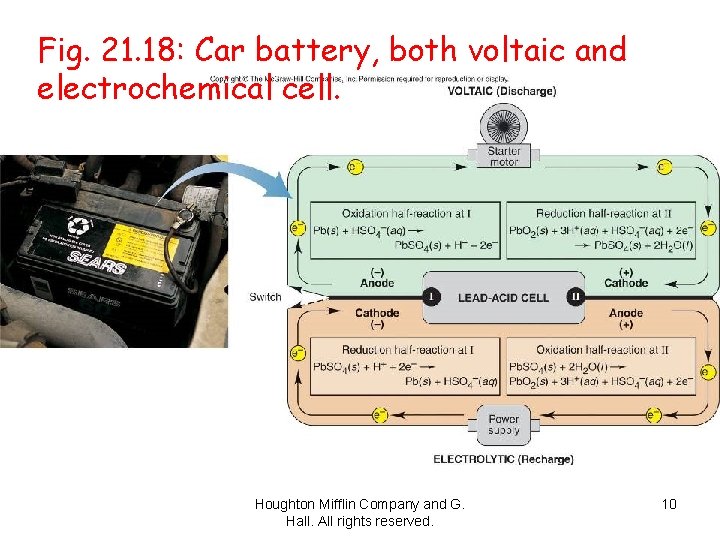
Fig. 21. 18: Car battery, both voltaic and electrochemical cell. Houghton Mifflin Company and G. Hall. All rights reserved. 10

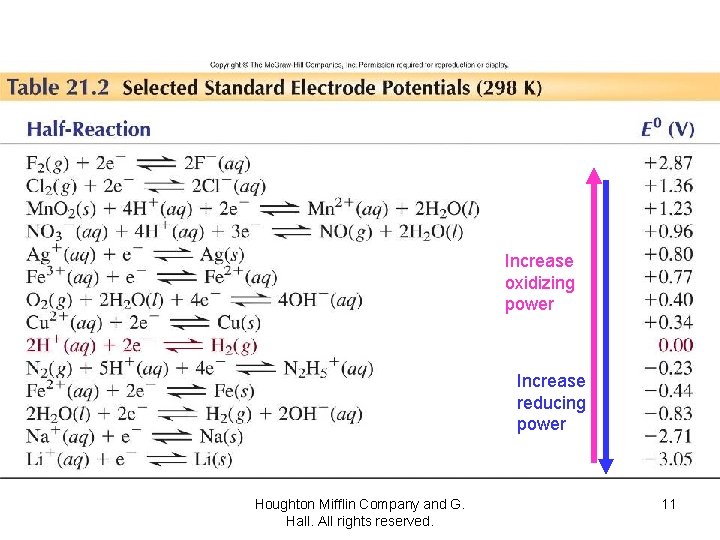
Increase oxidizing power Increase reducing power Houghton Mifflin Company and G. Hall. All rights reserved. 11

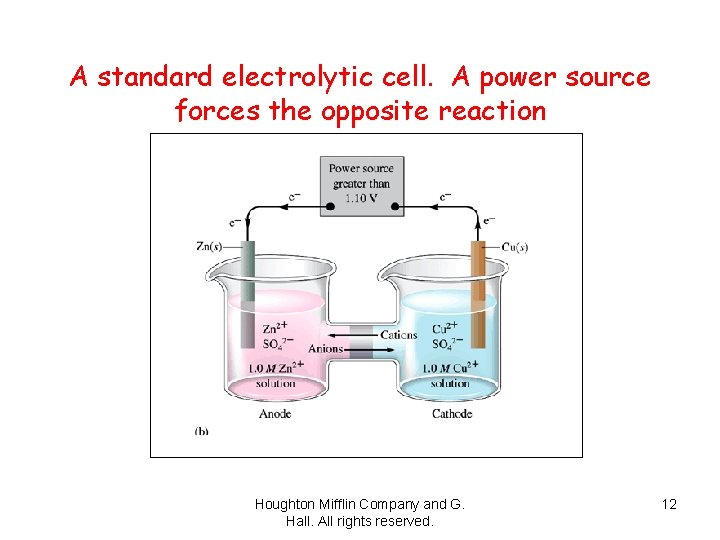
A standard electrolytic cell. A power source forces the opposite reaction Houghton Mifflin Company and G. Hall. All rights reserved. 12

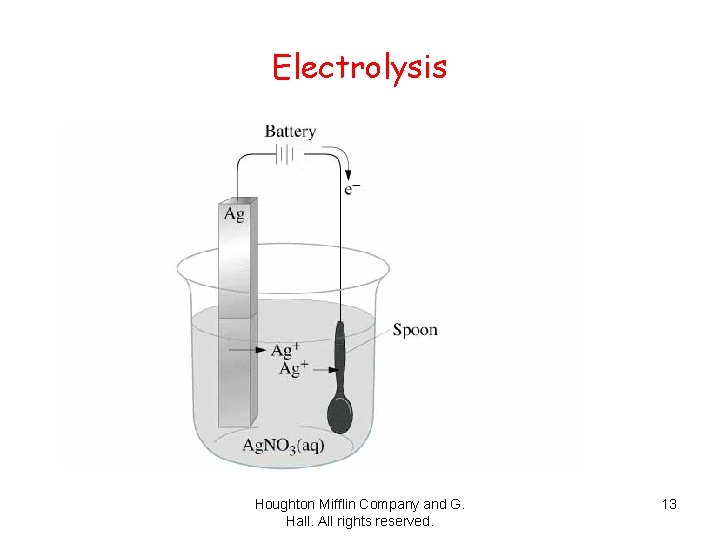
Electrolysis Houghton Mifflin Company and G. Hall. All rights reserved. 13

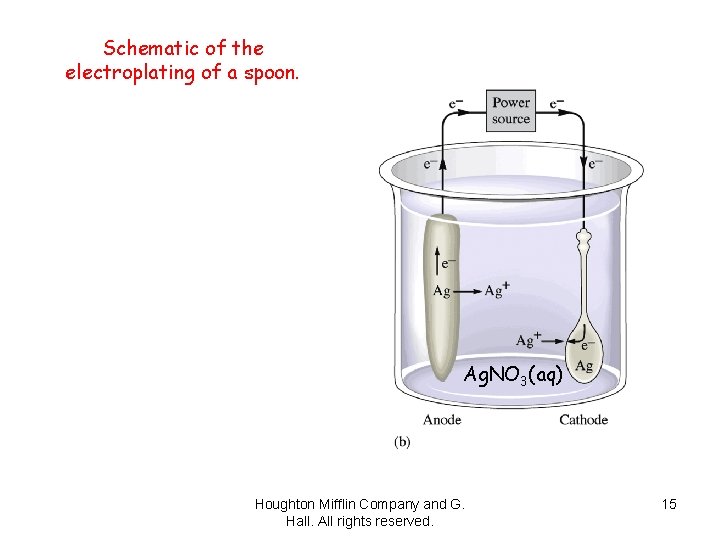
(a) A silver-plated teapot. (b) Schematic of the electroplating of a spoon. Houghton Mifflin Company and G. Hall. All rights reserved. 14

Schematic of the electroplating of a spoon. Ag. NO 3(aq) Houghton Mifflin Company and G. Hall. All rights reserved. 15

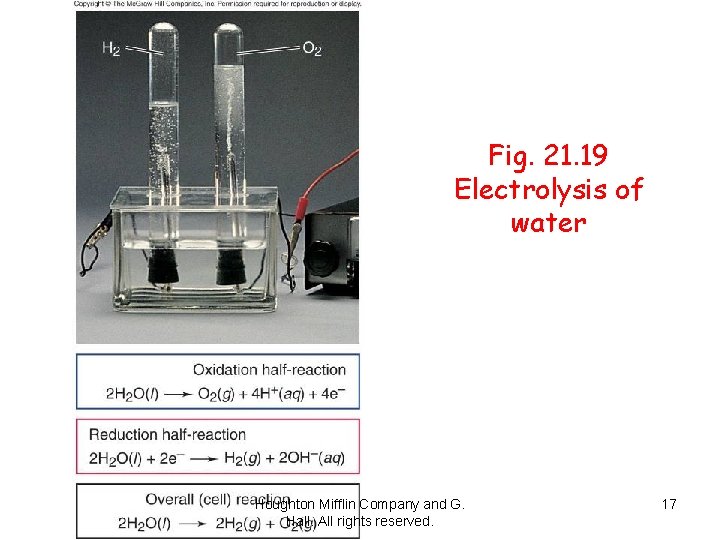
The electrolysis of water produces hydrogen gas at the cathode (on the right) and oxygen gas at the anode (on the left). Houghton Mifflin Company and G. Hall. All rights reserved. 16

Fig. 21. 19 Electrolysis of water Houghton Mifflin Company and G. Hall. All rights reserved. 17

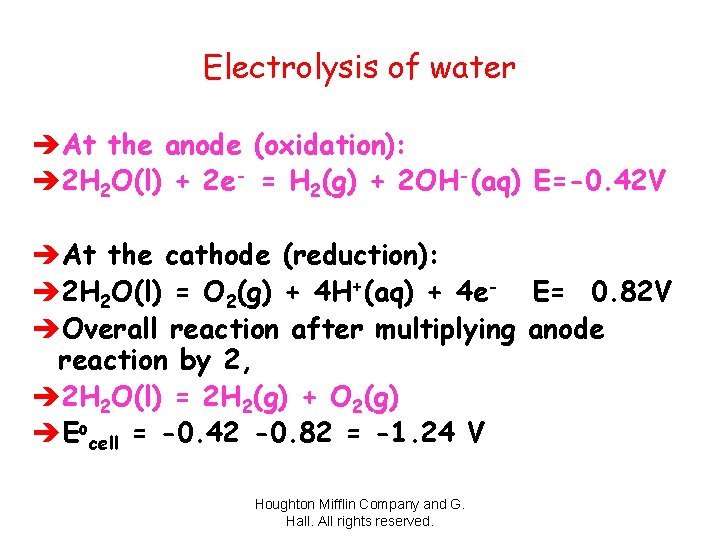
Electrolysis of water èAt the anode (oxidation): è 2 H 2 O(l) + 2 e- = H 2(g) + 2 OH-(aq) E=-0. 42 V èAt the cathode (reduction): è 2 H 2 O(l) = O 2(g) + 4 H+(aq) + 4 e- E= 0. 82 V èOverall reaction after multiplying anode reaction by 2, è 2 H 2 O(l) = 2 H 2(g) + O 2(g) èEocell = -0. 42 -0. 82 = -1. 24 V Houghton Mifflin Company and G. Hall. All rights reserved.

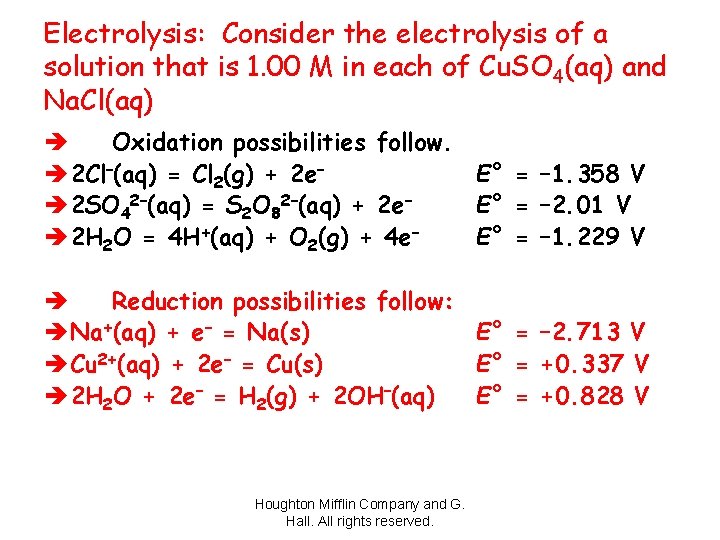
Electrolysis: Consider the electrolysis of a solution that is 1. 00 M in each of Cu. SO 4(aq) and Na. Cl(aq) è Oxidation possibilities follow. è 2 Cl–(aq) = Cl 2(g) + 2 e– E° = – 1. 358 V è 2 SO 42–(aq) = S 2 O 82–(aq) + 2 e– E° = – 2. 01 V è 2 H 2 O = 4 H+(aq) + O 2(g) + 4 e– E° = – 1. 229 V è Reduction possibilities follow: è Na+(aq) + e– = Na(s) E° = – 2. 713 V è Cu 2+(aq) + 2 e– = Cu(s) E° = +0. 337 V è 2 H 2 O + 2 e– = H 2(g) + 2 OH–(aq) E° = +0. 828 V Houghton Mifflin Company and G. Hall. All rights reserved.

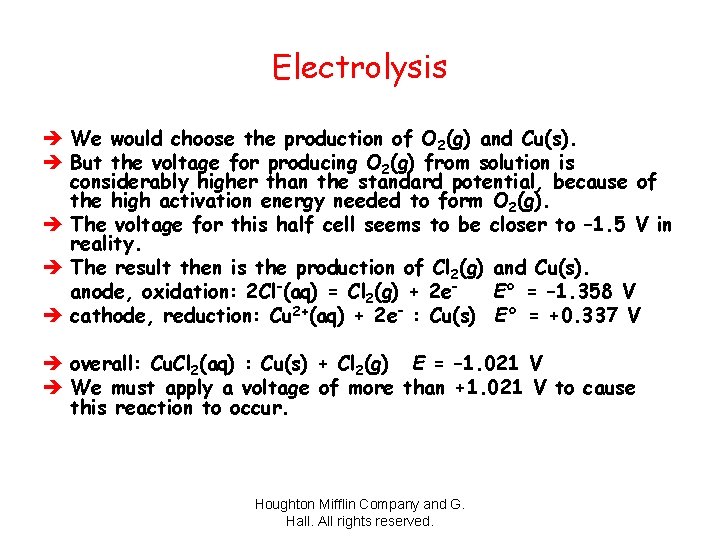
Electrolysis è We would choose the production of O 2(g) and Cu(s). è But the voltage for producing O 2(g) from solution is considerably higher than the standard potential, because of the high activation energy needed to form O 2(g). è The voltage for this half cell seems to be closer to – 1. 5 V in reality. è The result then is the production of Cl 2(g) and Cu(s). anode, oxidation: 2 Cl–(aq) = Cl 2(g) + 2 e– E° = – 1. 358 V è cathode, reduction: Cu 2+(aq) + 2 e– : Cu(s) E° = +0. 337 V è overall: Cu. Cl 2(aq) : Cu(s) + Cl 2(g) E = – 1. 021 V è We must apply a voltage of more than +1. 021 V to cause this reaction to occur. Houghton Mifflin Company and G. Hall. All rights reserved.

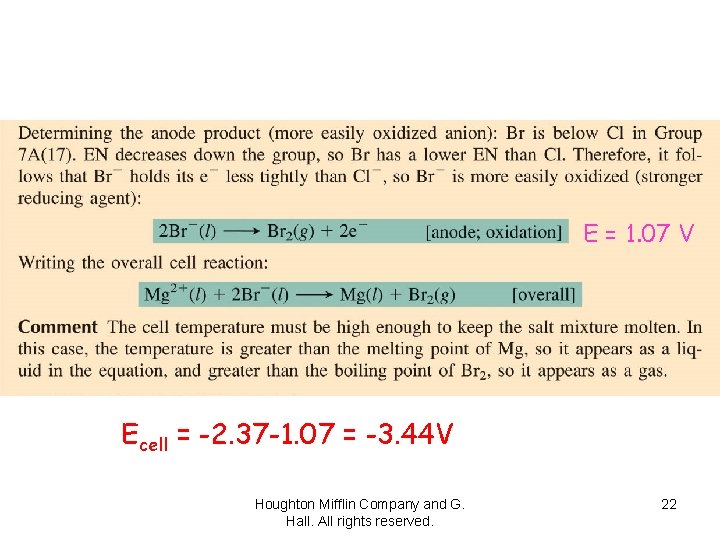
E = -2. 37 V Houghton Mifflin Company and G. Hall. All rights reserved. 21

E = 1. 07 V Ecell = -2. 37 -1. 07 = -3. 44 V Houghton Mifflin Company and G. Hall. All rights reserved. 22

Houghton Mifflin Company and G. Hall. All rights reserved. 23

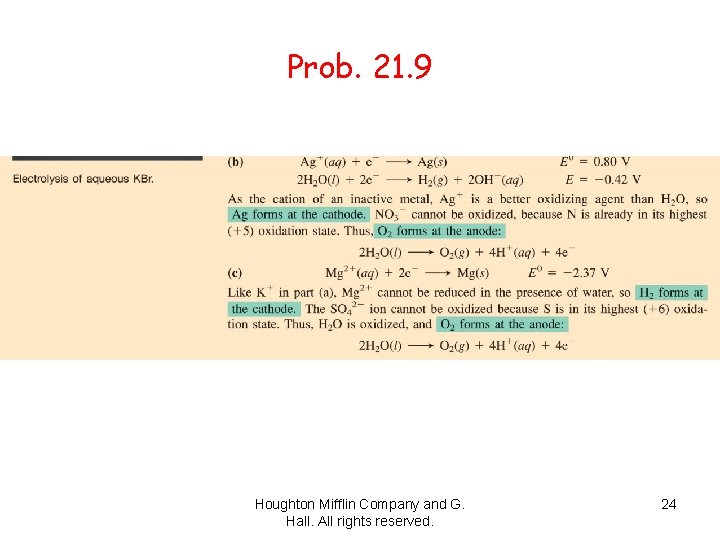
Prob. 21. 9 Houghton Mifflin Company and G. Hall. All rights reserved. 24

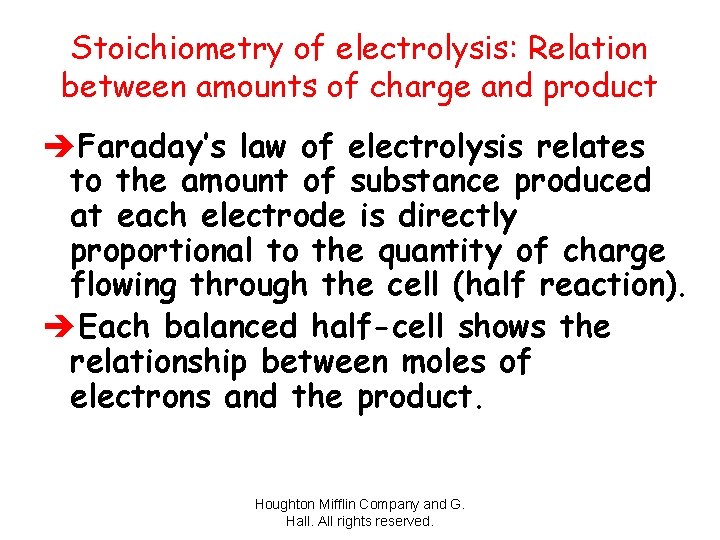
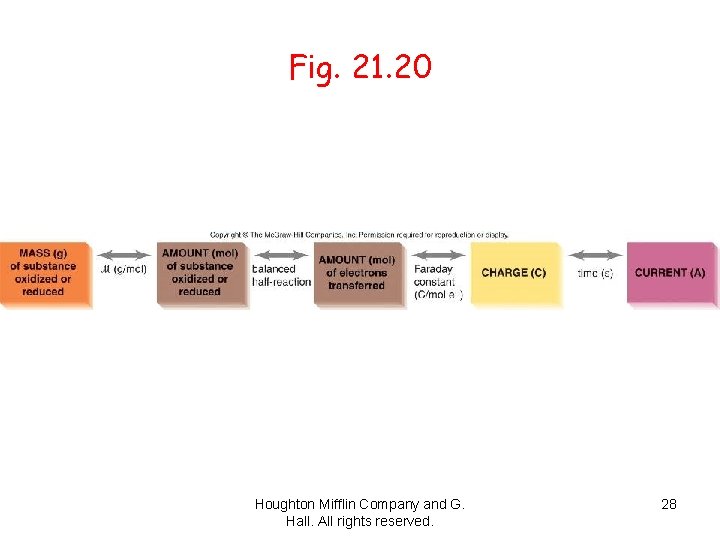
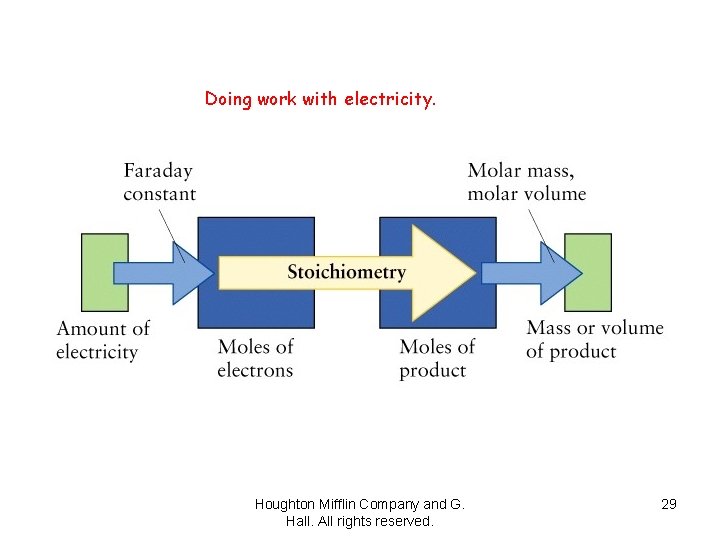
Stoichiometry of electrolysis: Relation between amounts of charge and product èFaraday’s law of electrolysis relates to the amount of substance produced at each electrode is directly proportional to the quantity of charge flowing through the cell (half reaction). èEach balanced half-cell shows the relationship between moles of electrons and the product. Houghton Mifflin Company and G. Hall. All rights reserved.

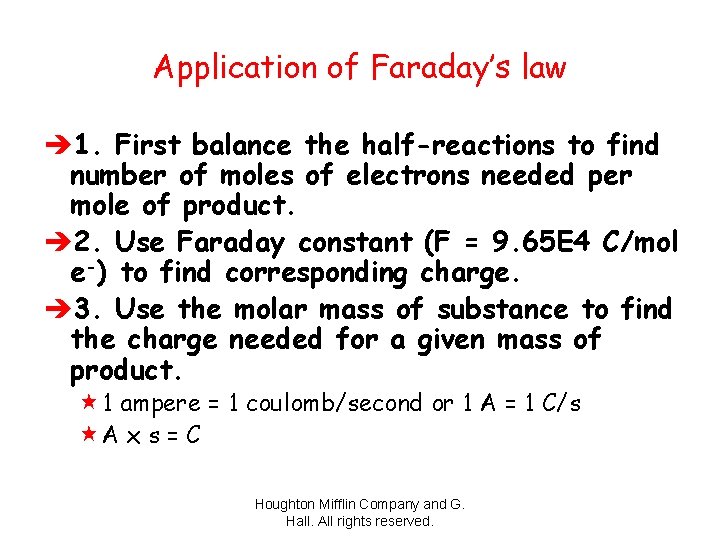
Application of Faraday’s law è 1. First balance the half-reactions to find number of moles of electrons needed per mole of product. è 2. Use Faraday constant (F = 9. 65 E 4 C/mol e-) to find corresponding charge. è 3. Use the molar mass of substance to find the charge needed for a given mass of product. « 1 ampere = 1 coulomb/second or 1 A = 1 C/s «A x s = C Houghton Mifflin Company and G. Hall. All rights reserved.

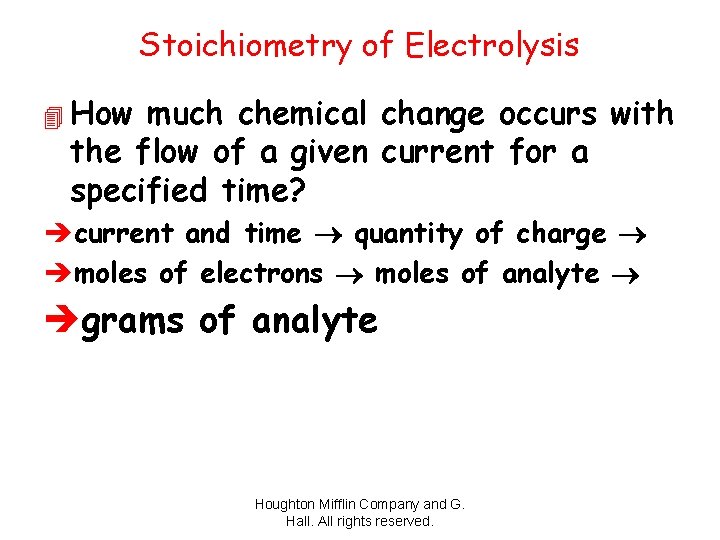
Stoichiometry of Electrolysis 4 How much chemical change occurs with the flow of a given current for a specified time? ècurrent and time quantity of charge èmoles of electrons moles of analyte ègrams of analyte Houghton Mifflin Company and G. Hall. All rights reserved.

Fig. 21. 20 Houghton Mifflin Company and G. Hall. All rights reserved. 28

Doing work with electricity. Houghton Mifflin Company and G. Hall. All rights reserved. 29

Houghton Mifflin Company and G. Hall. All rights reserved. 30

Industrial Applications of Electrolysis Houghton Mifflin Company and G. Hall. All rights reserved. 31

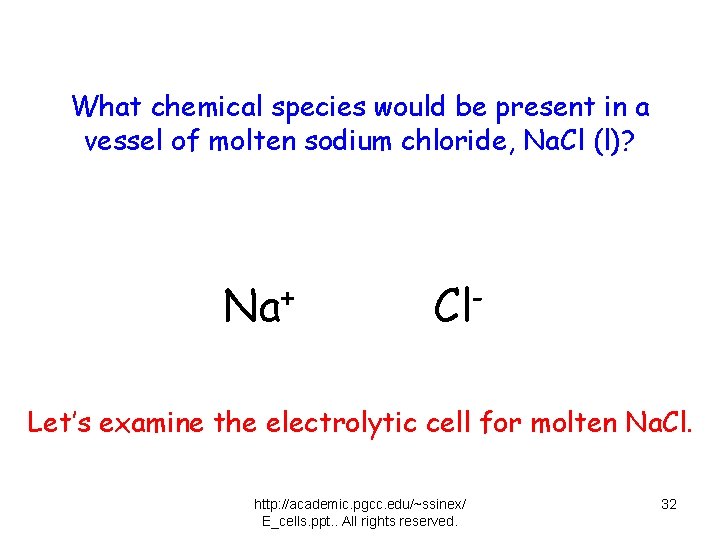
What chemical species would be present in a vessel of molten sodium chloride, Na. Cl (l)? Na+ Cl- Let’s examine the electrolytic cell for molten Na. Cl. http: //academic. pgcc. edu/~ssinex/ E_cells. ppt. . All rights reserved. 32

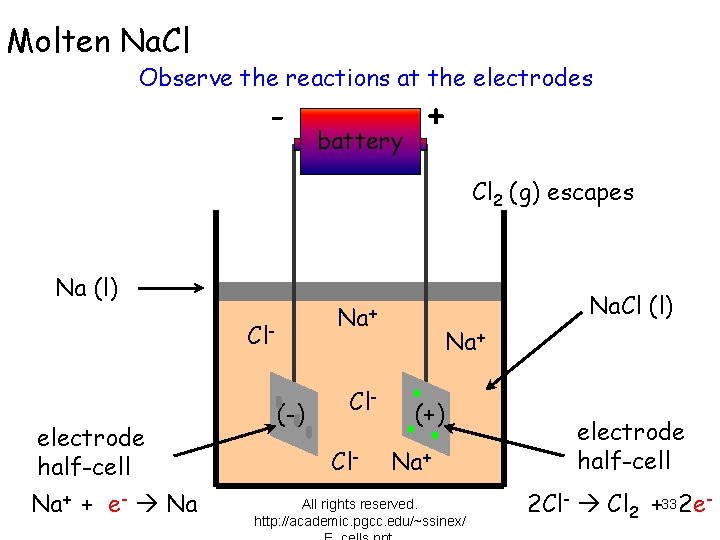
Molten Na. Cl Observe the reactions at the electrodes - battery + Cl 2 (g) escapes Na (l) Cl- electrode half-cell Na+ + e- Na Na. Cl (l) Na+ (-) Cl. Cl- Na+ (+) Na+ All rights reserved. http: //academic. pgcc. edu/~ssinex/ electrode half-cell 2 Cl- Cl 2 +33 2 e-

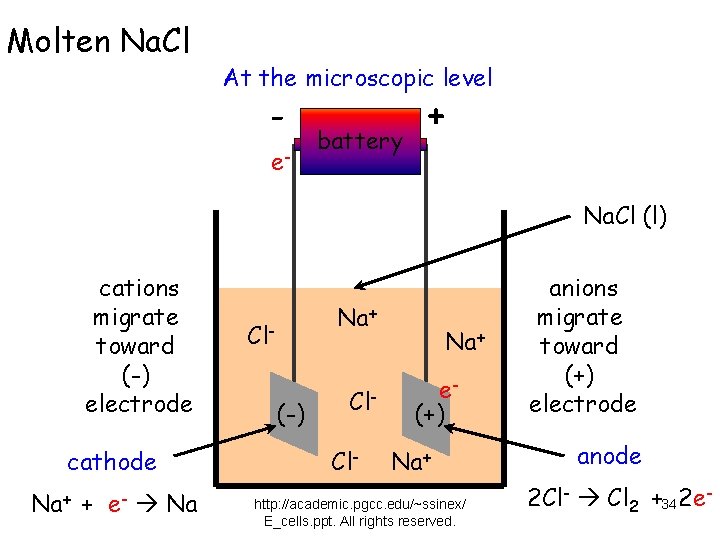
Molten Na. Cl At the microscopic level - e- battery + Na. Cl (l) cations migrate toward (-) electrode cathode Na+ + e- Na Na+ Cl(-) Cl. Cl- Na+ e(+) Na+ http: //academic. pgcc. edu/~ssinex/ E_cells. ppt. All rights reserved. anions migrate toward (+) electrode anode 2 Cl- Cl 2 +34 2 e-

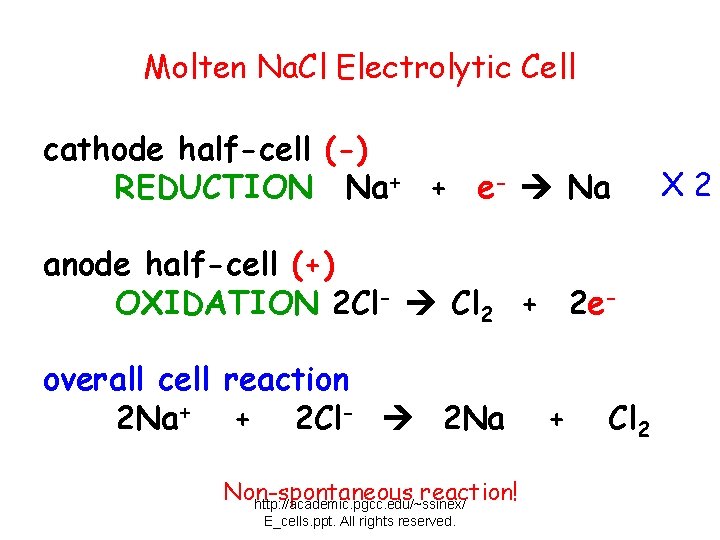
Molten Na. Cl Electrolytic Cell cathode half-cell (-) REDUCTION Na+ + e- Na anode half-cell (+) OXIDATION 2 Cl- Cl 2 + 2 eoverall cell reaction 2 Na+ + 2 Cl- 2 Na Non-spontaneous reaction! http: //academic. pgcc. edu/~ssinex/ E_cells. ppt. All rights reserved. + Cl 2 X 2

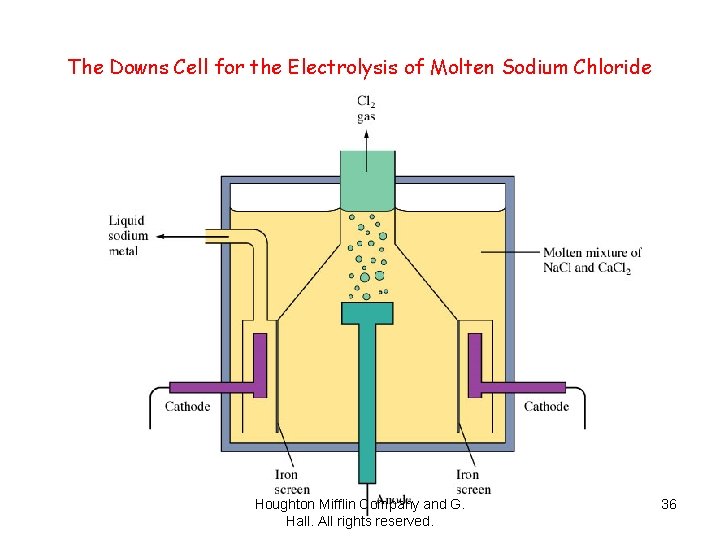
The Downs Cell for the Electrolysis of Molten Sodium Chloride Houghton Mifflin Company and G. Hall. All rights reserved. 36

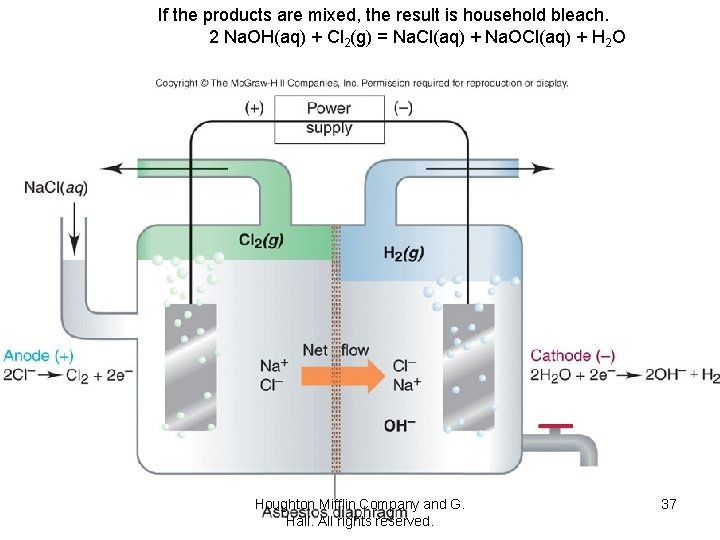
If the products are mixed, the result is household bleach. 2 Na. OH(aq) + Cl 2(g) = Na. Cl(aq) + Na. OCl(aq) + H 2 O Houghton Mifflin Company and G. Hall. All rights reserved. 37

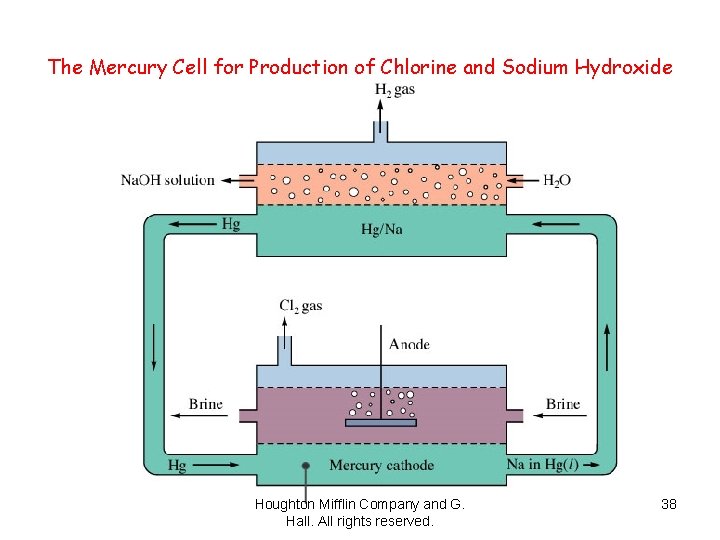
The Mercury Cell for Production of Chlorine and Sodium Hydroxide Houghton Mifflin Company and G. Hall. All rights reserved. 38

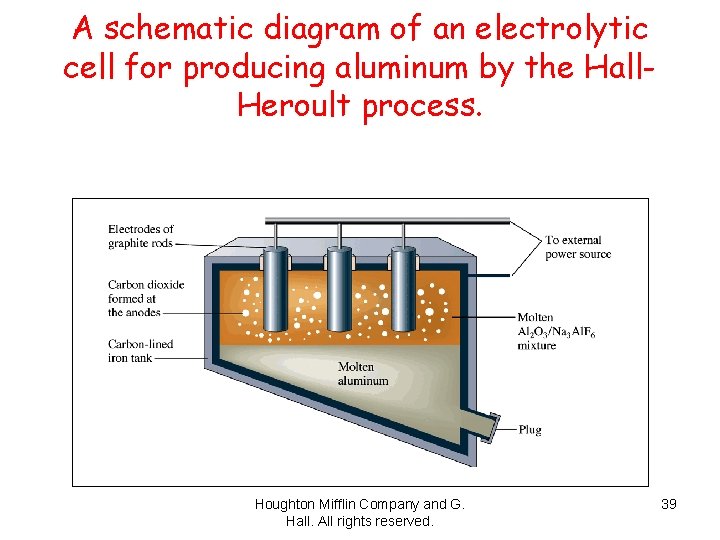
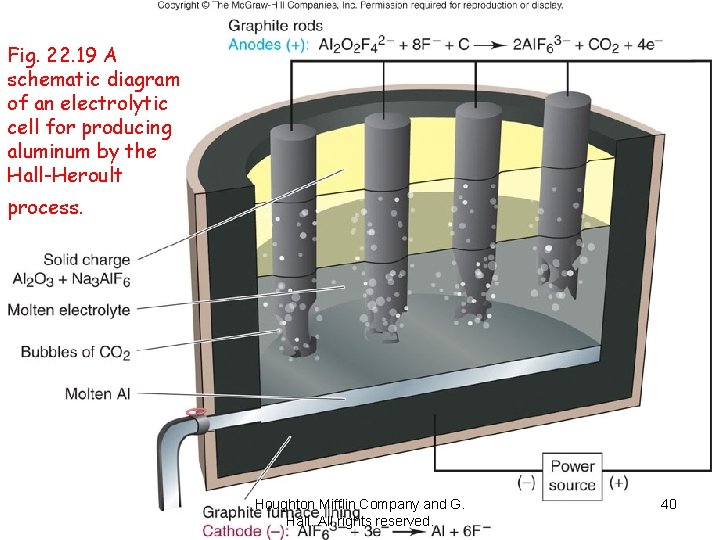
A schematic diagram of an electrolytic cell for producing aluminum by the Hall. Heroult process. Houghton Mifflin Company and G. Hall. All rights reserved. 39

Fig. 22. 19 A schematic diagram of an electrolytic cell for producing aluminum by the Hall-Heroult process. Houghton Mifflin Company and G. Hall. All rights reserved. 40

The Hall Process for Aluminum èElectrolysis of molten Al 2 O 3 mixed with cryolite – lowers melting point èCell operates at high temperature – 1000 o. C èAluminum was a precious metal in 1886. èA block of aluminum is at the tip of the Washington Monument! http: //academic. pgcc. edu/~ssinex/ E_cells. ppt.

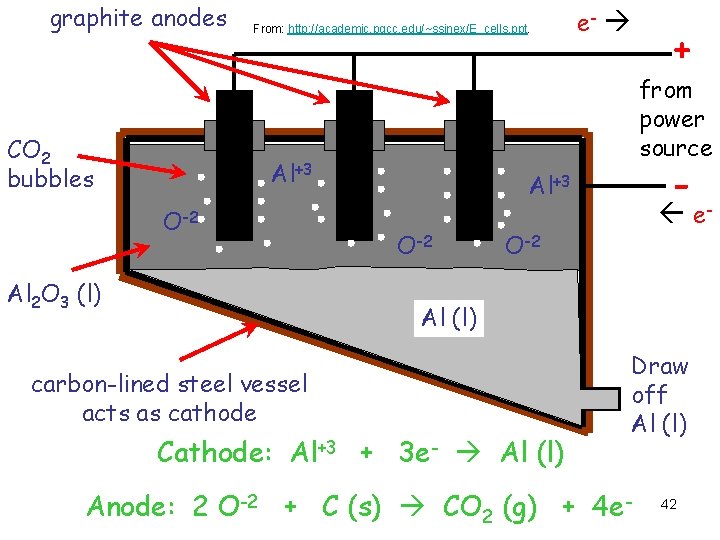
graphite anodes CO 2 bubbles From: http: //academic. pgcc. edu/~ssinex/E_cells. ppt. Al 2 O 3 (l) + from power source Al+3 O-2 e- - Al+3 O-2 e- O-2 Al (l) carbon-lined steel vessel acts as cathode Cathode: Al+3 + 3 e- Al (l) Draw off Al (l) Anode: 2 O-2 + C (s) CO 2 (g) + 4 e- 42

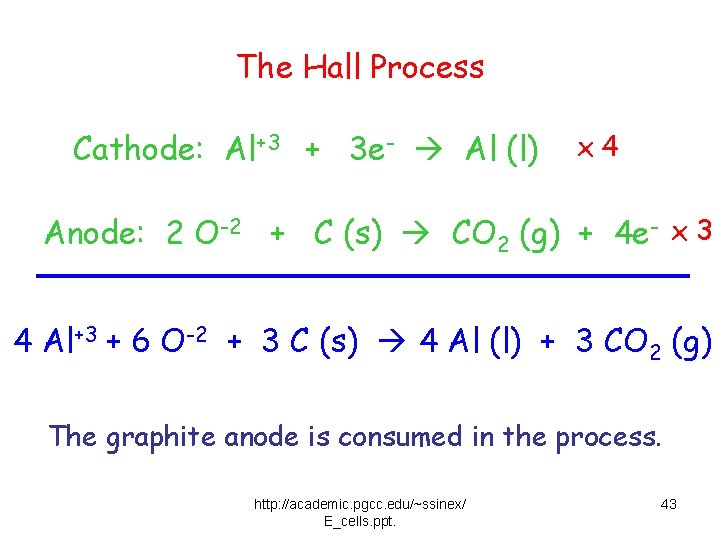
The Hall Process Cathode: Al+3 + 3 e- Al (l) x 4 Anode: 2 O-2 + C (s) CO 2 (g) + 4 e- x 3 4 Al+3 + 6 O-2 + 3 C (s) 4 Al (l) + 3 CO 2 (g) The graphite anode is consumed in the process. http: //academic. pgcc. edu/~ssinex/ E_cells. ppt. 43

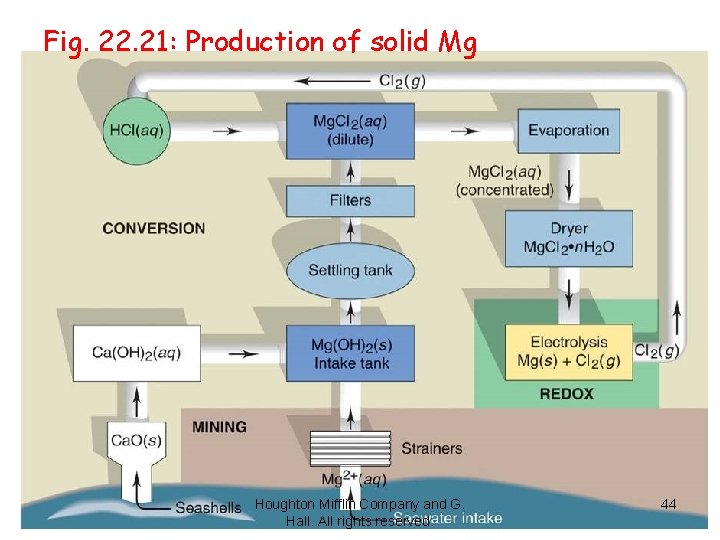
Fig. 22. 21: Production of solid Mg Houghton Mifflin Company and G. Hall. All rights reserved. 44

Corrosion èElectrochemistry plays a major role in corrosion and protection against it. Houghton Mifflin Company and G. Hall. All rights reserved.

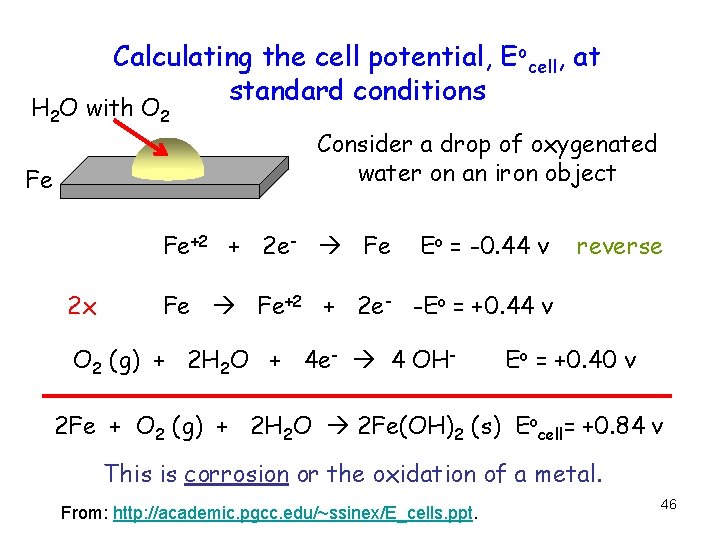
Calculating the cell potential, Eocell, at standard conditions H 2 O with O 2 Fe Consider a drop of oxygenated water on an iron object Fe+2 + 2 e- Fe 2 x Eo = -0. 44 v reverse Fe Fe+2 + 2 e- -Eo = +0. 44 v O 2 (g) + 2 H 2 O + 4 e- 4 OH- Eo = +0. 40 v 2 Fe + O 2 (g) + 2 H 2 O 2 Fe(OH)2 (s) Eocell= +0. 84 v This is corrosion or the oxidation of a metal. From: http: //academic. pgcc. edu/~ssinex/E_cells. ppt. 46

Cathodic Protection Against Corrosion Underground steel pipes offer the strength to transport fluids at high pressures, but they are vulnerable to corrosion driven by electrochemical processes. A measure of protection can be offered by driving a magnesium rod into the ground near the pipe and providing an electrical connection to the pipe. Since the magnesium has a standard potential of -2. 38 volts compared to -. 41 volts for iron, it can act as a anode of a voltaic cell with the steel pipe acting as the cathode. With damp soil serving as the electrolyte, a small current can flow in the wire connected to the pipe. The magnesium rod will be eventually consumed by the reaction Mg(s) -> + Mg 2+(aq) + 2 ewhile the steel pipe as the cathode will be protected by the reaction O 2(g) + 2 H 2 O(l) + 4 e- -> 4 OH-(aq). From: http: //hyperphysics. phy-astr. gsu. edu/hbase/chemical/corrosion. html#c 2 Houghton Mifflin Company and G. Hall. All rights reserved. 47

Lecture summary èElectrolysis is often the reverse of voltaic cell in that Ecell < 0, and DG >0 and reaction is non-spontaneous. èElectrolysis of water and to produce O 2 and H 2. èFaraday’s law allows us to determine how much current is needed to produce a certain amount of an element. èIndustrial applications are numerous for producing a variety of solid elements (Al, Mg, Na, etc). Houghton Mifflin Company and G. Hall. All rights reserved.