Lecture 2 Radioactive Decay Kinetics Basic Decay Equations

Lecture 2 Radioactive Decay Kinetics
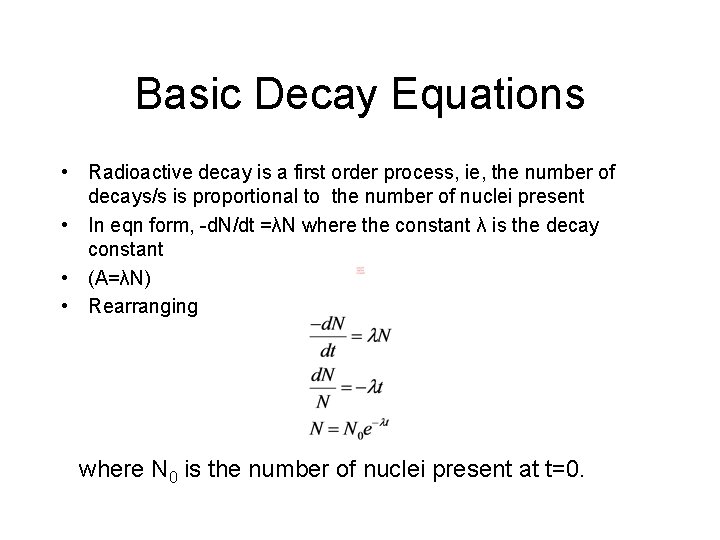
Basic Decay Equations • Radioactive decay is a first order process, ie, the number of decays/s is proportional to the number of nuclei present • In eqn form, -d. N/dt =λN where the constant λ is the decay constant • (A=λN) • Rearranging where N 0 is the number of nuclei present at t=0.
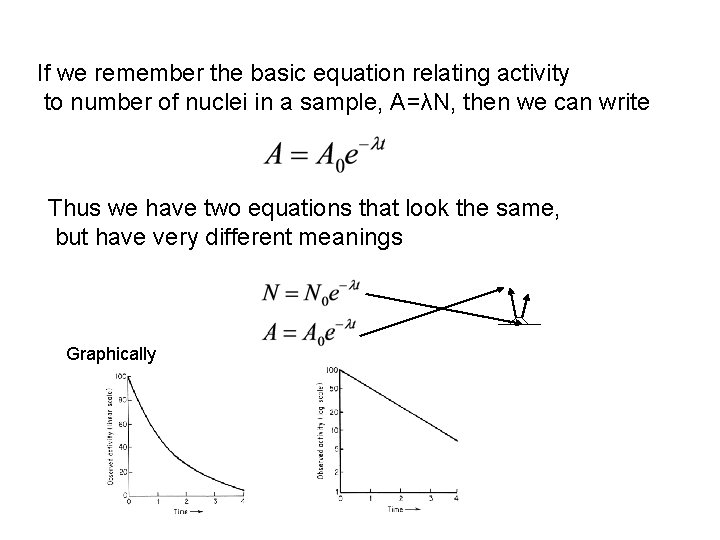
If we remember the basic equation relating activity to number of nuclei in a sample, A=λN, then we can write Thus we have two equations that look the same, but have very different meanings Graphically
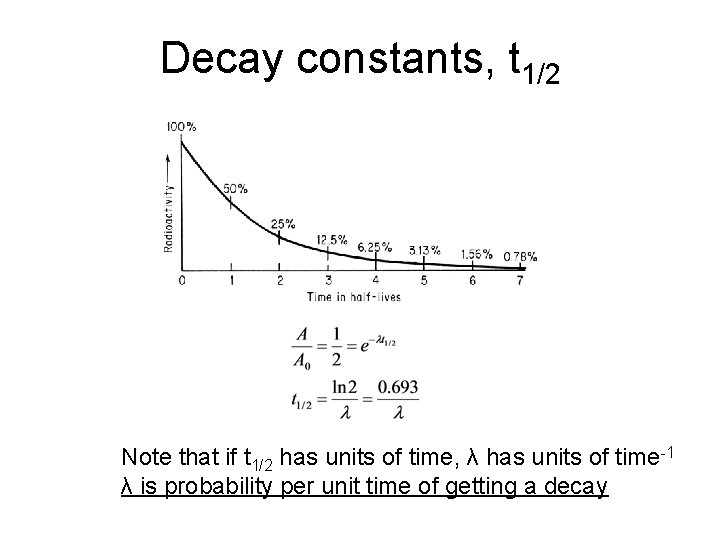
Decay constants, t 1/2 Note that if t 1/2 has units of time, λ has units of time-1 λ is probability per unit time of getting a decay
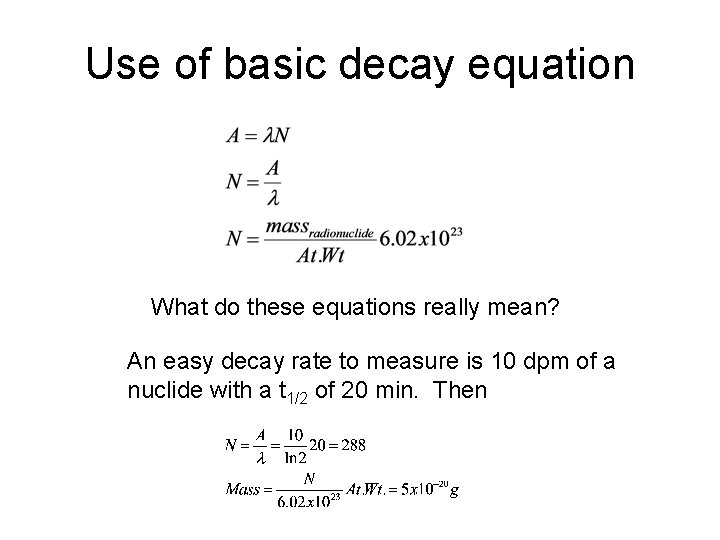
Use of basic decay equation What do these equations really mean? An easy decay rate to measure is 10 dpm of a nuclide with a t 1/2 of 20 min. Then
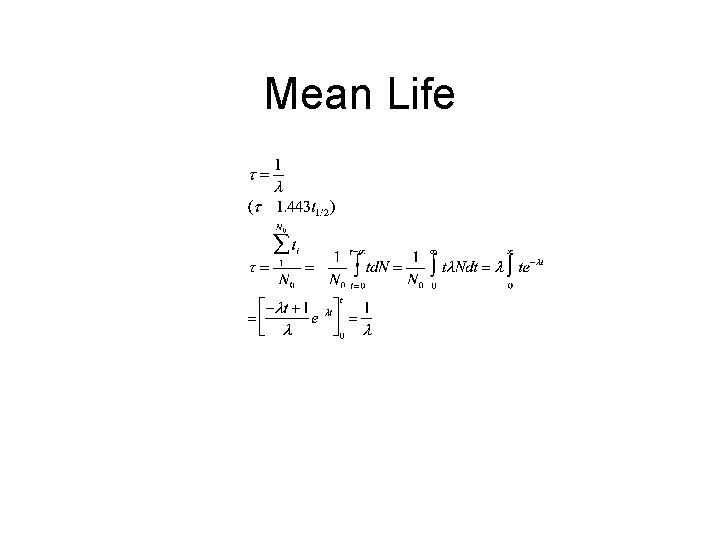
Mean Life
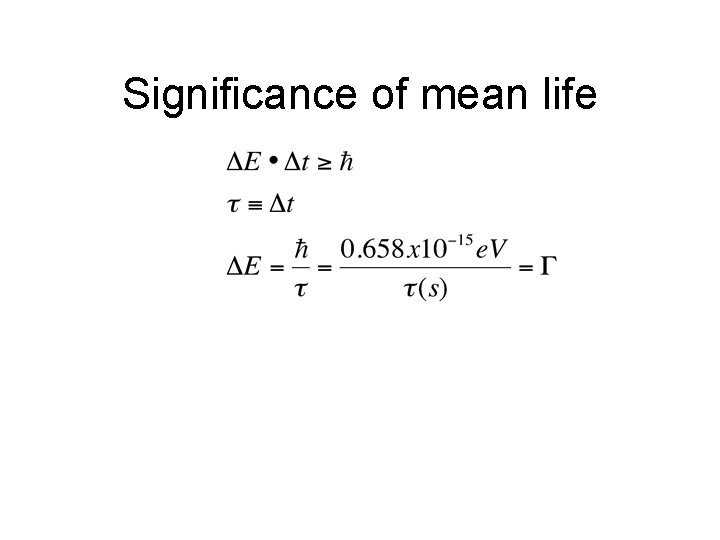
Significance of mean life

Units 1 Bq=1 Becqueral=1 d/s 1 Curie=1 Ci=3. 7 x 1010 Bq
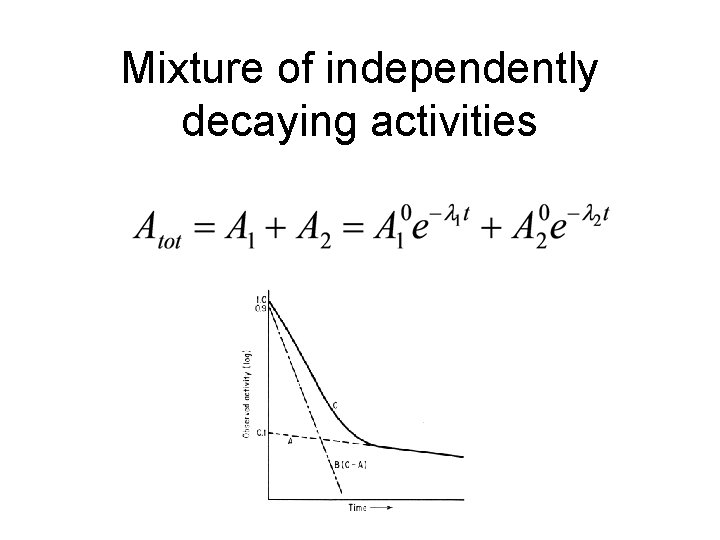
Mixture of independently decaying activities
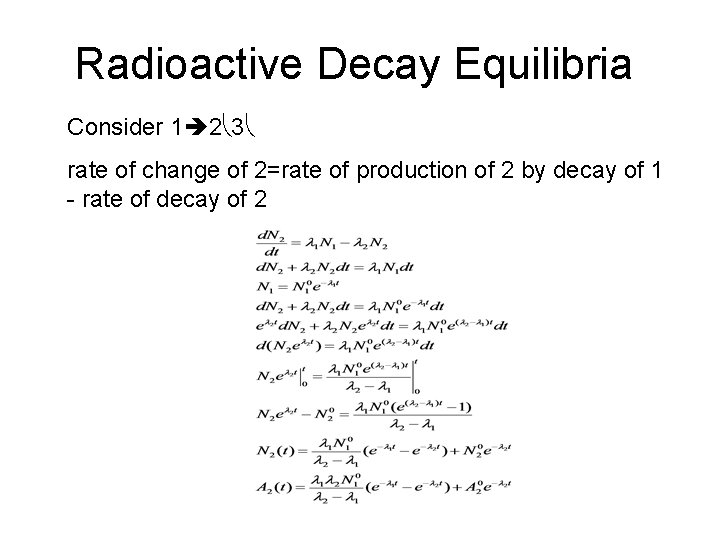
Radioactive Decay Equilibria Consider 1 2 3 rate of change of 2=rate of production of 2 by decay of 1 - rate of decay of 2
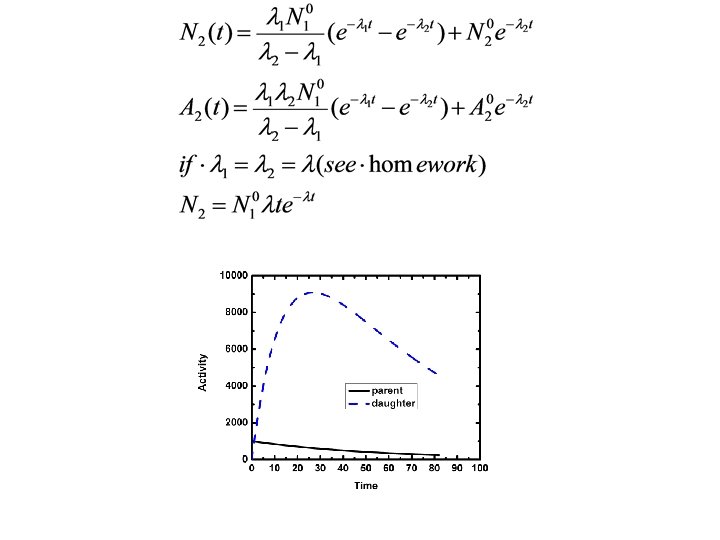
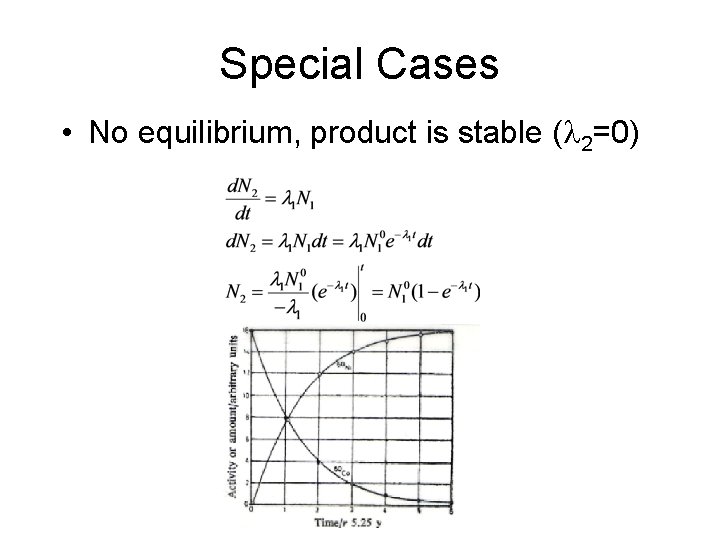
Special Cases • No equilibrium, product is stable ( 2=0)
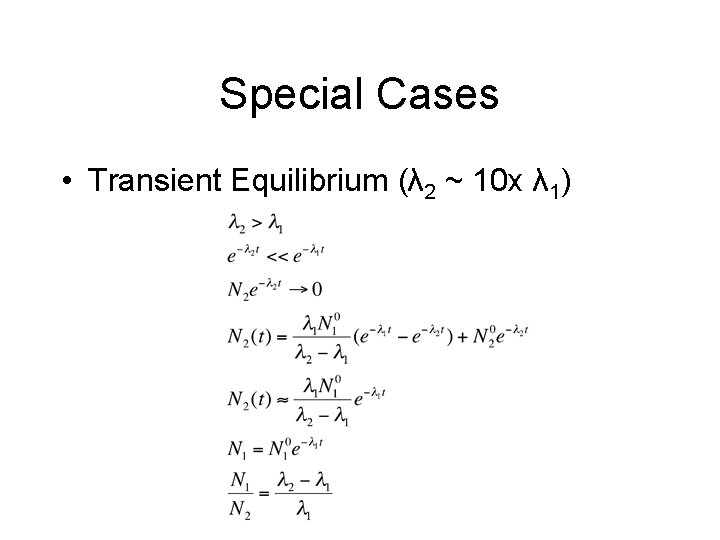
Special Cases • Transient Equilibrium (λ 2 ~ 10 x λ 1)
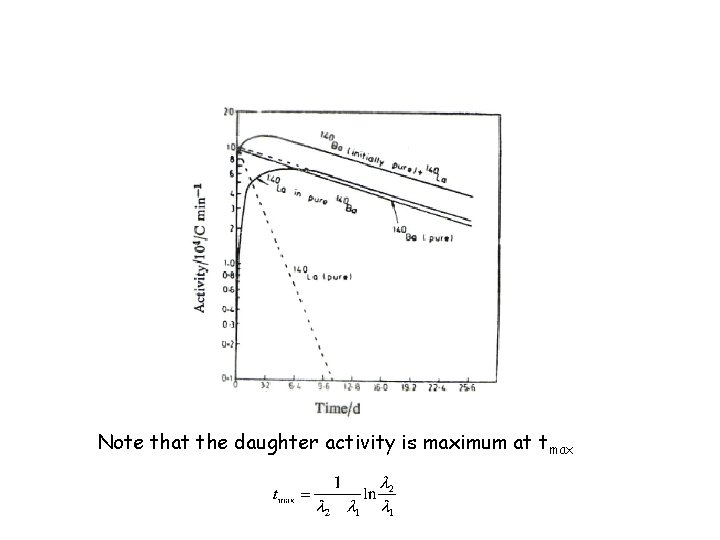
Note that the daughter activity is maximum at tmax
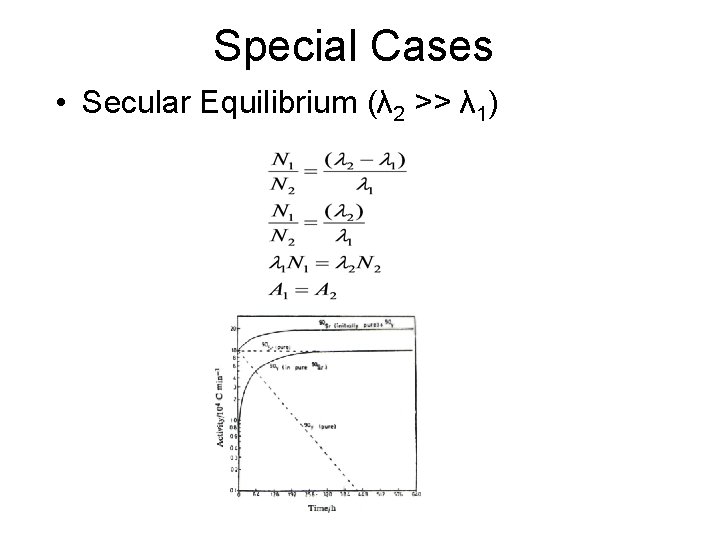
Special Cases • Secular Equilibrium (λ 2 >> λ 1)
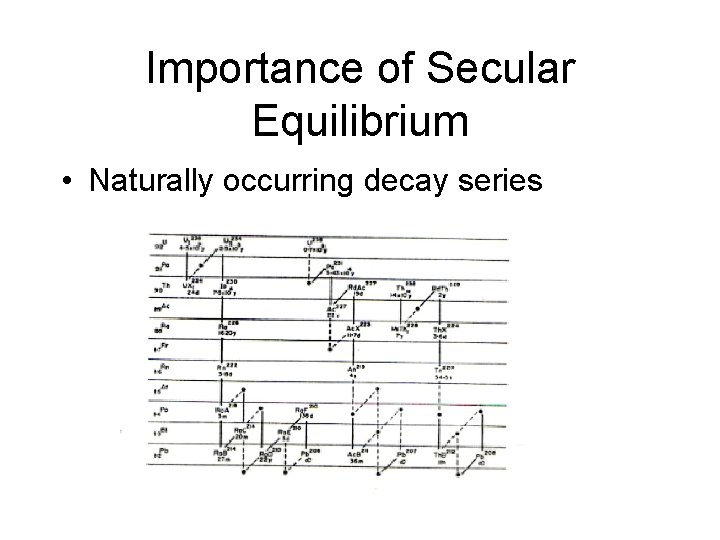
Importance of Secular Equilibrium • Naturally occurring decay series
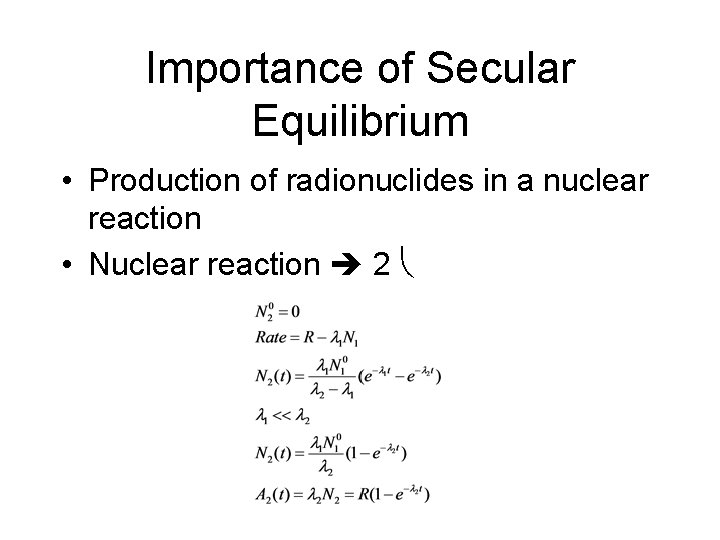
Importance of Secular Equilibrium • Production of radionuclides in a nuclear reaction • Nuclear reaction 2
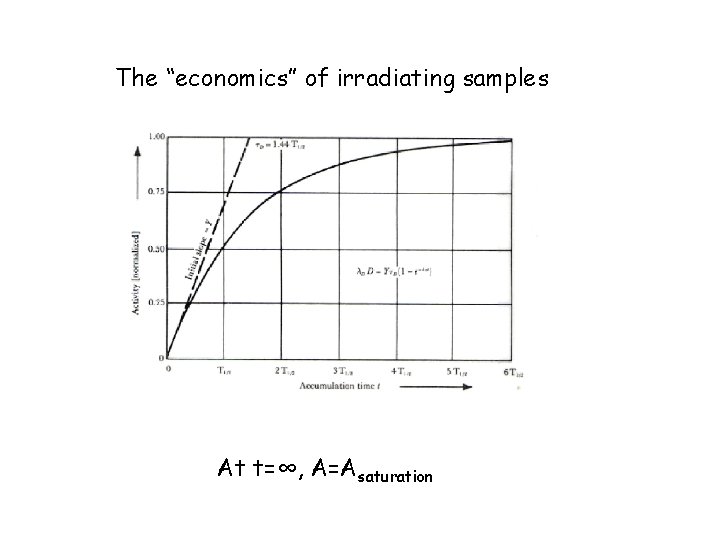
The “economics” of irradiating samples At t=∞, A=Asaturation
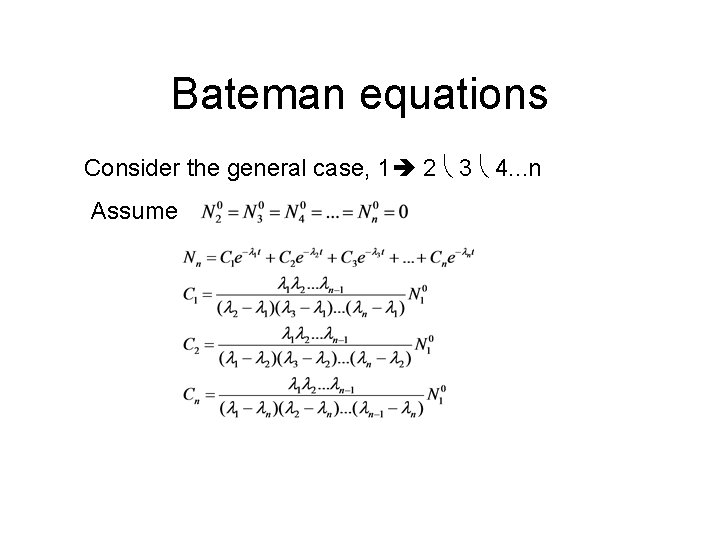
Bateman equations Consider the general case, 1 2 3 4. . . n Assume
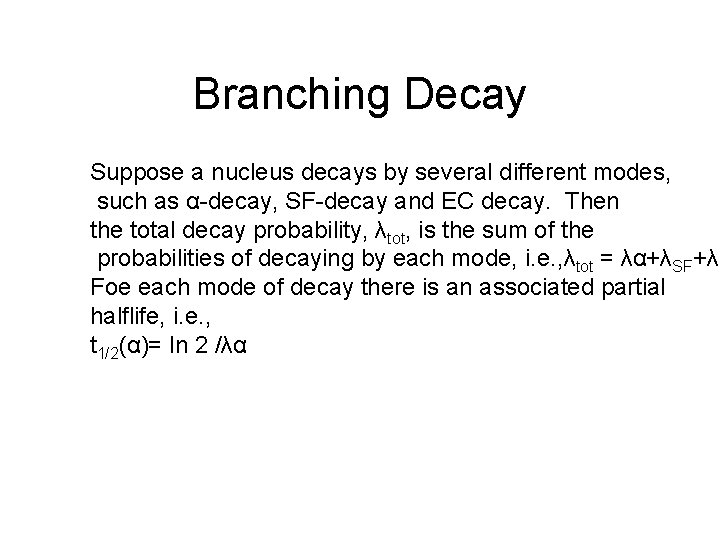
Branching Decay Suppose a nucleus decays by several different modes, such as α-decay, SF-decay and EC decay. Then the total decay probability, λtot, is the sum of the probabilities of decaying by each mode, i. e. , λtot = λα+λSF+λE Foe each mode of decay there is an associated partial halflife, i. e. , t 1/2(α)= ln 2 /λα
Naturally occurring radionuclides • Primordial • Cosmogenic • Anthropogenic
Environmentally interesting radionuclides • 222 Rn • 40 K • 3 H
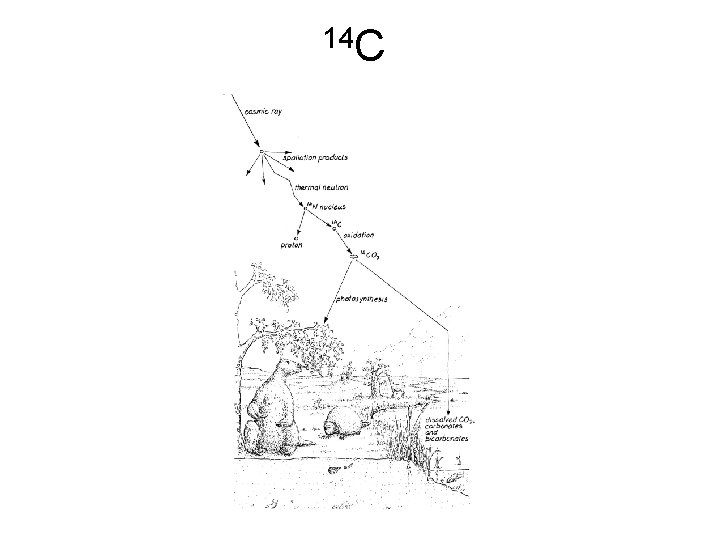
14 C
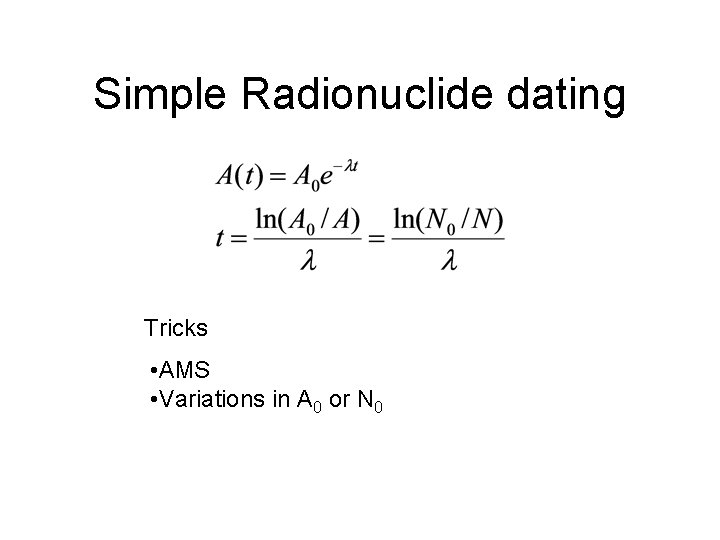
Simple Radionuclide dating Tricks • AMS • Variations in A 0 or N 0
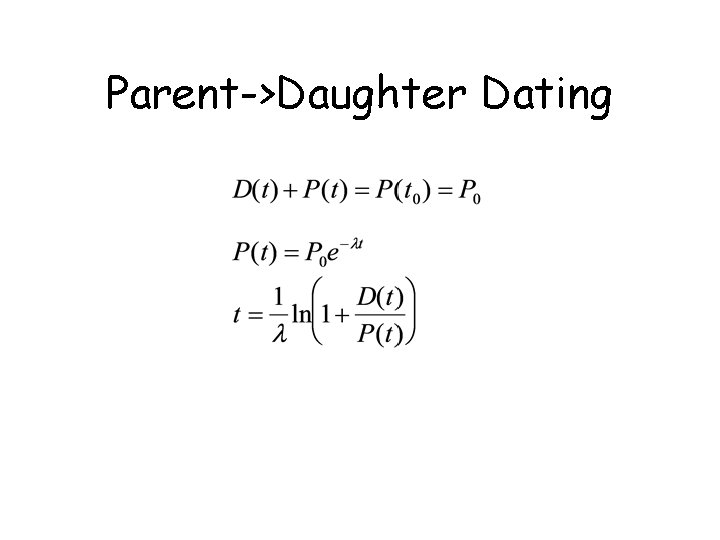
Parent->Daughter Dating
- Slides: 25