Lecture 2 Composition Structure of the Atmosphere Ch


- Slides: 12

Lecture 2: Composition & Structure of the Atmosphere (Ch 1) • Composition (continued) • Vertical structure of the atmosphere Hurricane Florence, a problem for Bermuda on Monday, was expected to bring strong winds and heavy rain to eastern Newfoundland on Wednesday. (NOAA)… by that time, Florence will probably be gearing down from a hurricane to a "very intense posttropical storm, " Environment Canada's Canadian Hurricane Centre said in a statement issued early Monday.

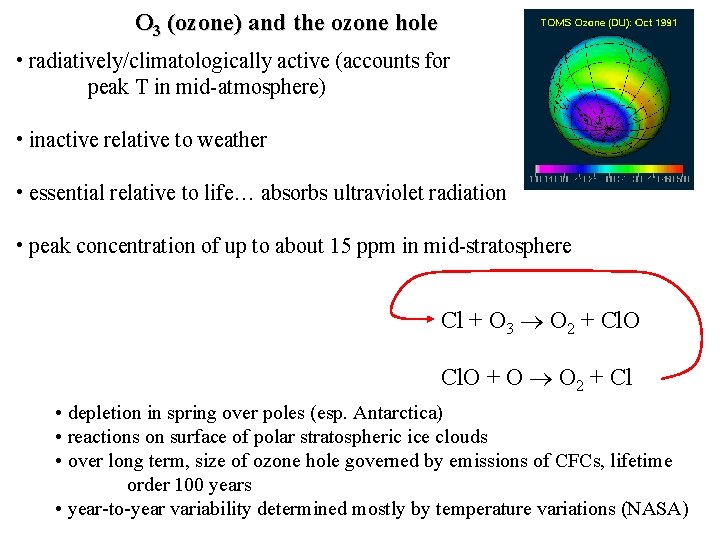
O 3 (ozone) and the ozone hole • radiatively/climatologically active (accounts for peak T in mid-atmosphere) • inactive relative to weather • essential relative to life… absorbs ultraviolet radiation • peak concentration of up to about 15 ppm in mid-stratosphere Cl + O 3 O 2 + Cl. O + O O 2 + Cl • depletion in spring over poles (esp. Antarctica) • reactions on surface of polar stratospheric ice clouds • over long term, size of ozone hole governed by emissions of CFCs, lifetime order 100 years • year-to-year variability determined mostly by temperature variations (NASA)

Aerosols • size 0. 1 m 100 m or larger (smallest formed from sulphate gases) • influence visibility (VV see Appendix C) • increase shortwave reflectivity • trap outgoing longwave radiation • form cloud condensation nuclei UA farm Ellerslie 28 May 2001 *An uncertain feedback in climatic modelling: DMS (dimethyl sulphide) gas released by decay of ocean biota generates aerosol with radiative impact as well as acting as cloud condensation nucleii (CCN) 1 o. C reduction in N. hemisphere sfc temp a year after Pinatubo eruption 1991

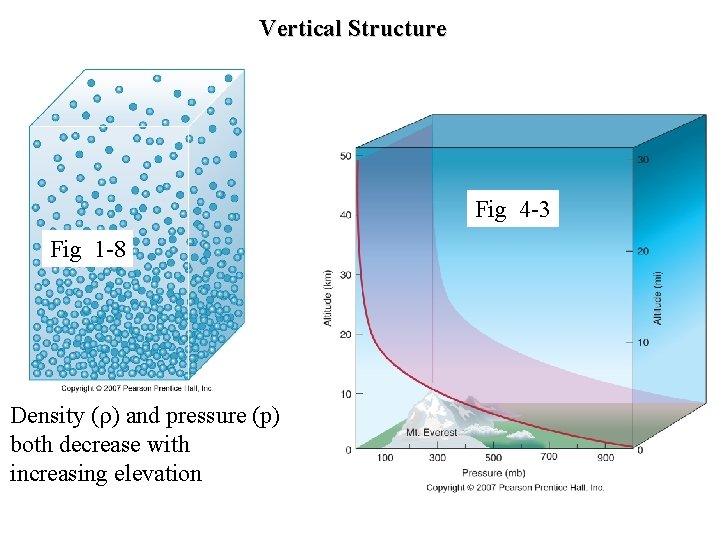
Vertical Structure Fig 4 -3 Fig 1 -8 Density ( ) and pressure (p) both decrease with increasing elevation

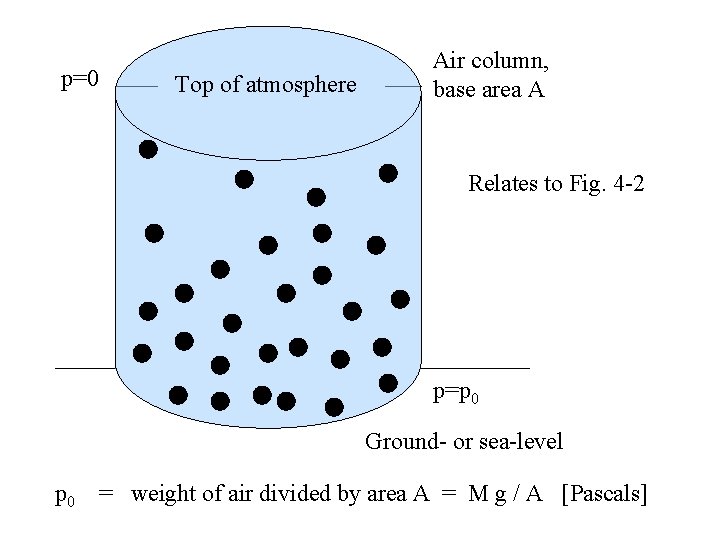
p=0 Top of atmosphere Air column, base area A Relates to Fig. 4 -2 p=p 0 Ground- or sea-level p 0 = weight of air divided by area A = M g / A [Pascals]

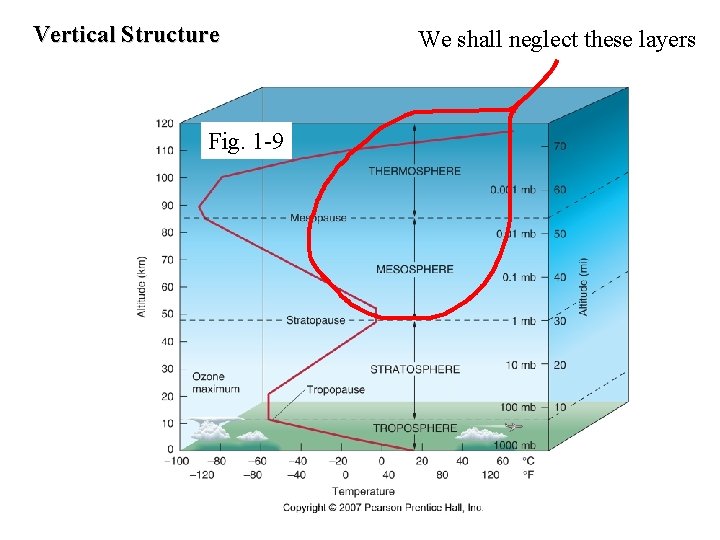
Vertical Structure Fig. 1 -9 We shall neglect these layers

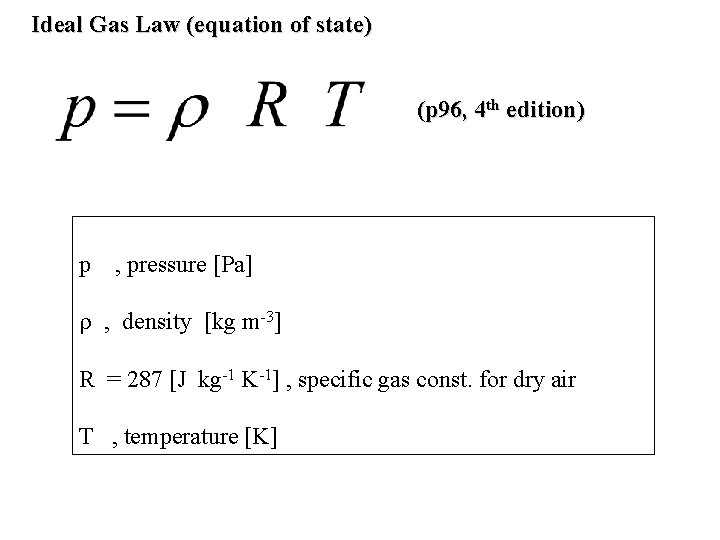
Ideal Gas Law (equation of state) (p 96, 4 th edition) p , pressure [Pa] , density [kg m-3] R = 287 [J kg-1 K-1] , specific gas const. for dry air T , temperature [K]

Question: these paragliders are flying at a height of 1000 m above sea-level. A pilot’s instrument reports so the air density at flight level is?

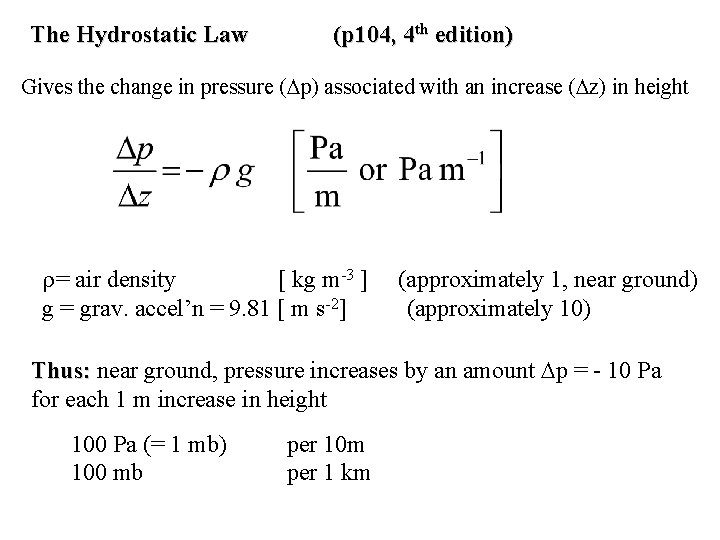
The Hydrostatic Law (p 104, 4 th edition) Gives the change in pressure ( p) associated with an increase ( z) in height = air density [ kg m-3 ] g = grav. accel’n = 9. 81 [ m s-2] (approximately 1, near ground) (approximately 10) Thus: near ground, pressure increases by an amount p = - 10 Pa for each 1 m increase in height 100 Pa (= 1 mb) 100 mb per 10 m per 1 km

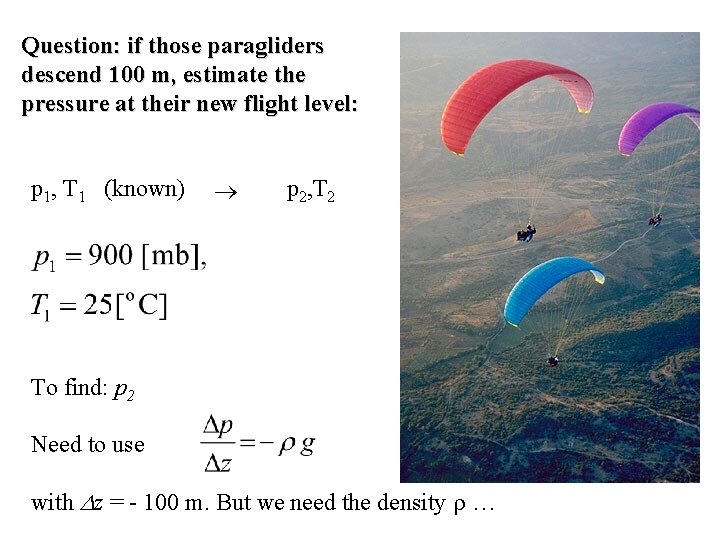
Question: if those paragliders descend 100 m, estimate the pressure at their new flight level: p 1, T 1 (known) To find: p 2, T 2

Question: if those paragliders descend 100 m, estimate the pressure at their new flight level: p 1, T 1 (known) p 2, T 2 To find: p 2 Need to use with z = - 100 m. But we need the density …

Approximate the density as (note the unit conversions; we work in MKS units) (implies) A negative step in height z gives us a positive step in pressure p