LECTURE 2 Structure and bonding in organic compounds

LECTURE 2

Structure and bonding in organic compounds
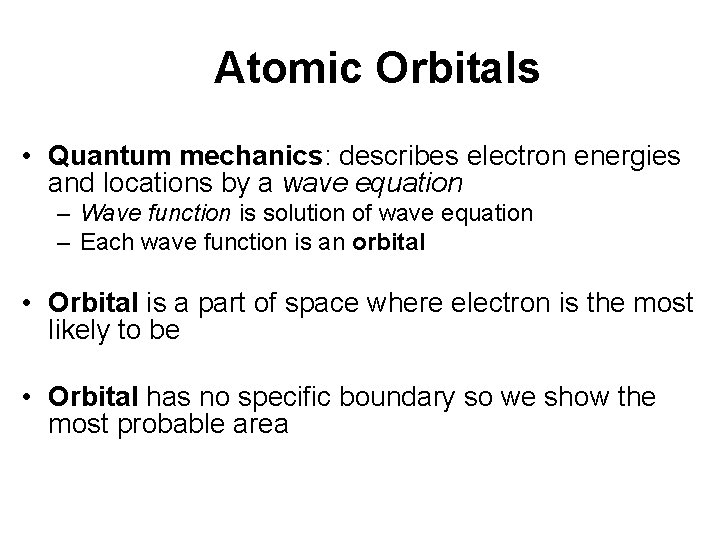
Atomic Orbitals • Quantum mechanics: describes electron energies and locations by a wave equation – Wave function is solution of wave equation – Each wave function is an orbital • Orbital is a part of space where electron is the most likely to be • Orbital has no specific boundary so we show the most probable area
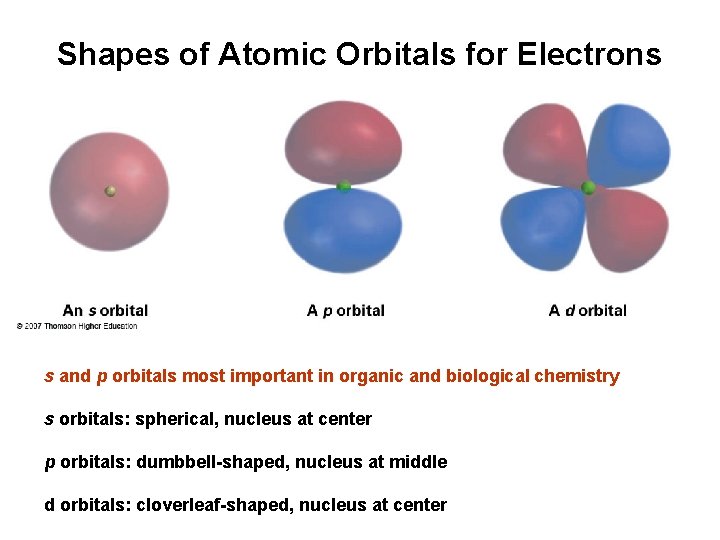
Shapes of Atomic Orbitals for Electrons s and p orbitals most important in organic and biological chemistry s orbitals: spherical, nucleus at center p orbitals: dumbbell-shaped, nucleus at middle d orbitals: cloverleaf-shaped, nucleus at center
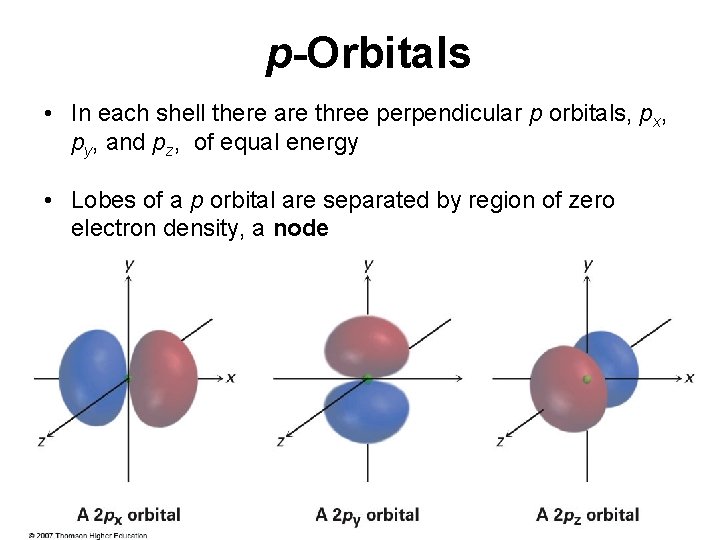
p-Orbitals • In each shell there are three perpendicular p orbitals, px, py, and pz, of equal energy • Lobes of a p orbital are separated by region of zero electron density, a node

Orbitals and Shells • Orbitals are grouped in shells of increasing size and energy • Different shells contain different numbers and kinds of orbitals • Each orbital can be occupied by maximum two electrons
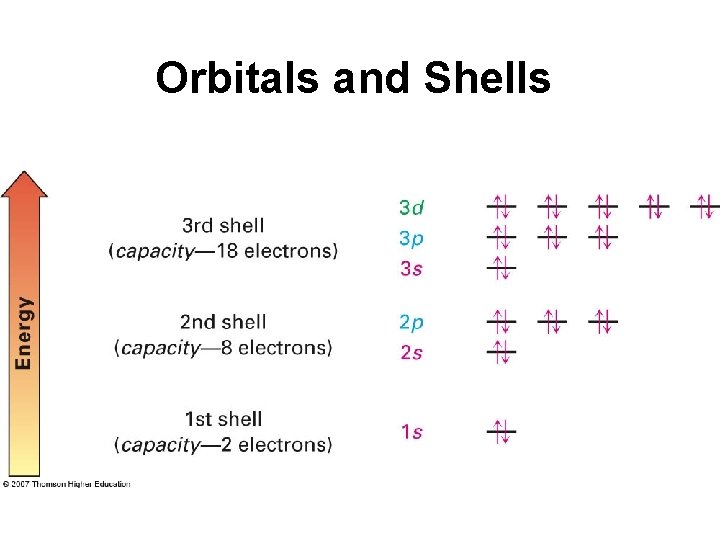
Orbitals and Shells

Orbitals and Shells • First shell contains one s orbital, denoted 1 s, holds only 2 electrons • Second shell contains one s orbital (2 s) and three p orbitals (2 p), capacity - 8 electrons • Third shell contains an s orbital (3 s), three p orbitals (3 p), and five d orbitals (3 d), capacity - 18 electrons
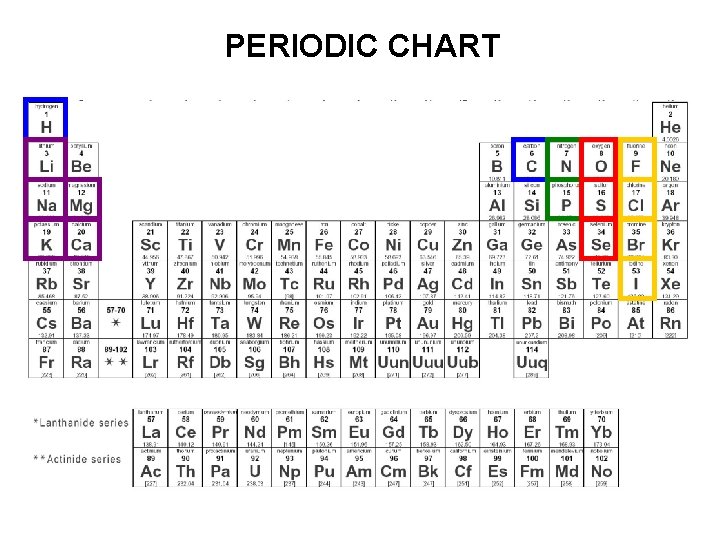
PERIODIC CHART
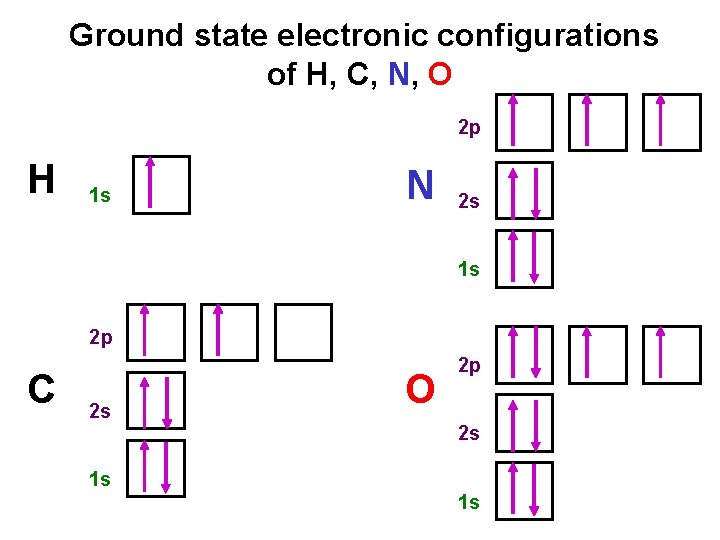
Ground state electronic configurations of H, C, N, O 2 p H 1 s N 2 s 1 s 2 p C 2 s O 2 p 2 s 1 s 1 s

• Atoms form bonds because the compound that results is more stable than the separate atoms • Ionic bonds in salts form as a result of electron transfer • Organic compounds have covalent bonds from sharing electrons (G. N. Lewis, 1916)
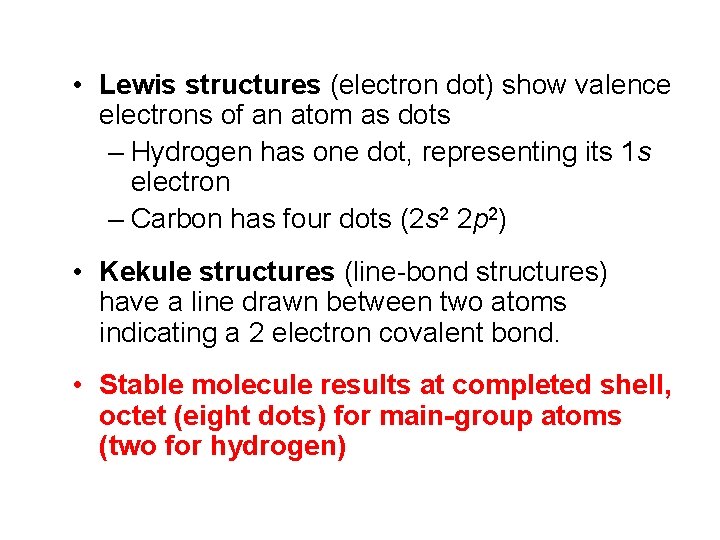
• Lewis structures (electron dot) show valence electrons of an atom as dots – Hydrogen has one dot, representing its 1 s electron – Carbon has four dots (2 s 2 2 p 2) • Kekule structures (line-bond structures) have a line drawn between two atoms indicating a 2 electron covalent bond. • Stable molecule results at completed shell, octet (eight dots) for main-group atoms (two for hydrogen)
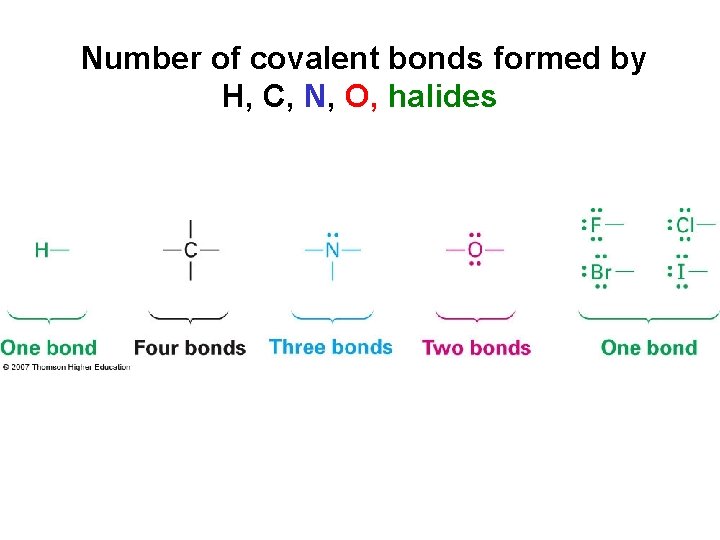
Number of covalent bonds formed by H, C, N, O, halides
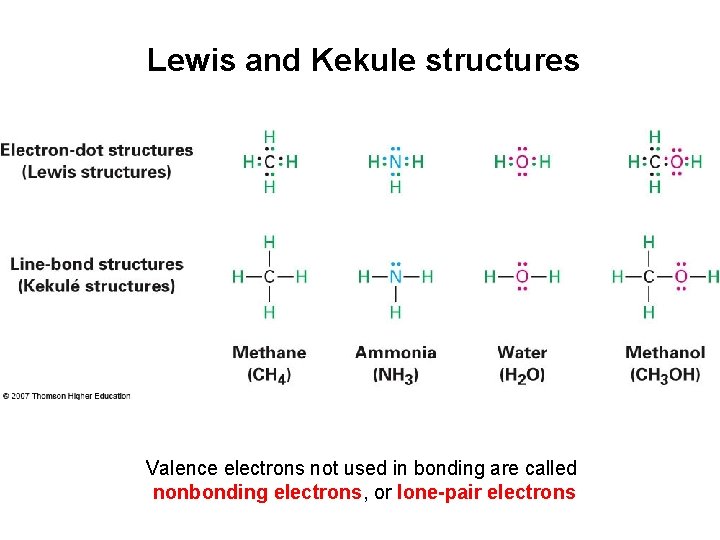
Lewis and Kekule structures Valence electrons not used in bonding are called nonbonding electrons, or lone-pair electrons
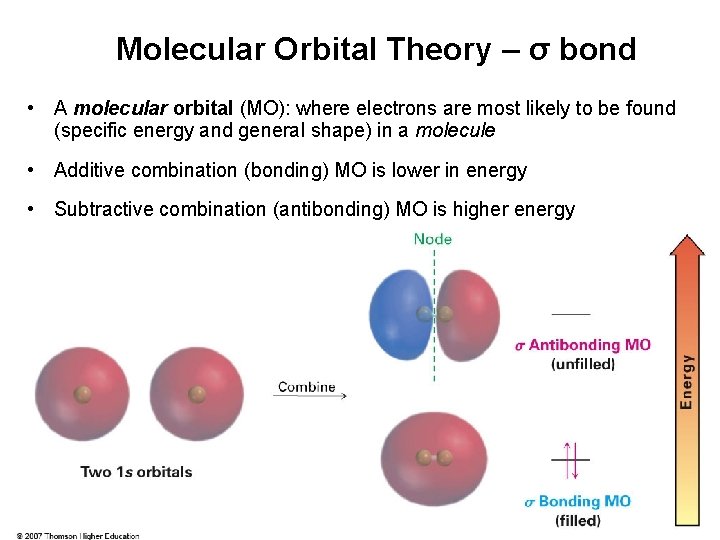
Molecular Orbital Theory – σ bond • A molecular orbital (MO): where electrons are most likely to be found (specific energy and general shape) in a molecule • Additive combination (bonding) MO is lower in energy • Subtractive combination (antibonding) MO is higher energy
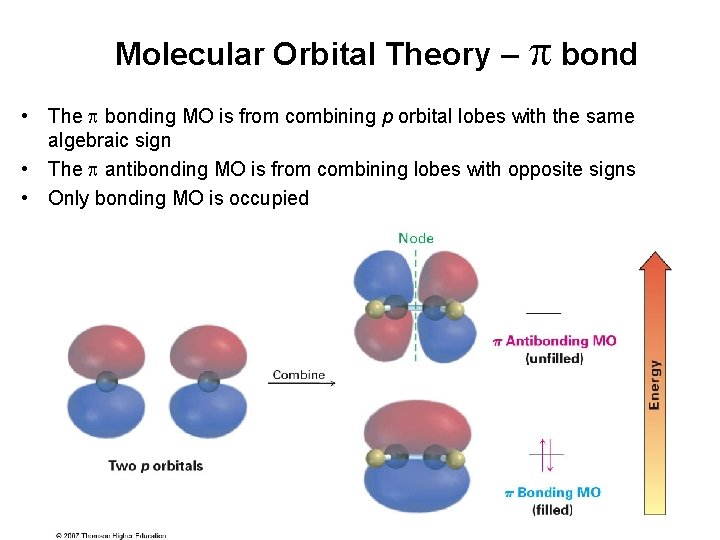
Molecular Orbital Theory – bond • The bonding MO is from combining p orbital lobes with the same algebraic sign • The antibonding MO is from combining lobes with opposite signs • Only bonding MO is occupied
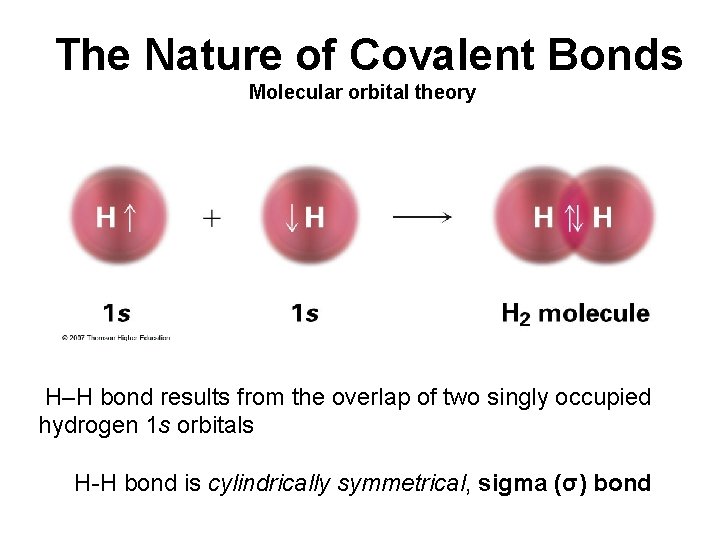
The Nature of Covalent Bonds Molecular orbital theory H–H bond results from the overlap of two singly occupied hydrogen 1 s orbitals H-H bond is cylindrically symmetrical, sigma (σ) bond
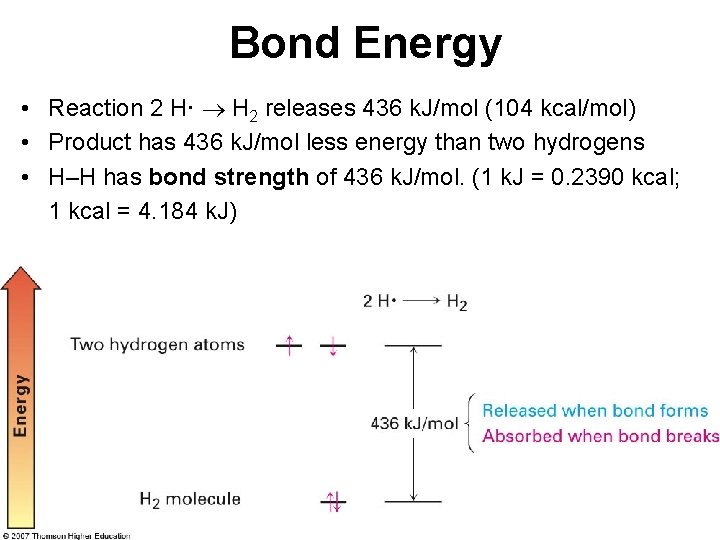
Bond Energy • Reaction 2 H· H 2 releases 436 k. J/mol (104 kcal/mol) • Product has 436 k. J/mol less energy than two hydrogens • H–H has bond strength of 436 k. J/mol. (1 k. J = 0. 2390 kcal; 1 kcal = 4. 184 k. J)
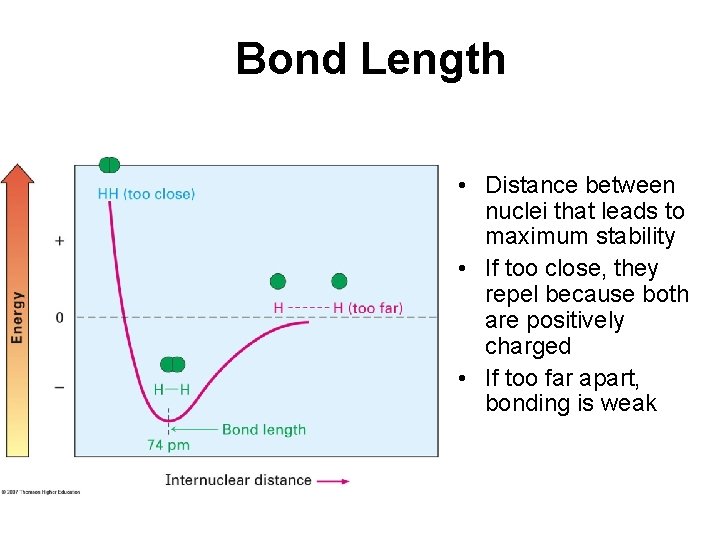
Bond Length • Distance between nuclei that leads to maximum stability • If too close, they repel because both are positively charged • If too far apart, bonding is weak
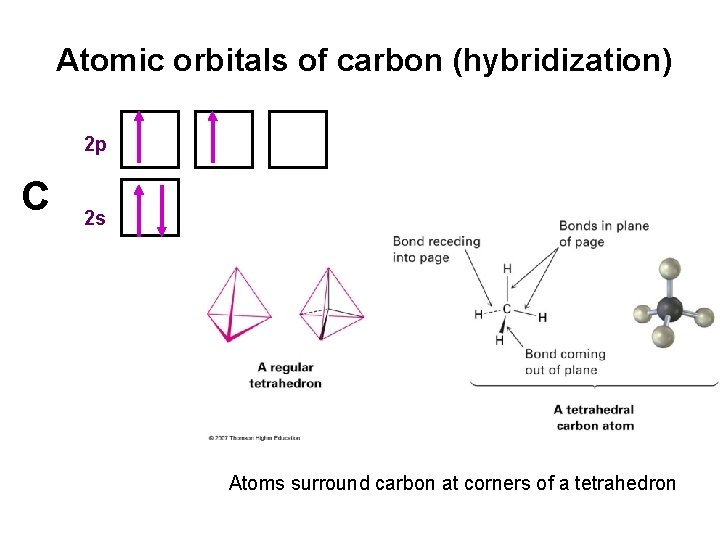
Atomic orbitals of carbon (hybridization) 2 p C 2 s Atoms surround carbon at corners of a tetrahedron
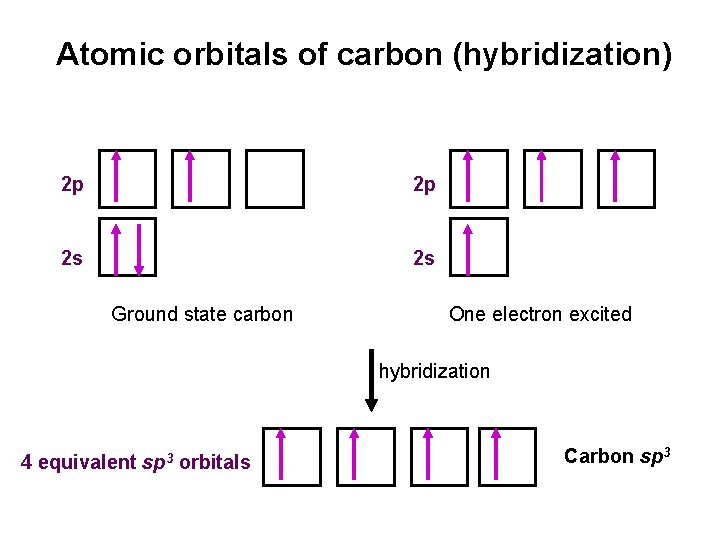
Atomic orbitals of carbon (hybridization) 2 p 2 p 2 s 2 s Ground state carbon One electron excited hybridization 4 equivalent sp 3 orbitals Carbon sp 3
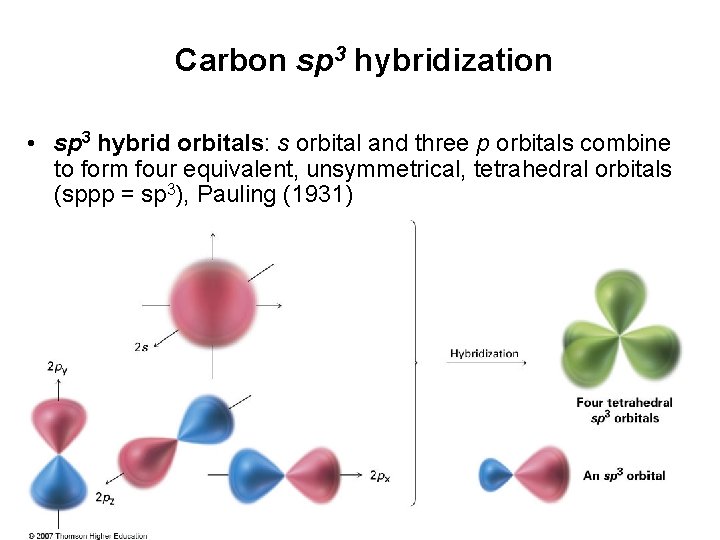
Carbon sp 3 hybridization • sp 3 hybrid orbitals: s orbital and three p orbitals combine to form four equivalent, unsymmetrical, tetrahedral orbitals (sppp = sp 3), Pauling (1931)
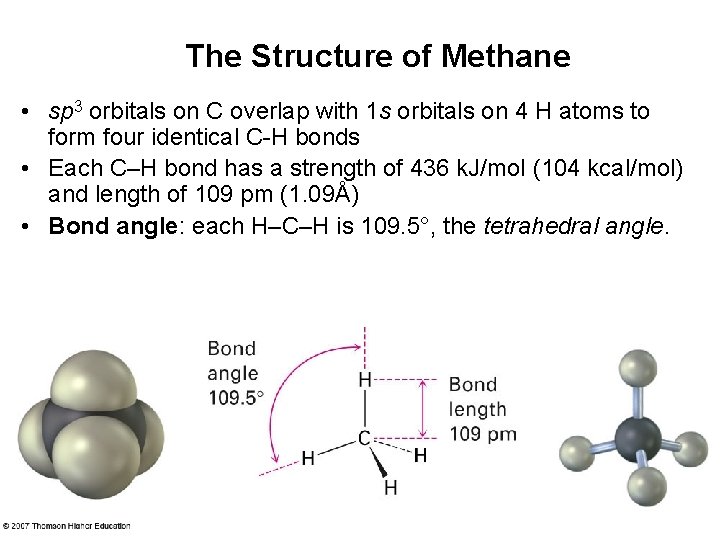
The Structure of Methane • sp 3 orbitals on C overlap with 1 s orbitals on 4 H atoms to form four identical C-H bonds • Each C–H bond has a strength of 436 k. J/mol (104 kcal/mol) and length of 109 pm (1. 09Å) • Bond angle: each H–C–H is 109. 5°, the tetrahedral angle.
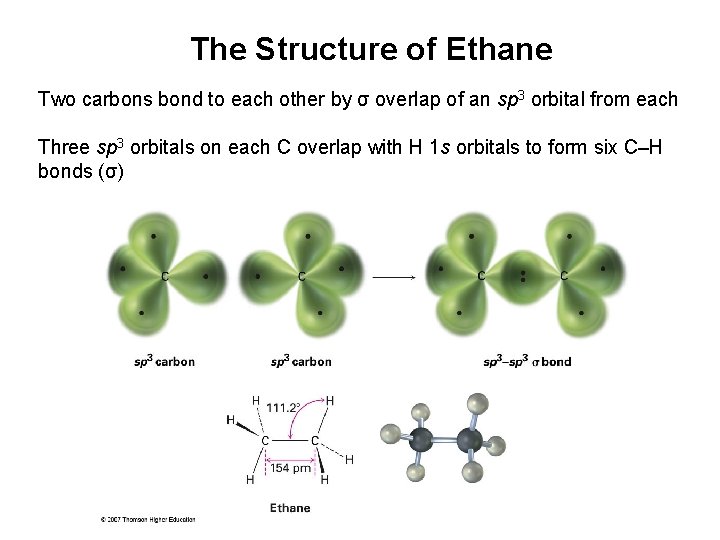
The Structure of Ethane Two carbons bond to each other by σ overlap of an sp 3 orbital from each Three sp 3 orbitals on each C overlap with H 1 s orbitals to form six C–H bonds (σ)
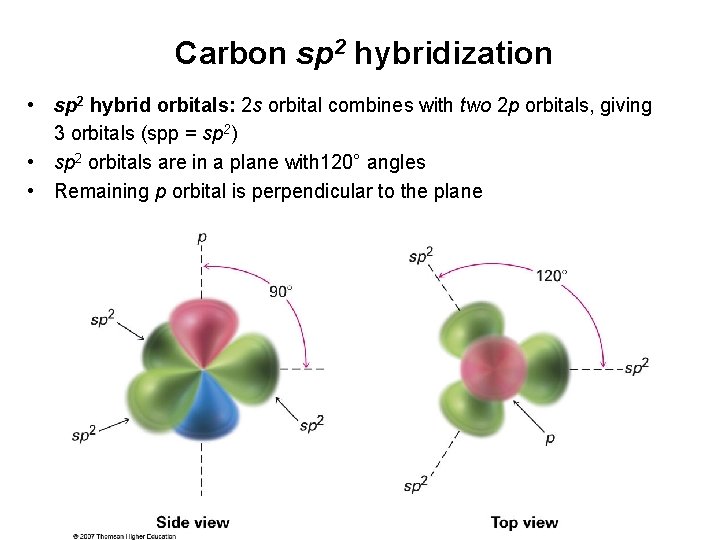
Carbon sp 2 hybridization • sp 2 hybrid orbitals: 2 s orbital combines with two 2 p orbitals, giving 3 orbitals (spp = sp 2) • sp 2 orbitals are in a plane with 120° angles • Remaining p orbital is perpendicular to the plane
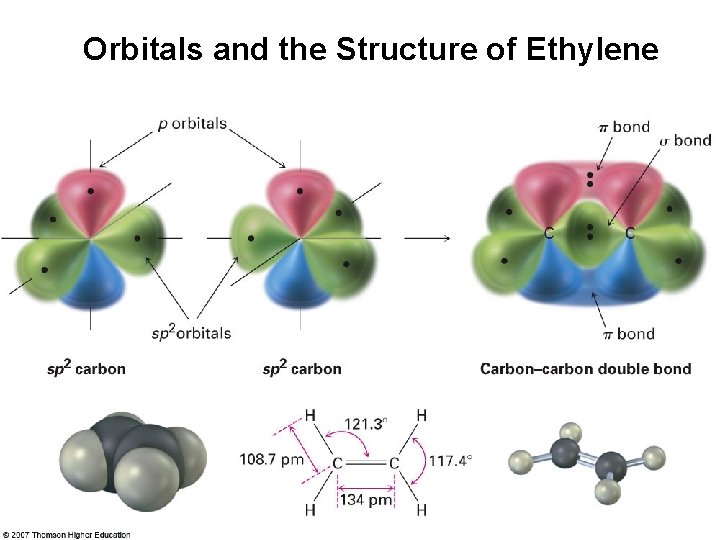
Orbitals and the Structure of Ethylene
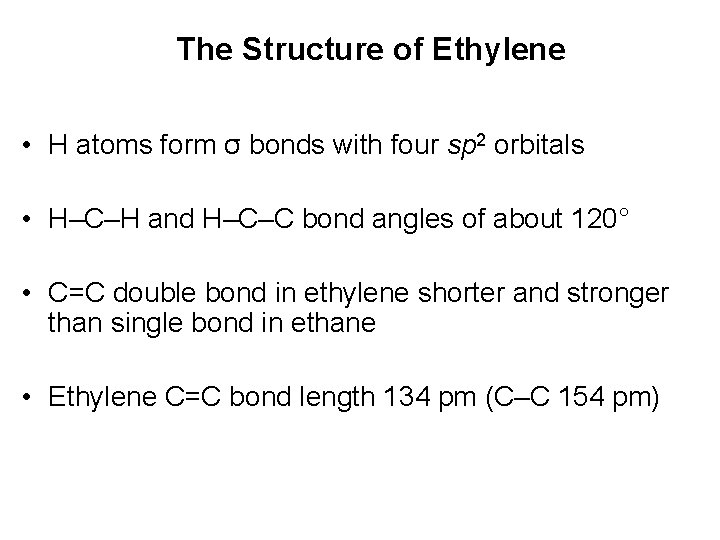
The Structure of Ethylene • H atoms form σ bonds with four sp 2 orbitals • H–C–H and H–C–C bond angles of about 120° • C=C double bond in ethylene shorter and stronger than single bond in ethane • Ethylene C=C bond length 134 pm (C–C 154 pm)
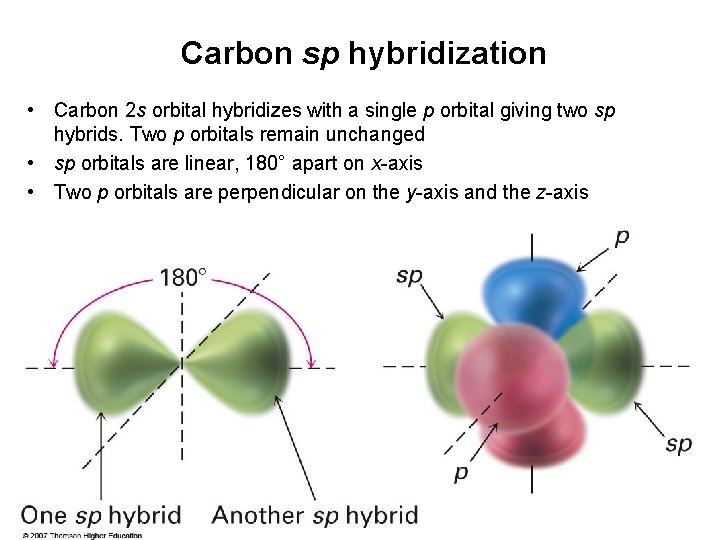
Carbon sp hybridization • Carbon 2 s orbital hybridizes with a single p orbital giving two sp hybrids. Two p orbitals remain unchanged • sp orbitals are linear, 180° apart on x-axis • Two p orbitals are perpendicular on the y-axis and the z-axis
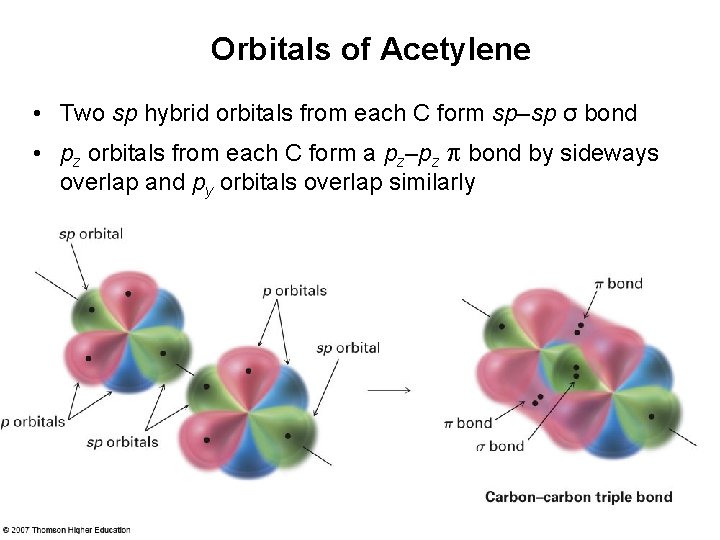
Orbitals of Acetylene • Two sp hybrid orbitals from each C form sp–sp σ bond • pz orbitals from each C form a pz–pz bond by sideways overlap and py orbitals overlap similarly
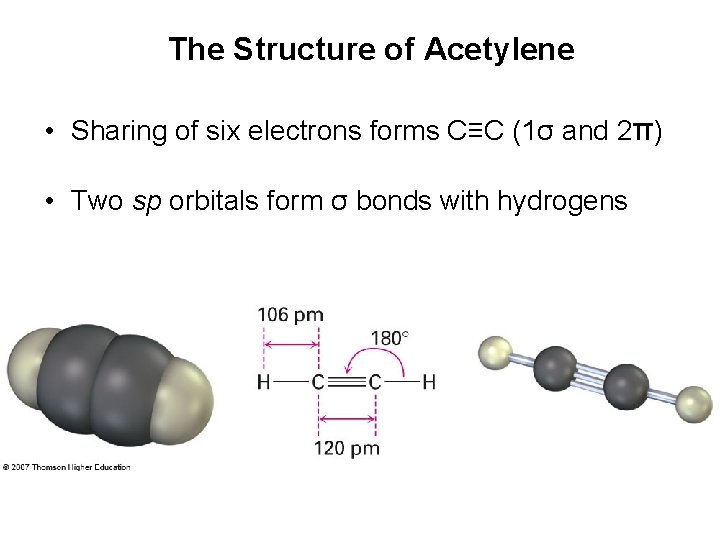
The Structure of Acetylene • Sharing of six electrons forms C≡C (1σ and 2π) • Two sp orbitals form σ bonds with hydrogens
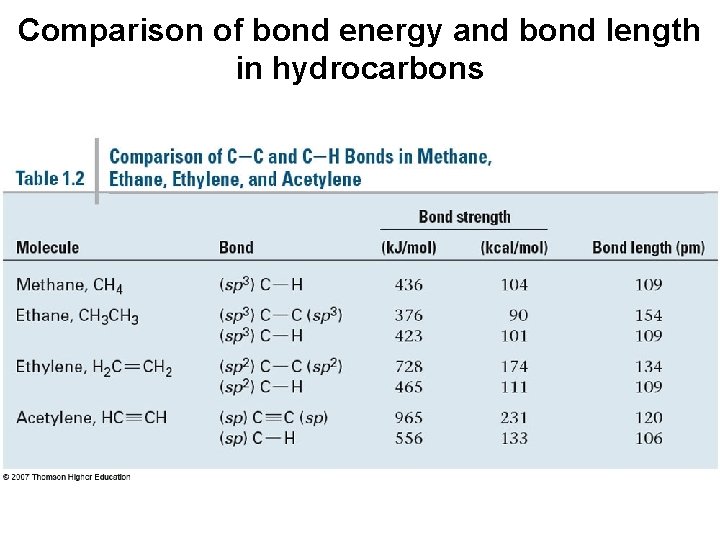
Comparison of bond energy and bond length in hydrocarbons
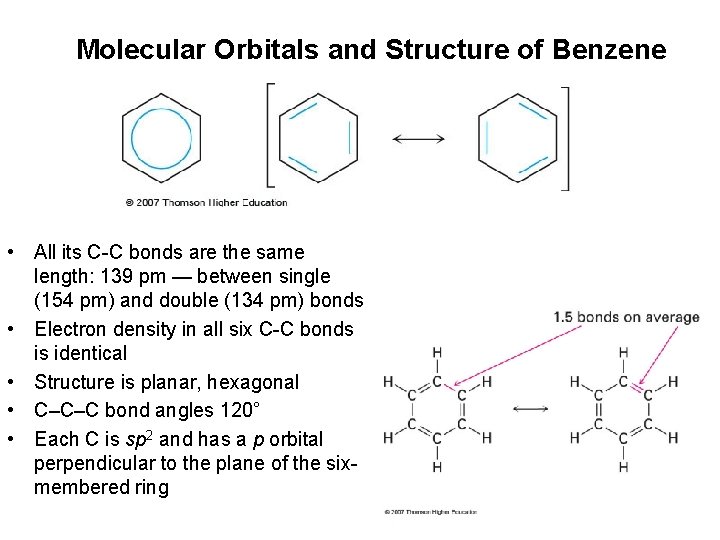
Molecular Orbitals and Structure of Benzene • All its C-C bonds are the same length: 139 pm — between single (154 pm) and double (134 pm) bonds • Electron density in all six C-C bonds is identical • Structure is planar, hexagonal • C–C–C bond angles 120° • Each C is sp 2 and has a p orbital perpendicular to the plane of the sixmembered ring
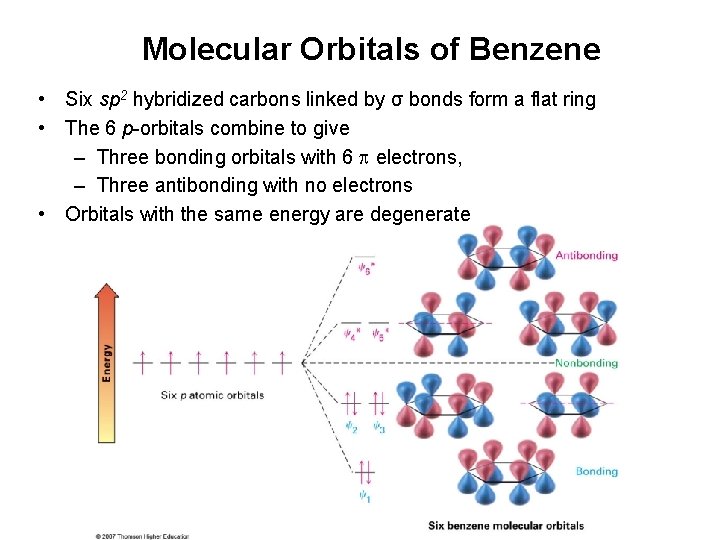
Molecular Orbitals of Benzene • Six sp 2 hybridized carbons linked by σ bonds form a flat ring • The 6 p-orbitals combine to give – Three bonding orbitals with 6 electrons, – Three antibonding with no electrons • Orbitals with the same energy are degenerate
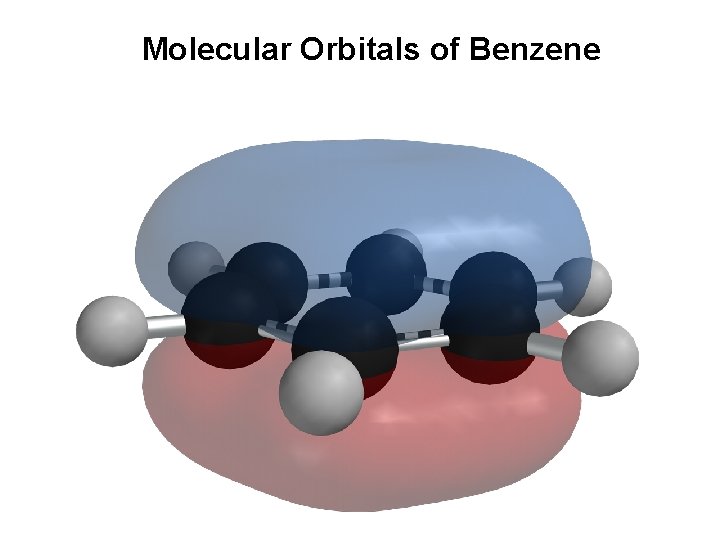
Molecular Orbitals of Benzene
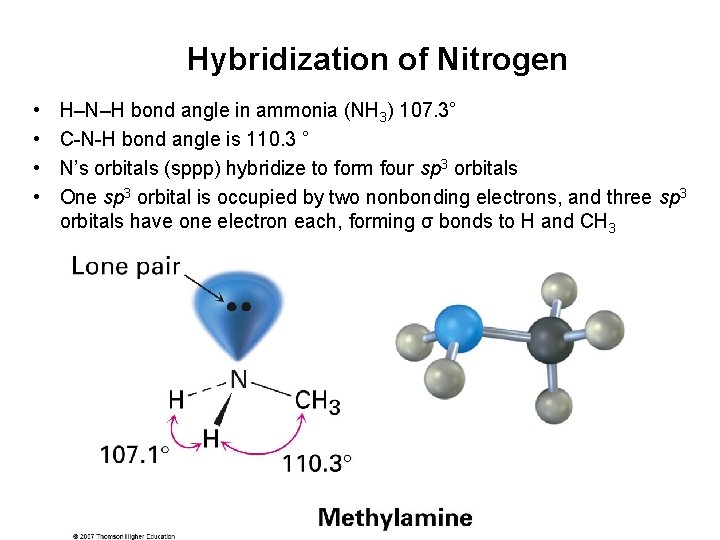
Hybridization of Nitrogen • • H–N–H bond angle in ammonia (NH 3) 107. 3° C-N-H bond angle is 110. 3 ° N’s orbitals (sppp) hybridize to form four sp 3 orbitals One sp 3 orbital is occupied by two nonbonding electrons, and three sp 3 orbitals have one electron each, forming σ bonds to H and CH 3
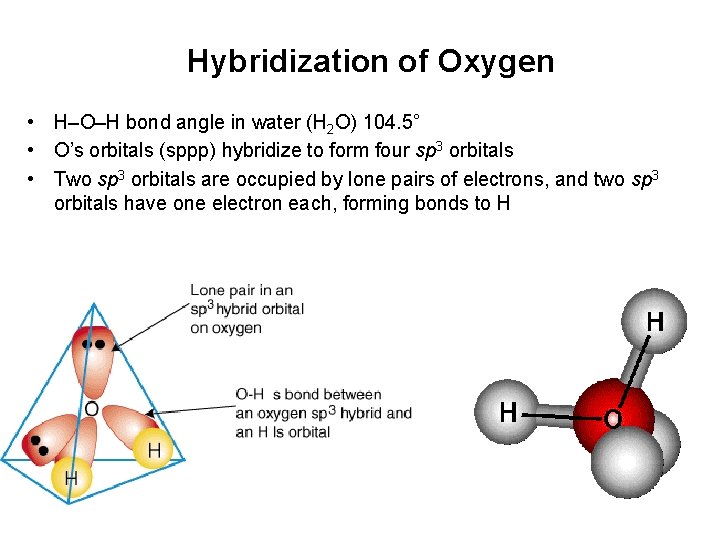
Hybridization of Oxygen • H–O–H bond angle in water (H 2 O) 104. 5° • O’s orbitals (sppp) hybridize to form four sp 3 orbitals • Two sp 3 orbitals are occupied by lone pairs of electrons, and two sp 3 orbitals have one electron each, forming bonds to H
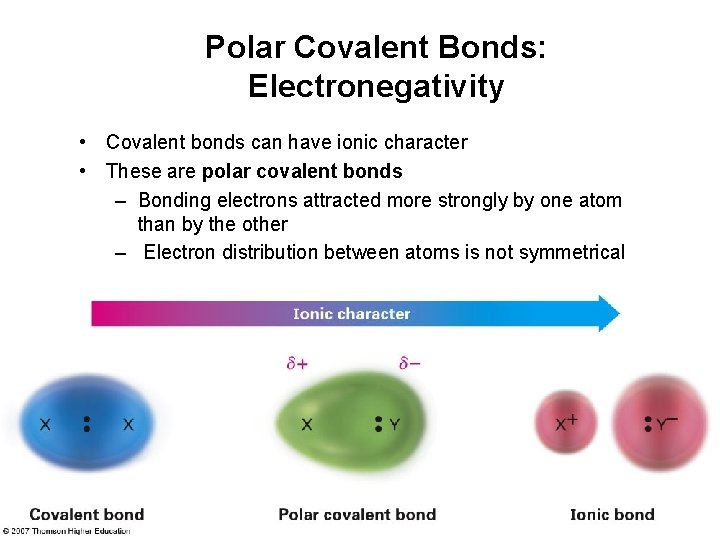
Polar Covalent Bonds: Electronegativity • Covalent bonds can have ionic character • These are polar covalent bonds – Bonding electrons attracted more strongly by one atom than by the other – Electron distribution between atoms is not symmetrical
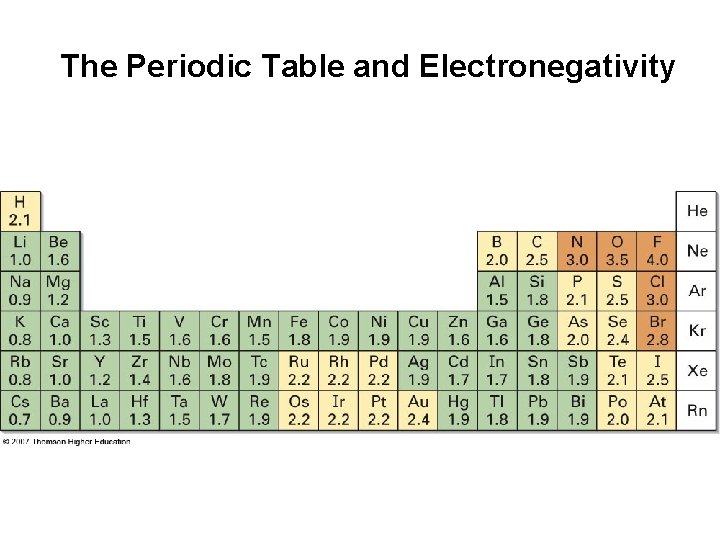
The Periodic Table and Electronegativity
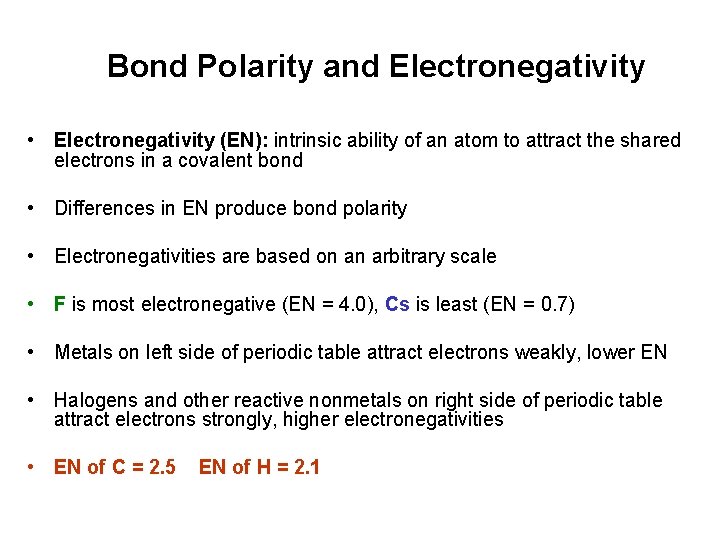
Bond Polarity and Electronegativity • Electronegativity (EN): intrinsic ability of an atom to attract the shared electrons in a covalent bond • Differences in EN produce bond polarity • Electronegativities are based on an arbitrary scale • F is most electronegative (EN = 4. 0), Cs is least (EN = 0. 7) • Metals on left side of periodic table attract electrons weakly, lower EN • Halogens and other reactive nonmetals on right side of periodic table attract electrons strongly, higher electronegativities • EN of C = 2. 5 EN of H = 2. 1
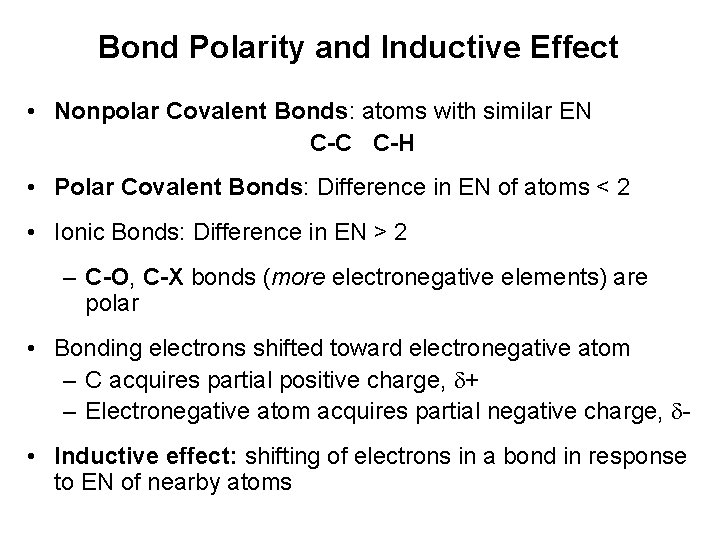
Bond Polarity and Inductive Effect • Nonpolar Covalent Bonds: atoms with similar EN C-C C-H • Polar Covalent Bonds: Difference in EN of atoms < 2 • Ionic Bonds: Difference in EN > 2 – C-O, C-X bonds (more electronegative elements) are polar • Bonding electrons shifted toward electronegative atom – C acquires partial positive charge, + – Electronegative atom acquires partial negative charge, • Inductive effect: shifting of electrons in a bond in response to EN of nearby atoms
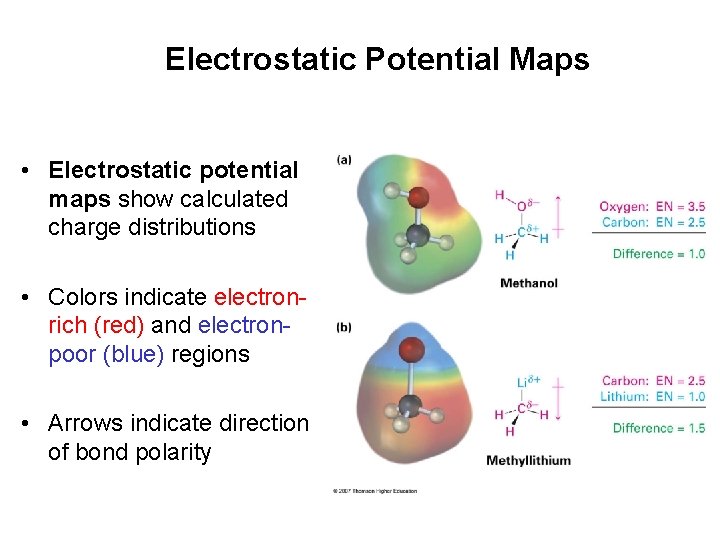
Electrostatic Potential Maps • Electrostatic potential maps show calculated charge distributions • Colors indicate electronrich (red) and electronpoor (blue) regions • Arrows indicate direction of bond polarity
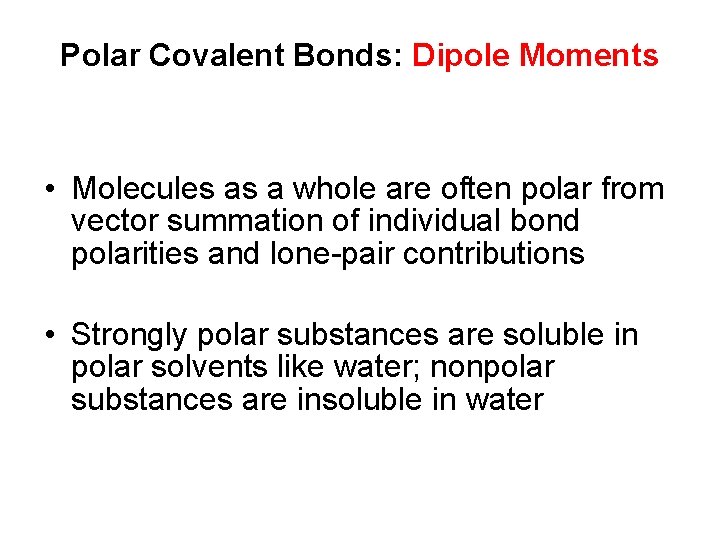
Polar Covalent Bonds: Dipole Moments • Molecules as a whole are often polar from vector summation of individual bond polarities and lone-pair contributions • Strongly polar substances are soluble in polar solvents like water; nonpolar substances are insoluble in water
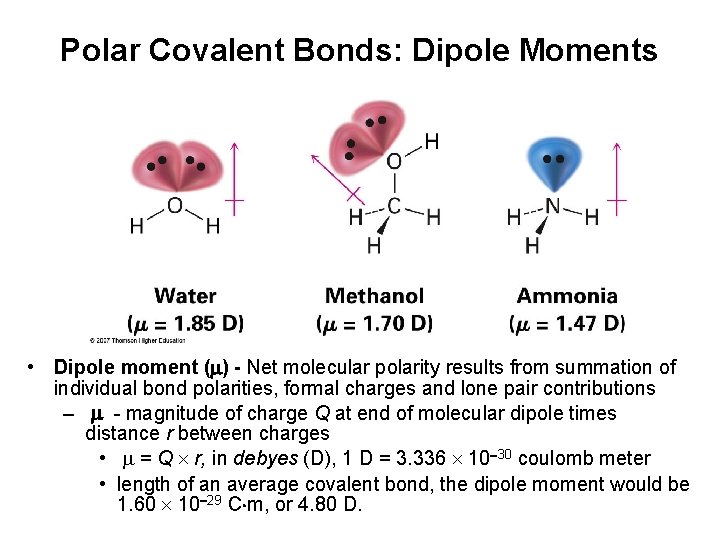
Polar Covalent Bonds: Dipole Moments • Dipole moment ( ) - Net molecular polarity results from summation of individual bond polarities, formal charges and lone pair contributions – - magnitude of charge Q at end of molecular dipole times distance r between charges • = Q r, in debyes (D), 1 D = 3. 336 10 30 coulomb meter • length of an average covalent bond, the dipole moment would be 1. 60 10 29 C m, or 4. 80 D.
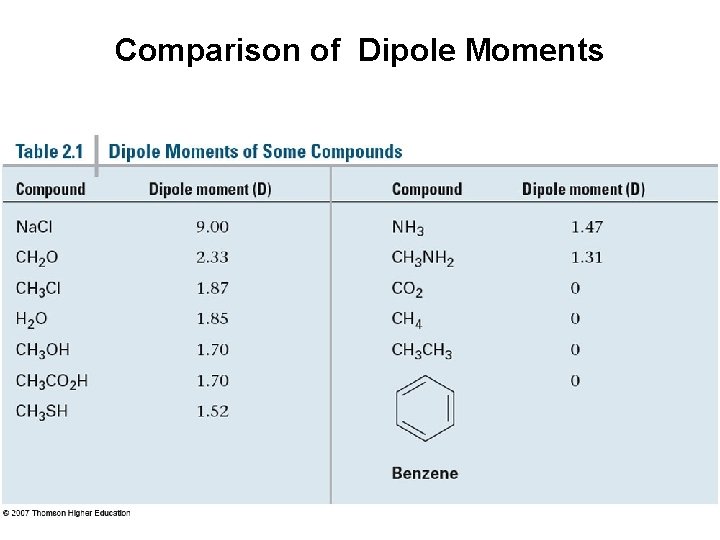
Comparison of Dipole Moments
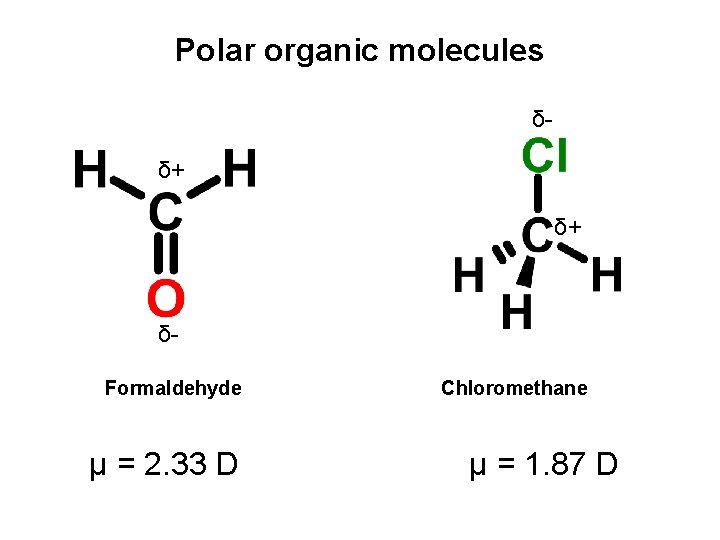
Polar organic molecules δδ+ δ+ δFormaldehyde μ = 2. 33 D Chloromethane μ = 1. 87 D
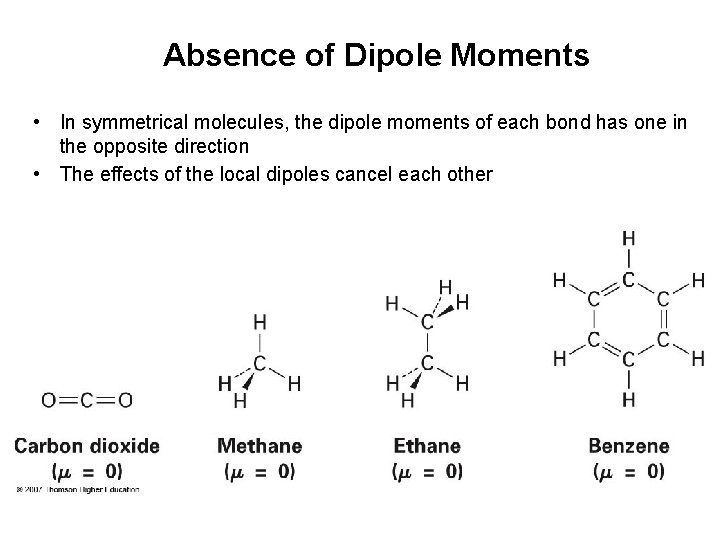
Absence of Dipole Moments • In symmetrical molecules, the dipole moments of each bond has one in the opposite direction • The effects of the local dipoles cancel each other
Noncovalent Interactions (Intermolecular weak forces) - Dipole-dipole forces - Dispersion forces - Hydrogen bonds
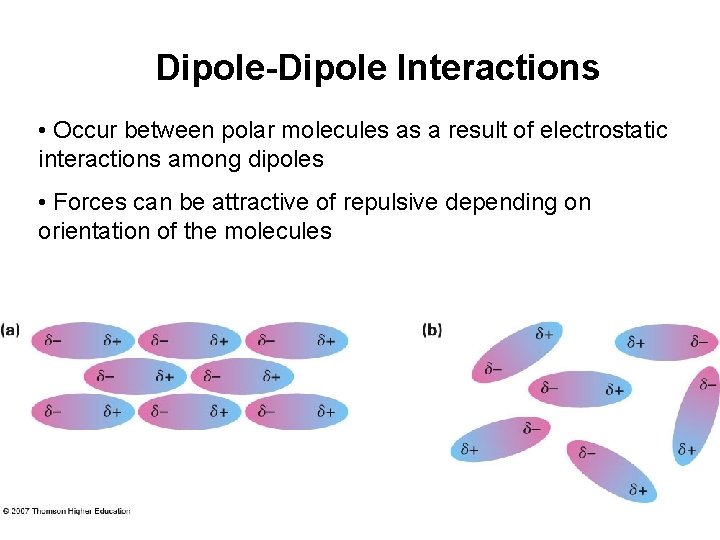
Dipole-Dipole Interactions • Occur between polar molecules as a result of electrostatic interactions among dipoles • Forces can be attractive of repulsive depending on orientation of the molecules
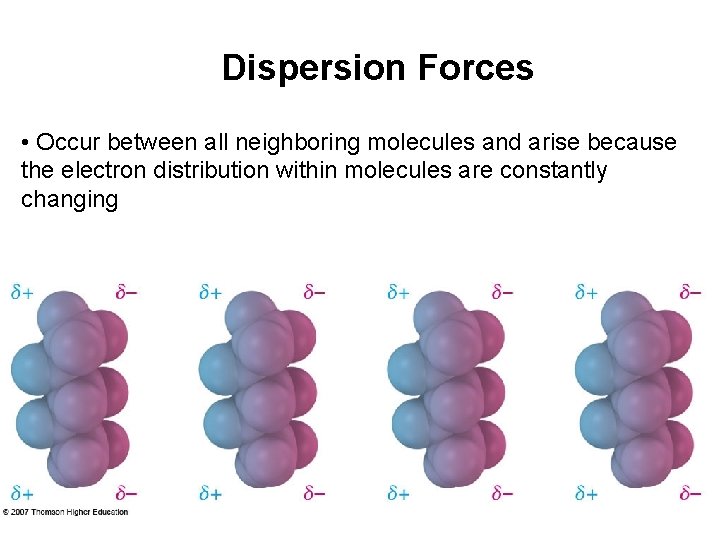
Dispersion Forces • Occur between all neighboring molecules and arise because the electron distribution within molecules are constantly changing
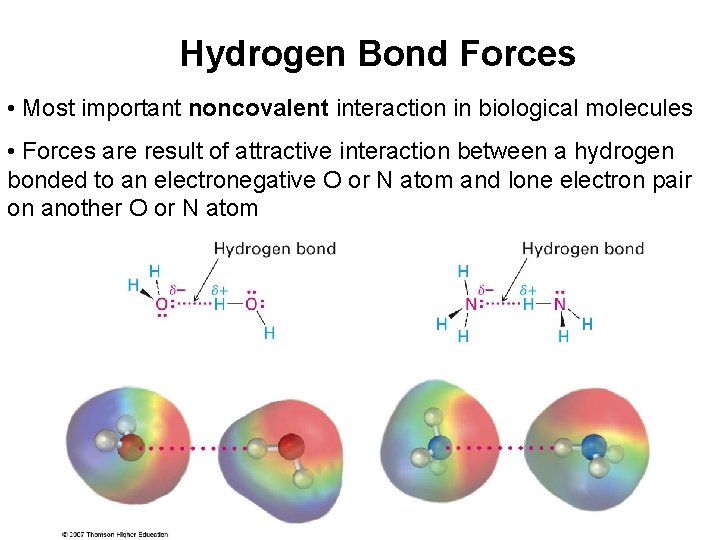
Hydrogen Bond Forces • Most important noncovalent interaction in biological molecules • Forces are result of attractive interaction between a hydrogen bonded to an electronegative O or N atom and lone electron pair on another O or N atom
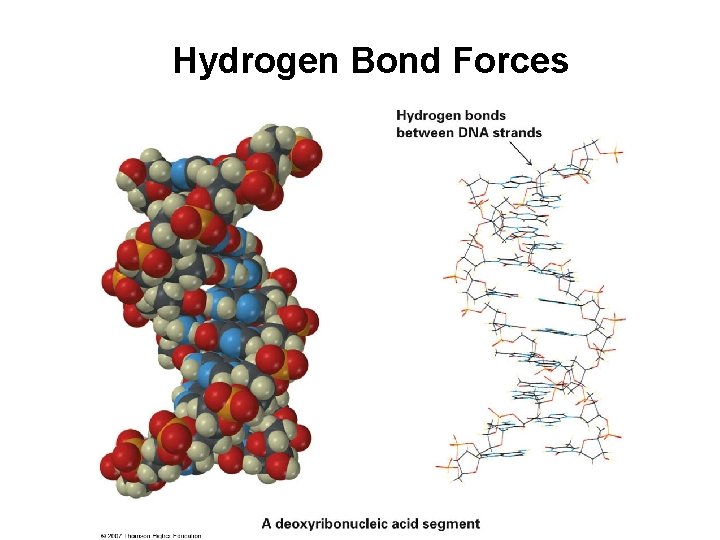
Hydrogen Bond Forces
Acids and Bases: The Brønsted–Lowry Definition • “Brønsted-Lowry” is usually shortened to “Brønsted” • A Brønsted acid is a substance that donates a proton (H+) in the reaction (it is proton donor) • A Brønsted base is a substance that accepts a proton (H+) in the reaction (it is proton acceptor) – “proton” is a synonym for H+ - loss of an electron from hydrogen atom (H) leaving the bare nucleus - a proton
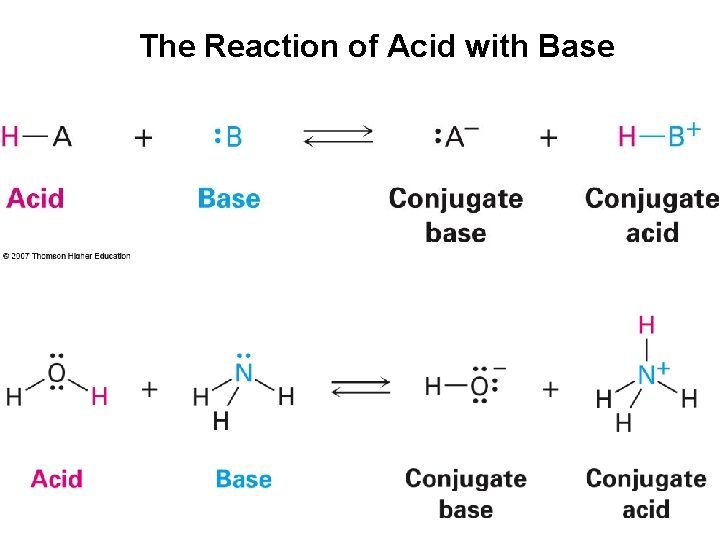
The Reaction of Acid with Base
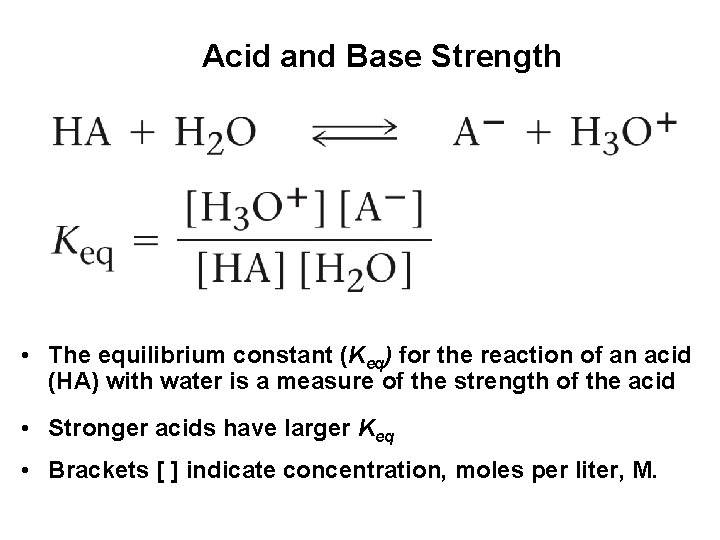
Acid and Base Strength • The equilibrium constant (Keq) for the reaction of an acid (HA) with water is a measure of the strength of the acid • Stronger acids have larger Keq • Brackets [ ] indicate concentration, moles per liter, M.
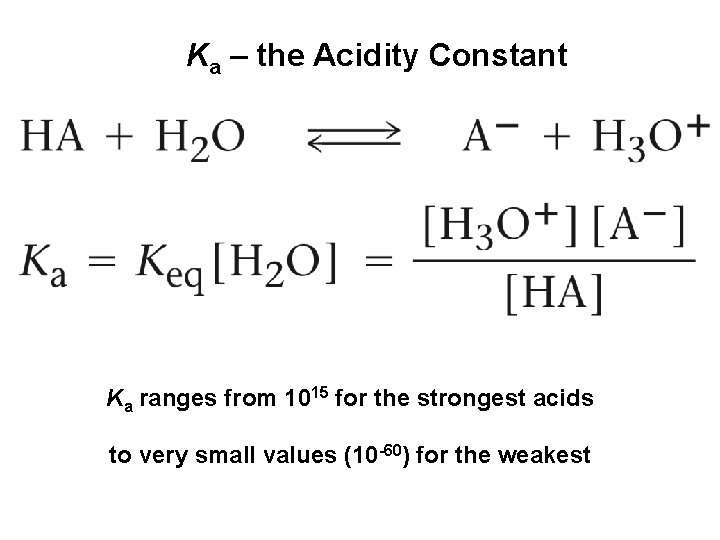
Ka – the Acidity Constant Ka ranges from 1015 for the strongest acids to very small values (10 -60) for the weakest
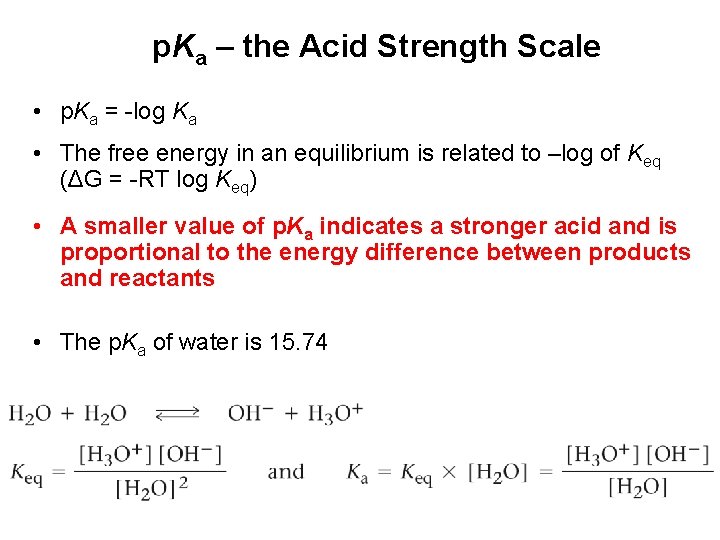
p. Ka – the Acid Strength Scale • p. Ka = -log Ka • The free energy in an equilibrium is related to –log of Keq (ΔG = -RT log Keq) • A smaller value of p. Ka indicates a stronger acid and is proportional to the energy difference between products and reactants • The p. Ka of water is 15. 74
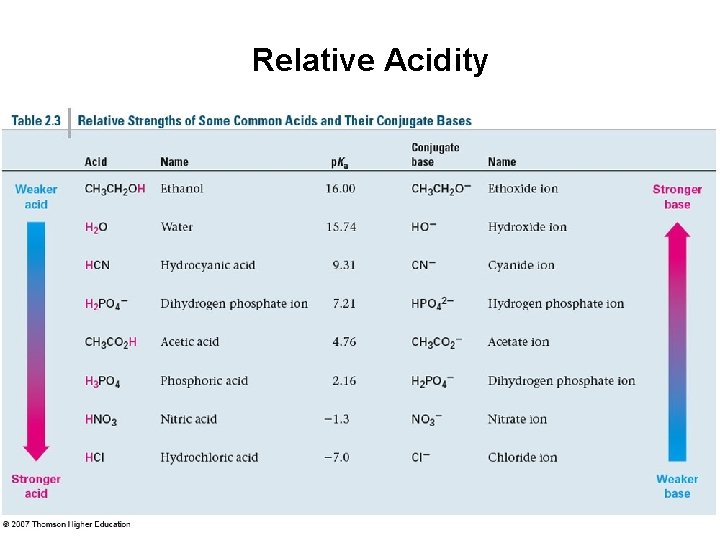
Relative Acidity
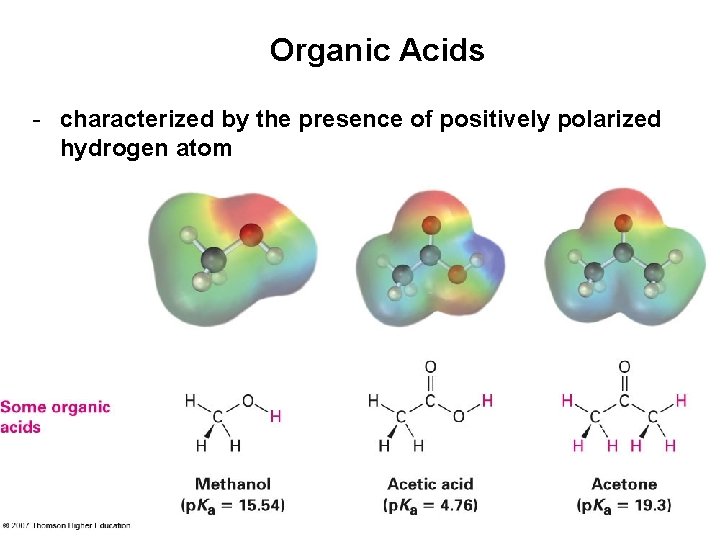
Organic Acids - characterized by the presence of positively polarized hydrogen atom
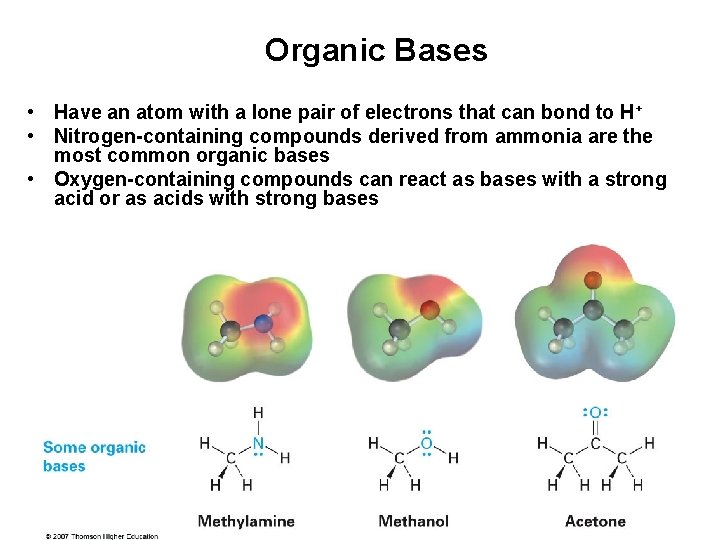
Organic Bases • Have an atom with a lone pair of electrons that can bond to H + • Nitrogen-containing compounds derived from ammonia are the most common organic bases • Oxygen-containing compounds can react as bases with a strong acid or as acids with strong bases
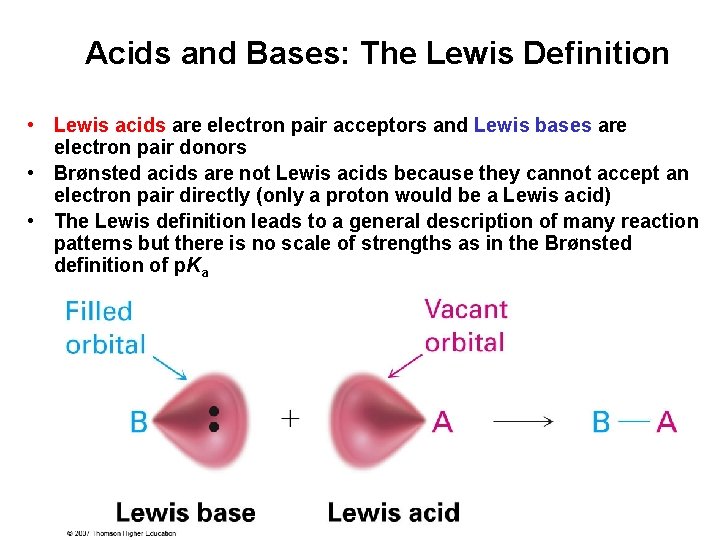
Acids and Bases: The Lewis Definition • Lewis acids are electron pair acceptors and Lewis bases are electron pair donors • Brønsted acids are not Lewis acids because they cannot accept an electron pair directly (only a proton would be a Lewis acid) • The Lewis definition leads to a general description of many reaction patterns but there is no scale of strengths as in the Brønsted definition of p. Ka

Lewis Acids (electrophiles) • The Lewis definition of acidity includes metal cations, such as Mg 2+ – They accept a pair of electrons when they form a bond to a base • Compounds such as BF 3 and Al. Cl 3, are Lewis acids because they have unfilled valence orbitals and can accept electron pairs from Lewis bases • Transition-metal compounds, such as Ti. Cl 4, Fe. Cl 3, Zn. Cl 2, and Sn. Cl 4, are Lewis acids • Organic compounds that undergo addition reactions with Lewis bases are called electrophiles or Lewis Acids • The combination of a Lewis acid and a Lewis base can be shown with a curved arrow from base to acid
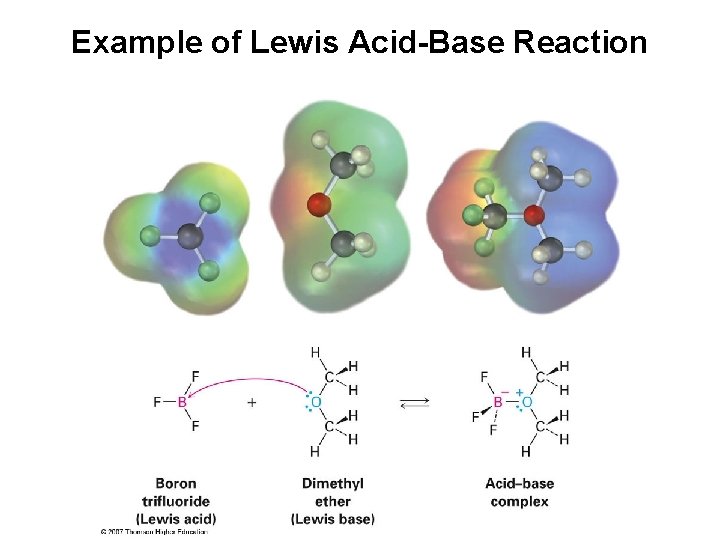
Example of Lewis Acid-Base Reaction

Lewis Bases (nucleophiles) • Lewis bases can accept protons as well as Lewis acids, therefore the definition encompasses that for Brønsted bases • Most oxygen- and nitrogen-containing organic compounds are Lewis bases because they have lone pairs of electrons • Some compounds can act as both acids and bases, depending on the reaction
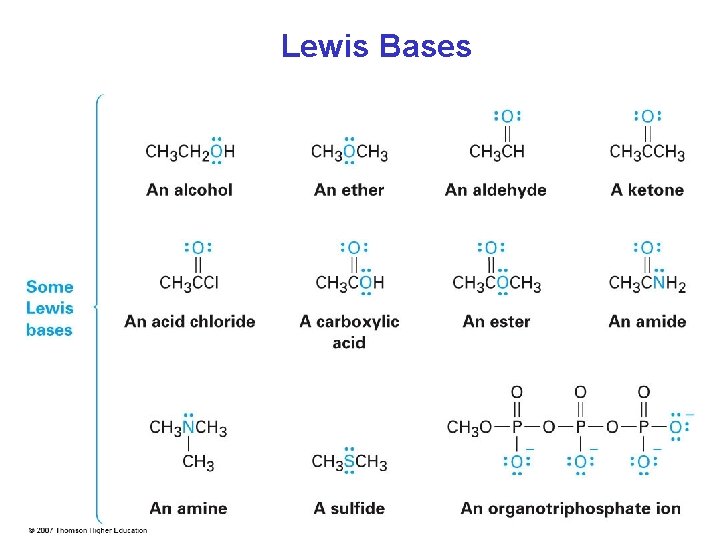
Lewis Bases
- Slides: 64