Lecture 2 The Principles of Microscopy BMS 524

Lecture 2 The Principles of Microscopy BMS 524 - “Introduction to Confocal Microscopy and Image Analysis” Purdue University Department of Basic Medical Sciences, School of Veterinary Medicine J. Paul Robinson, Ph. D. Professor of Immunopharmacology Director, Purdue University Cytometry Laboratories These slides are intended for use in a lecture series. Copies of the graphics are distributed and students encouraged to take their notes on these graphics. The intent is to have the student NOT try to reproduce the figures, but to LISTEN and UNDERSTAND the material. All material copyright J. Paul Robinson unless otherwise stated, however, the material may be freely used for lectures, tutorials and workshops. It may not be used for any commercial purpose. The text for this course is Pawley “Introduction to Confocal Microscopy”, Plenum Press, 2 nd Ed. A number of the ideas and figures in these lecture notes are taken from this text. UPDATED January, 2000 © 1995 -2000 J. Paul Robinson - Purdue University Cytometry Laboratories Slide 1 t: /classes/BMS 524/524 lect 2. ppt
Review • Microscope Basics, Magnification, Optical systems Properties of Light • • Refraction A Lens Refractive Index Numerical Aperture Resolution Aberrations Fluorescence © 1995 -2000 J. Paul Robinson - Purdue University Cytometry Laboratories Slide 2 t: /classes/BMS 524/524 lect 2. ppt
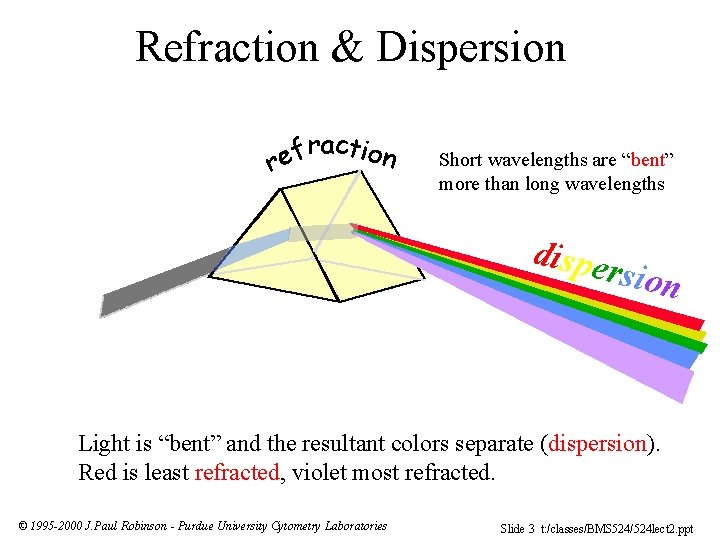
Refraction & Dispersion raction f e r Short wavelengths are “bent” more than long wavelengths dispe rsion Light is “bent” and the resultant colors separate (dispersion). Red is least refracted, violet most refracted. © 1995 -2000 J. Paul Robinson - Purdue University Cytometry Laboratories Slide 3 t: /classes/BMS 524/524 lect 2. ppt
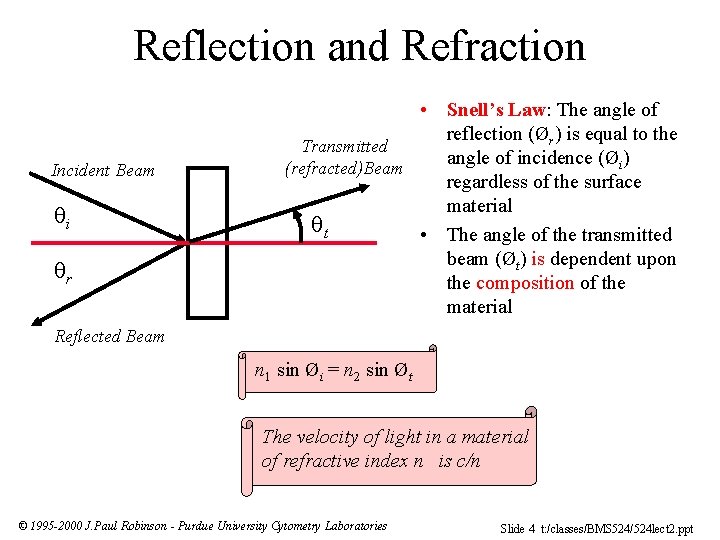
Reflection and Refraction Incident Beam i r • Snell’s Law: The angle of reflection (Ør) is equal to the Transmitted angle of incidence (Øi) (refracted)Beam regardless of the surface material t • The angle of the transmitted beam (Øt) is dependent upon the composition of the material Reflected Beam n 1 sin Øi = n 2 sin Øt The velocity of light in a material of refractive index n is c/n © 1995 -2000 J. Paul Robinson - Purdue University Cytometry Laboratories Slide 4 t: /classes/BMS 524/524 lect 2. ppt
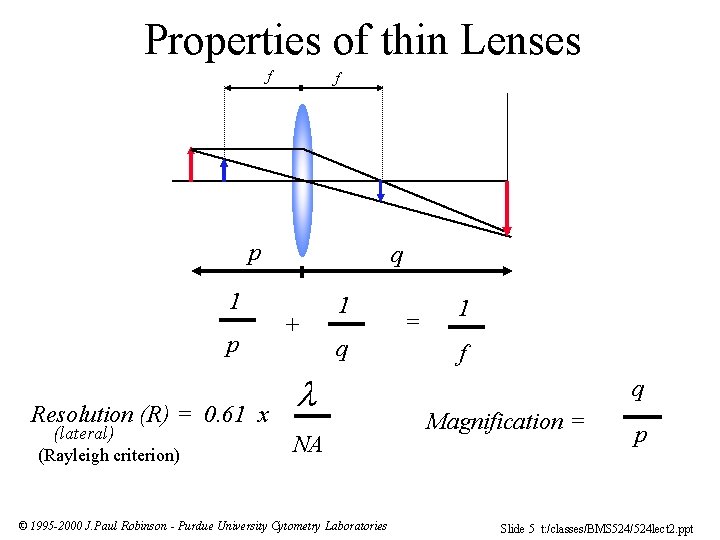
Properties of thin Lenses f f p 1 p Resolution (R) = 0. 61 x (lateral) (Rayleigh criterion) q + 1 q l NA © 1995 -2000 J. Paul Robinson - Purdue University Cytometry Laboratories = 1 f q Magnification = p Slide 5 t: /classes/BMS 524/524 lect 2. ppt
Microscope Components • • Ocular Objectives Condenser Numerical Aperture Refractive Index Aberrations Optical Filters © 1995 -2000 J. Paul Robinson - Purdue University Cytometry Laboratories Slide 6 t: /classes/BMS 524/524 lect 2. ppt
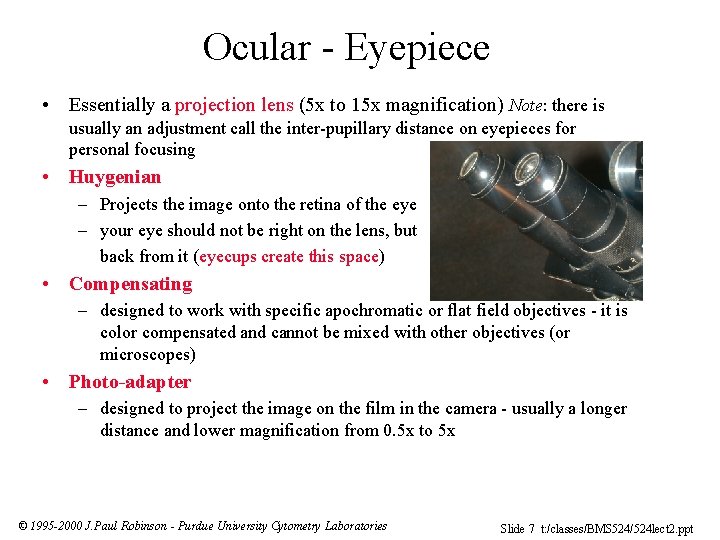
Ocular - Eyepiece • Essentially a projection lens (5 x to 15 x magnification) Note: there is usually an adjustment call the inter-pupillary distance on eyepieces for personal focusing • Huygenian – Projects the image onto the retina of the eye – your eye should not be right on the lens, but back from it (eyecups create this space) • Compensating – designed to work with specific apochromatic or flat field objectives - it is color compensated and cannot be mixed with other objectives (or microscopes) • Photo-adapter – designed to project the image on the film in the camera - usually a longer distance and lower magnification from 0. 5 x to 5 x © 1995 -2000 J. Paul Robinson - Purdue University Cytometry Laboratories Slide 7 t: /classes/BMS 524/524 lect 2. ppt
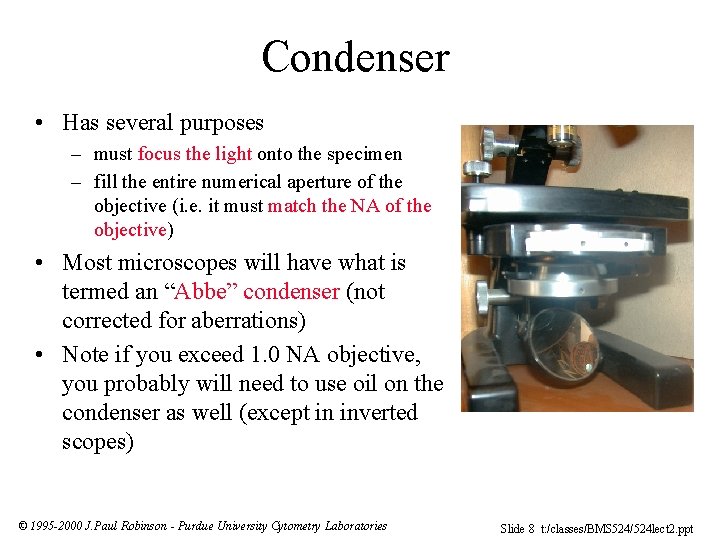
Condenser • Has several purposes – must focus the light onto the specimen – fill the entire numerical aperture of the objective (i. e. it must match the NA of the objective) • Most microscopes will have what is termed an “Abbe” condenser (not corrected for aberrations) • Note if you exceed 1. 0 NA objective, you probably will need to use oil on the condenser as well (except in inverted scopes) © 1995 -2000 J. Paul Robinson - Purdue University Cytometry Laboratories Slide 8 t: /classes/BMS 524/524 lect 2. ppt

Microscope Objectives © 1995 -2000 J. Paul Robinson - Purdue University Cytometry Laboratories Slide 9 t: /classes/BMS 524/524 lect 2. ppt
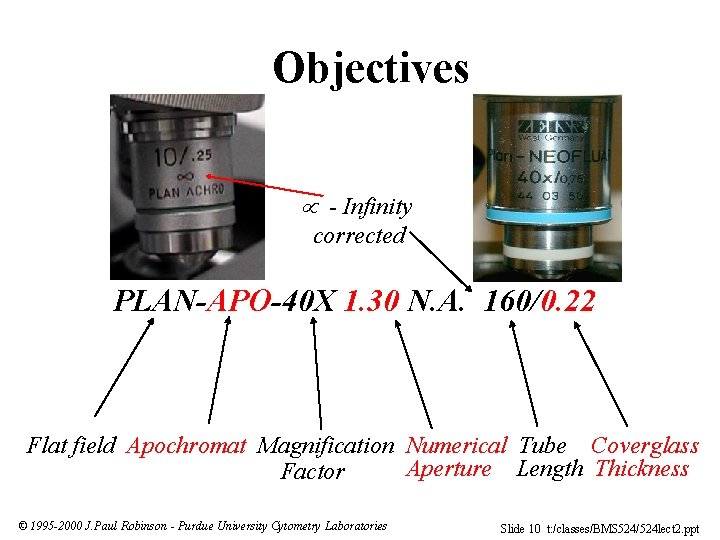
Objectives - Infinity corrected PLAN-APO-40 X 1. 30 N. A. 160/0. 22 Flat field Apochromat Magnification Numerical Tube Coverglass Aperture Length Thickness Factor © 1995 -2000 J. Paul Robinson - Purdue University Cytometry Laboratories Slide 10 t: /classes/BMS 524/524 lect 2. ppt
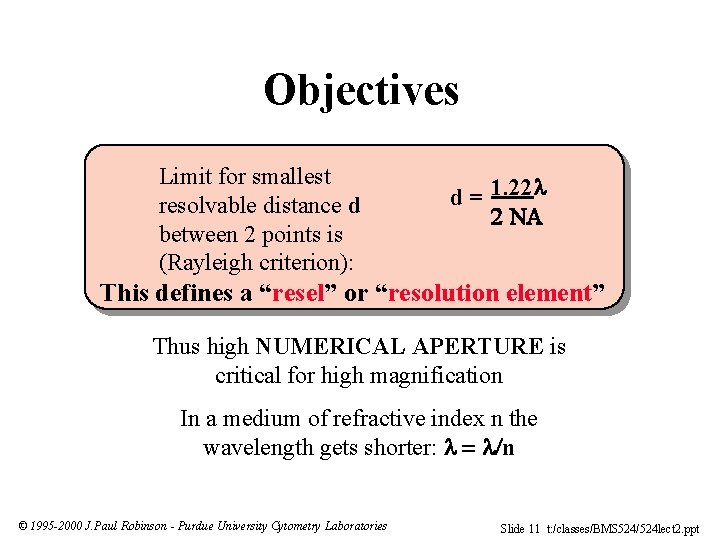
Objectives Limit for smallest resolvable distance d between 2 points is (Rayleigh criterion): d = 1. 22 This defines a “resel” or “resolution element” Thus high NUMERICAL APERTURE is critical for high magnification In a medium of refractive index n the wavelength gets shorter: n © 1995 -2000 J. Paul Robinson - Purdue University Cytometry Laboratories Slide 11 t: /classes/BMS 524/524 lect 2. ppt
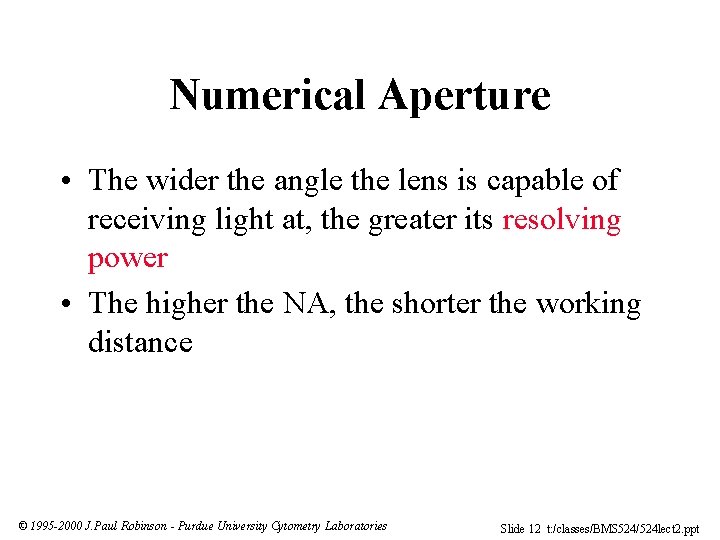
Numerical Aperture • The wider the angle the lens is capable of receiving light at, the greater its resolving power • The higher the NA, the shorter the working distance © 1995 -2000 J. Paul Robinson - Purdue University Cytometry Laboratories Slide 12 t: /classes/BMS 524/524 lect 2. ppt
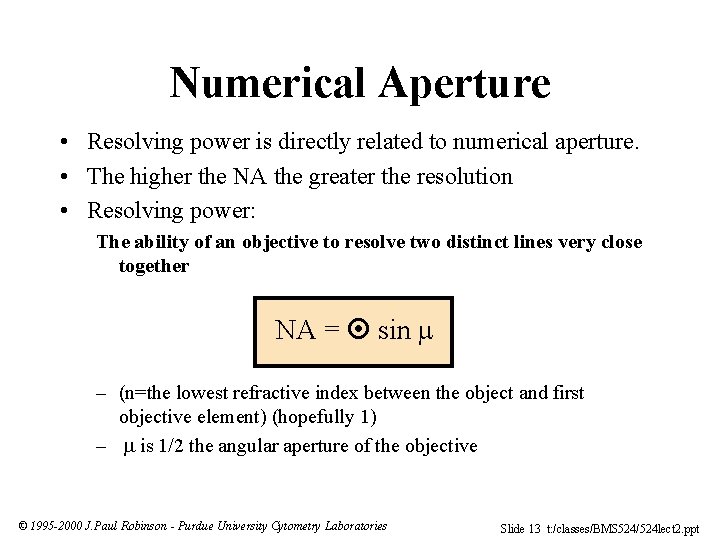
Numerical Aperture • Resolving power is directly related to numerical aperture. • The higher the NA the greater the resolution • Resolving power: The ability of an objective to resolve two distinct lines very close together NA = sin – (n=the lowest refractive index between the object and first objective element) (hopefully 1) – is 1/2 the angular aperture of the objective © 1995 -2000 J. Paul Robinson - Purdue University Cytometry Laboratories Slide 13 t: /classes/BMS 524/524 lect 2. ppt
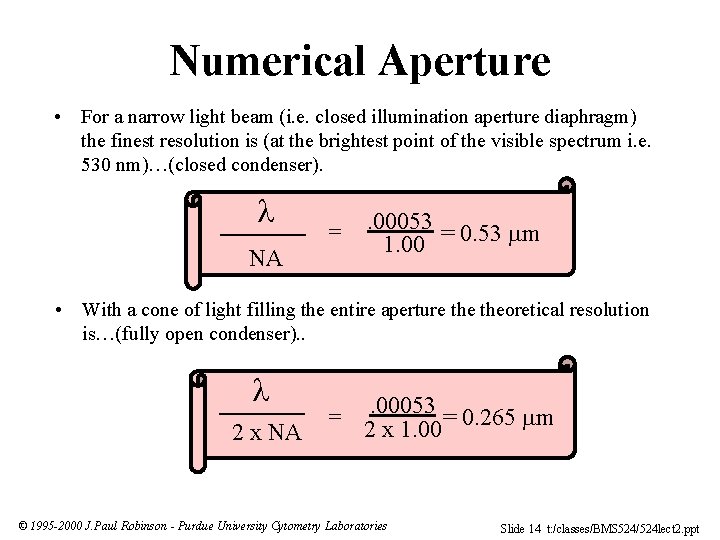
Numerical Aperture • For a narrow light beam (i. e. closed illumination aperture diaphragm) the finest resolution is (at the brightest point of the visible spectrum i. e. 530 nm)…(closed condenser). NA = . 00053 1. 00 = 0. 53 m • With a cone of light filling the entire aperture theoretical resolution is…(fully open condenser). . 2 x NA = . 00053 = 0. 265 m 2 x 1. 00 © 1995 -2000 J. Paul Robinson - Purdue University Cytometry Laboratories Slide 14 t: /classes/BMS 524/524 lect 2. ppt
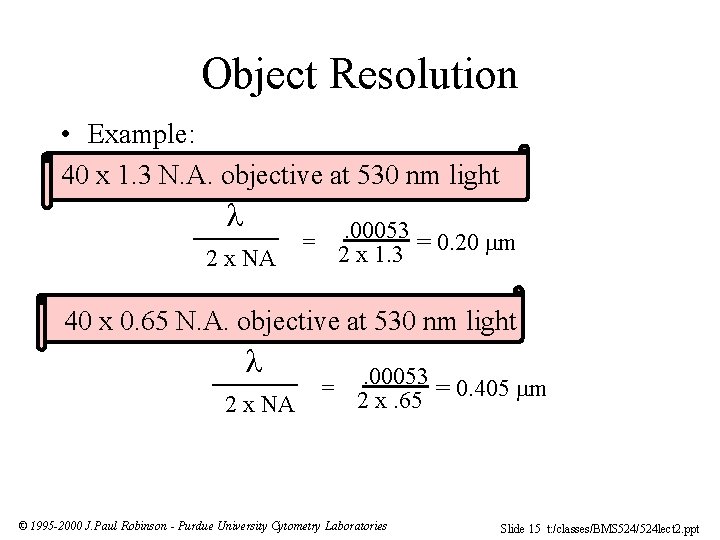
Object Resolution • Example: 40 x 1. 3 N. A. objective at 530 nm light 2 x NA . 00053 = 0. 20 m 2 x 1. 3 = 40 x 0. 65 N. A. objective at 530 nm light 2 x NA = . 00053 = 0. 405 m 2 x. 65 © 1995 -2000 J. Paul Robinson - Purdue University Cytometry Laboratories Slide 15 t: /classes/BMS 524/524 lect 2. ppt
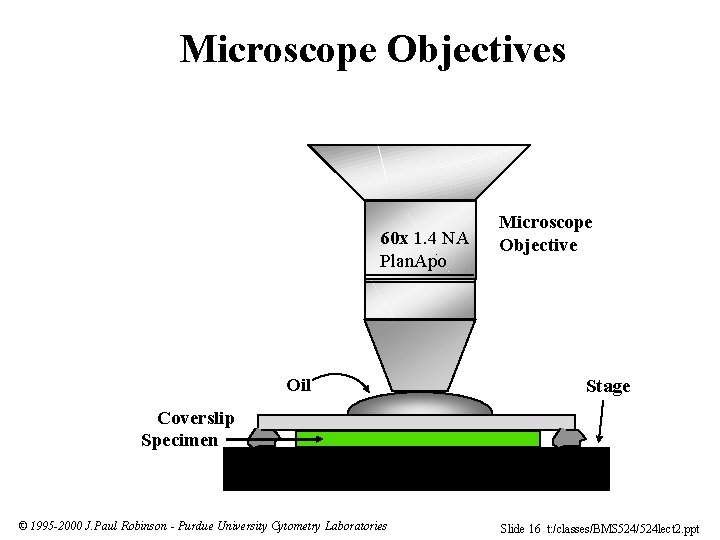
Microscope Objectives 60 x 1. 4 NA Plan. Apo Oil Microscope Objective Stage Coverslip Specimen © 1995 -2000 J. Paul Robinson - Purdue University Cytometry Laboratories Slide 16 t: /classes/BMS 524/524 lect 2. ppt

Refractive Index Objective n = 1. 52 n=1. 52 Oil n = 1. 5 n = 1. 0 Air n = 1. 52 Coverslip Specimen © 1995 -2000 J. Paul Robinson - Purdue University Cytometry Laboratories Water n=1. 33 Slide 17 t: /classes/BMS 524/524 lect 2. ppt
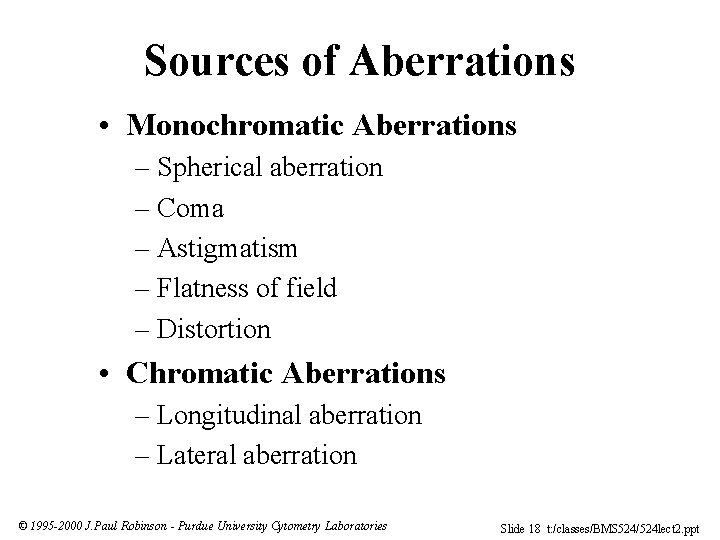
Sources of Aberrations • Monochromatic Aberrations – Spherical aberration – Coma – Astigmatism – Flatness of field – Distortion • Chromatic Aberrations – Longitudinal aberration – Lateral aberration © 1995 -2000 J. Paul Robinson - Purdue University Cytometry Laboratories Slide 18 t: /classes/BMS 524/524 lect 2. ppt
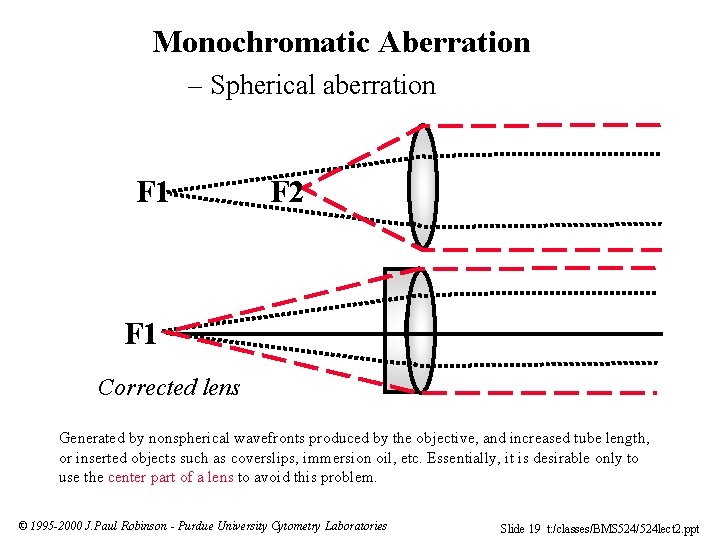
Monochromatic Aberration – Spherical aberration F 1 F 2 F 1 Corrected lens Generated by nonspherical wavefronts produced by the objective, and increased tube length, or inserted objects such as coverslips, immersion oil, etc. Essentially, it is desirable only to use the center part of a lens to avoid this problem. © 1995 -2000 J. Paul Robinson - Purdue University Cytometry Laboratories Slide 19 t: /classes/BMS 524/524 lect 2. ppt
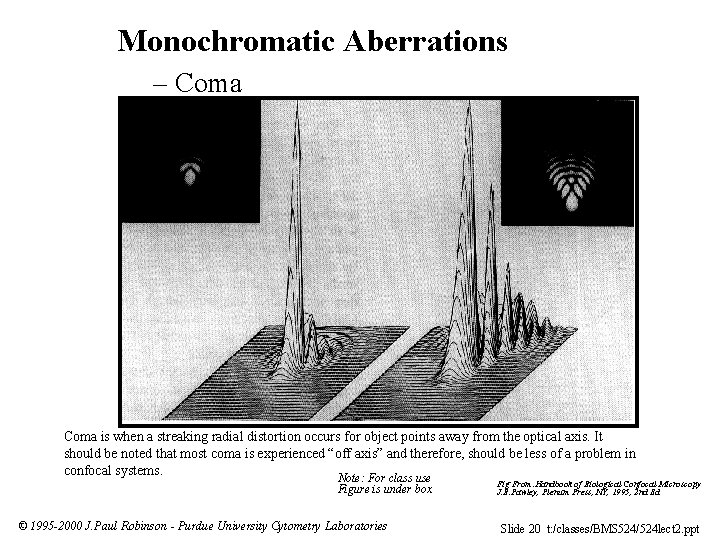
Monochromatic Aberrations – Coma Fig 12 p 117 From: ”Handbook of Biological Confocal Microscopy” J. B. Pawley, Plenum Press, NY, 1995, 2 nd Ed The figure is not reproduced in this presentation because we do not have permission to place this figure onto a public site. Coma is when a streaking radial distortion occurs for object points away from the optical axis. It should be noted that most coma is experienced “off axis” and therefore, should be less of a problem in confocal systems. Note: For class use Figure is under box © 1995 -2000 J. Paul Robinson - Purdue University Cytometry Laboratories Fig From: Handbook of Biological Confocal Microscopy J. B. Pawley, Plenum Press, NY, 1995, 2 nd Ed Slide 20 t: /classes/BMS 524/524 lect 2. ppt
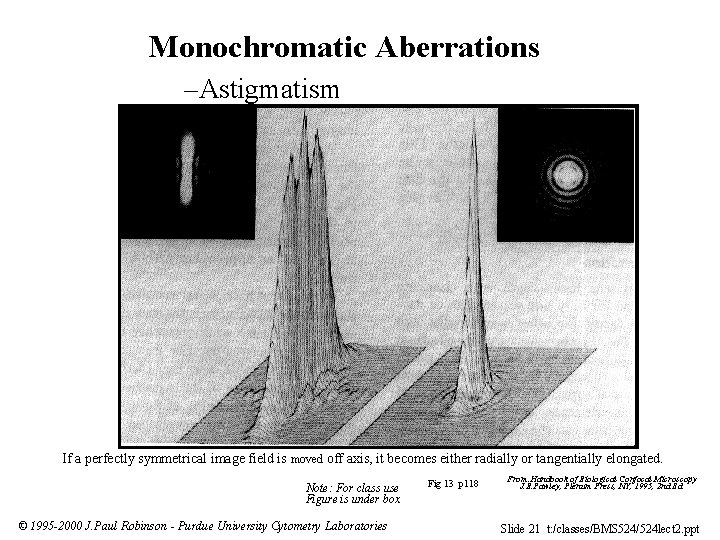
Monochromatic Aberrations –Astigmatism Fig 13 p 118 From: ”Handbook of Biological Confocal Microscopy” J. B. Pawley, Plenum Press, NY, 1995, 2 nd Ed The figure is not reproduced in this presentation because we do not have permission to place this figure onto a public site. If a perfectly symmetrical image field is moved off axis, it becomes either radially or tangentially elongated. Note: For class use Figure is under box © 1995 -2000 J. Paul Robinson - Purdue University Cytometry Laboratories Fig 13 p 118 From: Handbook of Biological Confocal Microscopy J. B. Pawley, Plenum Press, NY, 1995, 2 nd Ed. Slide 21 t: /classes/BMS 524/524 lect 2. ppt
• Monochromatic Aberrations – Flatness of Field – Distortion Lenses are spherical and since points of a flat image are focused onto a spherical dish, the central and peripheral zones will not be in focus. Complex Achromat and PLANAPOCHROMAT lenses partially solve this problem but at reduced transmission. DISTORTION occurs for objects components out of axis. Most objectives correct to reduce distortion to less than 2% of the radial distance from the axis. © 1995 -2000 J. Paul Robinson - Purdue University Cytometry Laboratories Slide 22 t: /classes/BMS 524/524 lect 2. ppt
Useful Factoids The intensity of light collected decreases as the square of the magnification The intensity of light increases as the square of the numerical aperture Thus when possible, use low magnification and high NA objectives © 1995 -2000 J. Paul Robinson - Purdue University Cytometry Laboratories Slide 23 t: /classes/BMS 524/524 lect 2. ppt
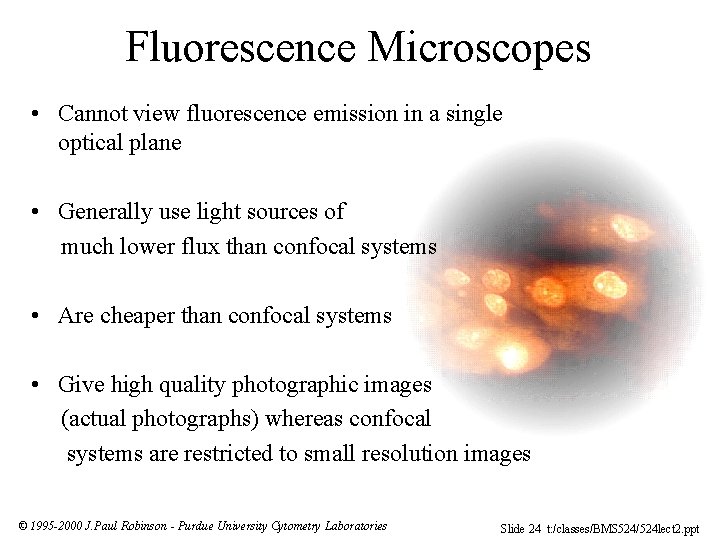
Fluorescence Microscopes • Cannot view fluorescence emission in a single optical plane • Generally use light sources of much lower flux than confocal systems • Are cheaper than confocal systems • Give high quality photographic images (actual photographs) whereas confocal systems are restricted to small resolution images © 1995 -2000 J. Paul Robinson - Purdue University Cytometry Laboratories Slide 24 t: /classes/BMS 524/524 lect 2. ppt
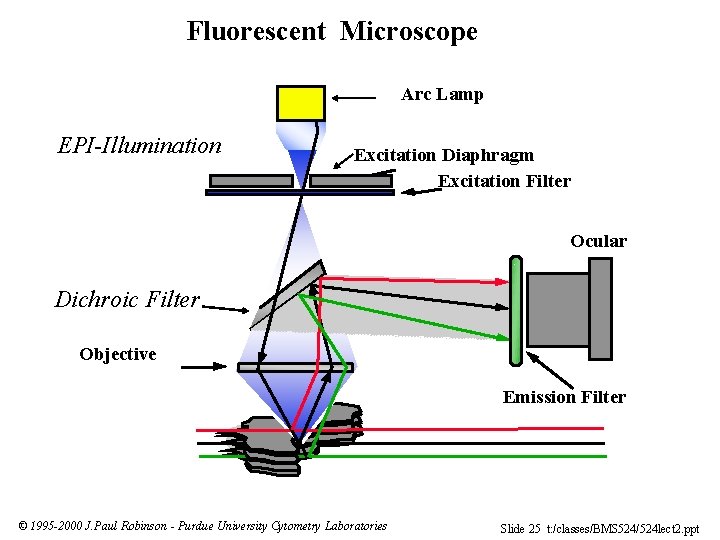
Fluorescent Microscope Arc Lamp EPI-Illumination Excitation Diaphragm Excitation Filter Ocular Dichroic Filter Objective Emission Filter © 1995 -2000 J. Paul Robinson - Purdue University Cytometry Laboratories Slide 25 t: /classes/BMS 524/524 lect 2. ppt
Interference in Thin Films • Small amounts of incident light are reflected at the interface between two material of different RI • Thickness of the material will alter the constructive or destructive interference patterns - increasing or decreasing certain wavelengths • Optical filters can thus be created that “interfere” with the normal transmission of light © 1995 -2000 J. Paul Robinson - Purdue University Cytometry Laboratories Slide 26 t: /classes/BMS 524/524 lect 2. ppt
Interference and Diffraction: Gratings • Diffraction essentially describes a departure from theoretical geometric optics • Thus a sharp objet casts an alternating shadow of light and dark “patterns” because of interference • Diffraction is the component that limits resolution © 1995 -2000 J. Paul Robinson - Purdue University Cytometry Laboratories Slide 27 t: /classes/BMS 524/524 lect 2. ppt
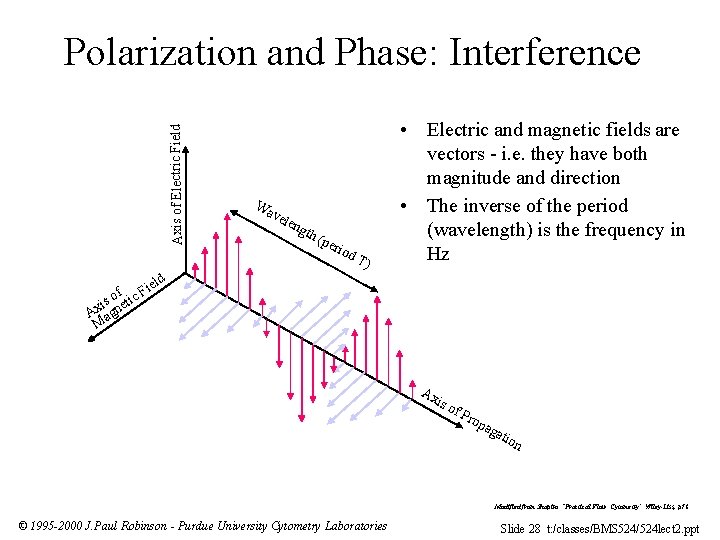
Axis of Electric Field Polarization and Phase: Interference i of tic F s i e Ax agn M Wa vel eng th ( per iod T) • Electric and magnetic fields are vectors - i. e. they have both magnitude and direction • The inverse of the period (wavelength) is the frequency in Hz eld Ax is o f. P rop aga tio n Modified from Shapiro “Practical Flow Cytometry” Wiley-Liss, p 78 © 1995 -2000 J. Paul Robinson - Purdue University Cytometry Laboratories Slide 28 t: /classes/BMS 524/524 lect 2. ppt
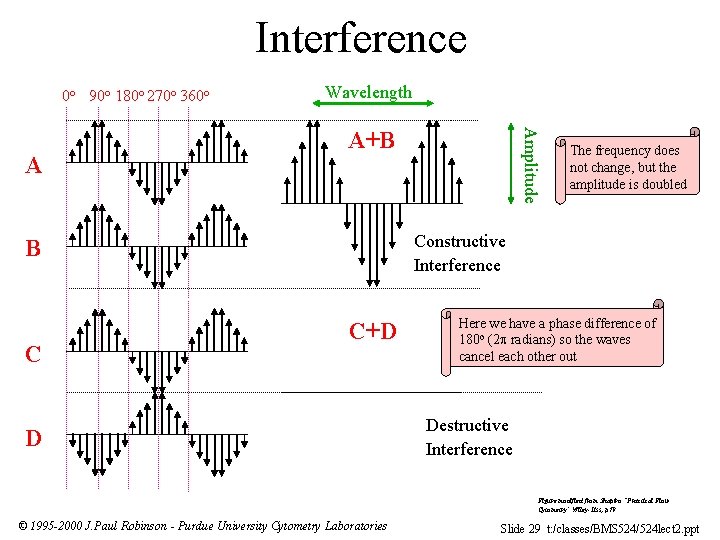
Interference 0 o 90 o 180 o 270 o 360 o A+B The frequency does not change, but the amplitude is doubled Constructive Interference B C Amplitude A Wavelength C+D D Here we have a phase difference of 180 o (2 radians) so the waves cancel each other out Destructive Interference Figure modified from Shapiro “Practical Flow Cytometry” Wiley-Liss, p 79 © 1995 -2000 J. Paul Robinson - Purdue University Cytometry Laboratories Slide 29 t: /classes/BMS 524/524 lect 2. ppt
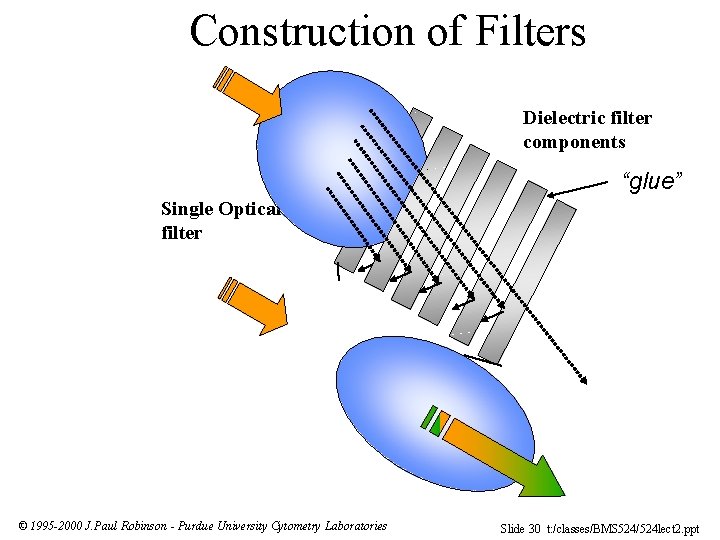
Construction of Filters Dielectric filter components “glue” Single Optical filter © 1995 -2000 J. Paul Robinson - Purdue University Cytometry Laboratories Slide 30 t: /classes/BMS 524/524 lect 2. ppt
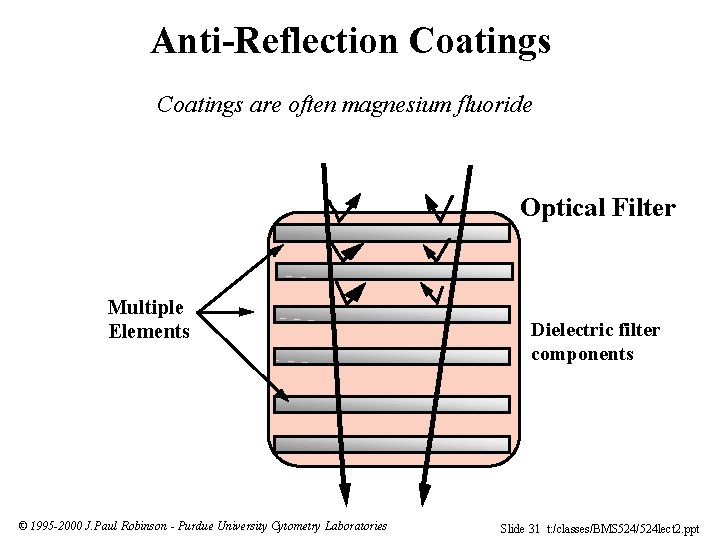
Anti-Reflection Coatings are often magnesium fluoride Optical Filter Multiple Elements © 1995 -2000 J. Paul Robinson - Purdue University Cytometry Laboratories Dielectric filter components Slide 31 t: /classes/BMS 524/524 lect 2. ppt

Standard Band Pass Filters 630 nm Band. Pass Filter White Light Source Transmitted Light 620 -640 nm Light © 1995 -2000 J. Paul Robinson - Purdue University Cytometry Laboratories Slide 32 t: /classes/BMS 524/524 lect 2. ppt
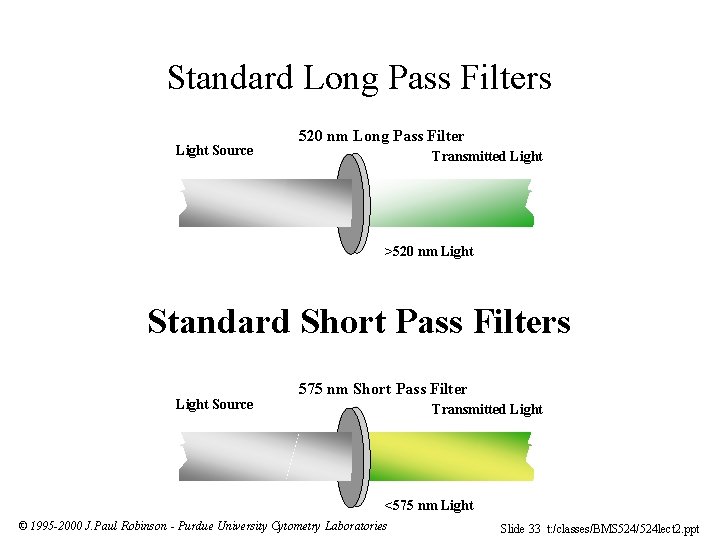
Standard Long Pass Filters Light Source 520 nm Long Pass Filter Transmitted Light >520 nm Light Standard Short Pass Filters Light Source 575 nm Short Pass Filter Transmitted Light <575 nm Light © 1995 -2000 J. Paul Robinson - Purdue University Cytometry Laboratories Slide 33 t: /classes/BMS 524/524 lect 2. ppt
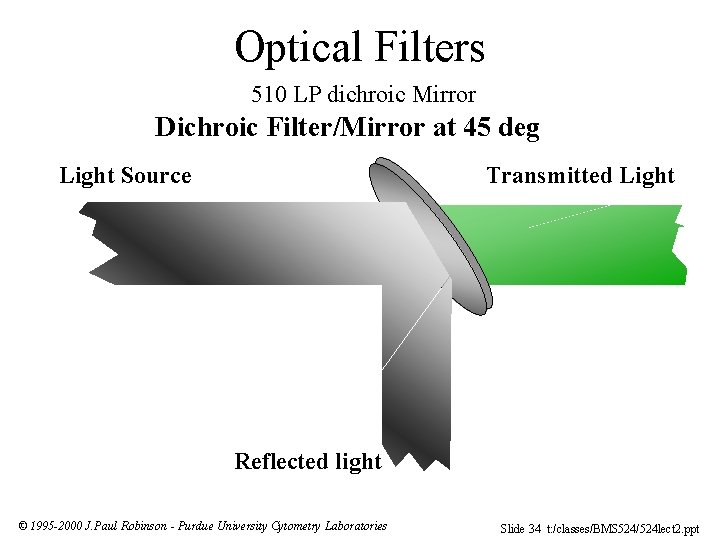
Optical Filters 510 LP dichroic Mirror Dichroic Filter/Mirror at 45 deg Light Source Transmitted Light Reflected light © 1995 -2000 J. Paul Robinson - Purdue University Cytometry Laboratories Slide 34 t: /classes/BMS 524/524 lect 2. ppt
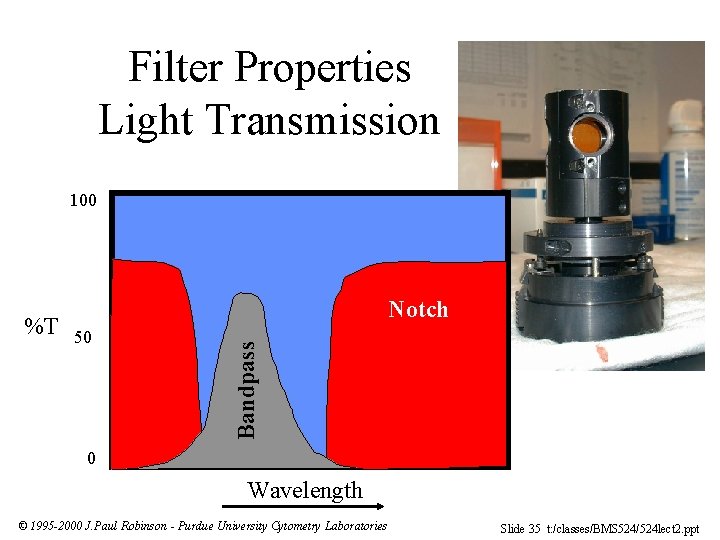
Filter Properties Light Transmission 100 50 Bandpass %T Notch 0 Wavelength © 1995 -2000 J. Paul Robinson - Purdue University Cytometry Laboratories Slide 35 t: /classes/BMS 524/524 lect 2. ppt

Summary Lecture 2 • • Parts of the microscope (ocular, condenser) Objectives Numerical Aperture (NA) Refractive Index/refraction (RI) Aberrations Fluorescence microscope Properties of optical filters © 1995 -2000 J. Paul Robinson - Purdue University Cytometry Laboratories Slide 36 t: /classes/BMS 524/524 lect 2. ppt
- Slides: 36