Lecture 19 The Ocean Nitrogen Cycle SinksSources Sink










- Slides: 41

Lecture 19 The Ocean Nitrogen Cycle Sinks/Sources Sink - Denitrification Reactions Distributions Source - Nitrogen Fixation Reactions Distributions

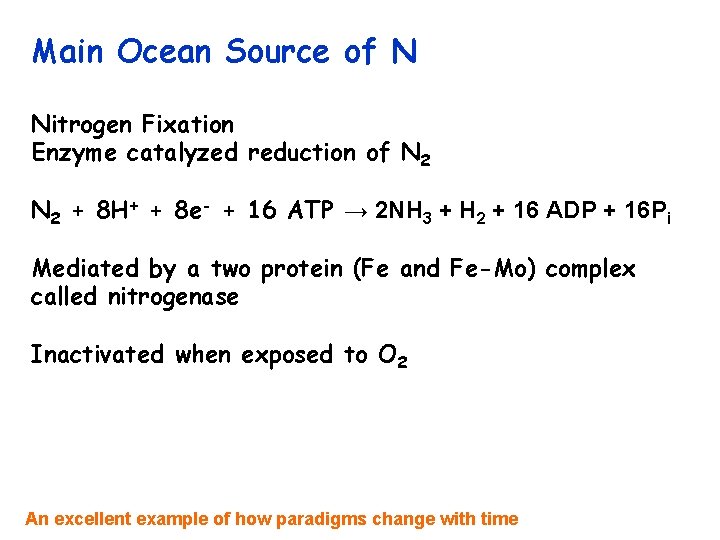
Main Ocean Source of N Nitrogen Fixation Enzyme catalyzed reduction of N 2 + 8 H+ + 8 e- + 16 ATP → 2 NH 3 + H 2 + 16 ADP + 16 Pi Mediated by a two protein (Fe and Fe-Mo) complex called nitrogenase Inactivated when exposed to O 2 An excellent example of how paradigms change with time

Main Ocean Sink of N Fixed Nitrogen (NO 3 -, NO 2 -, NH 4+) is converted to N 2 in low oxygen zones of the ocean Two Pathways Denitrification ( <2 to 10 m. M O 2): 2 NO 3 - + organic matter → N 2 Anammox (<2 m. M O 2) NH 4+ + NO 2 - → N 2 + H 2 O

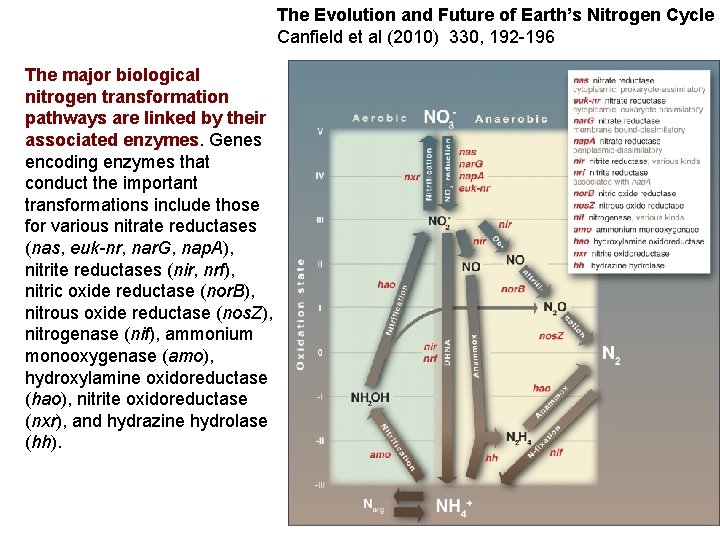
The Evolution and Future of Earth’s Nitrogen Cycle Canfield et al (2010) 330, 192 -196 The major biological nitrogen transformation pathways are linked by their associated enzymes. Genes encoding enzymes that conduct the important transformations include those for various nitrate reductases (nas, euk-nr, nar. G, nap. A), nitrite reductases (nir, nrf), nitric oxide reductase (nor. B), nitrous oxide reductase (nos. Z), nitrogenase (nif), ammonium monooxygenase (amo), hydroxylamine oxidoreductase (hao), nitrite oxidoreductase (nxr), and hydrazine hydrolase (hh).

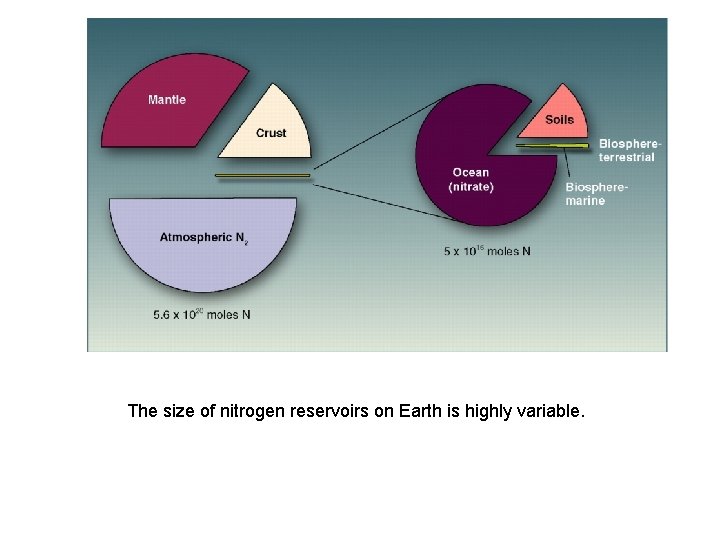
The size of nitrogen reservoirs on Earth is highly variable.

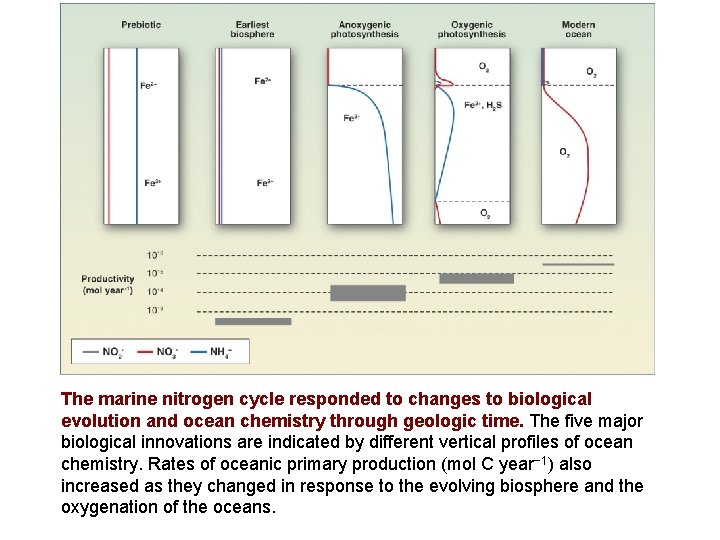
The marine nitrogen cycle responded to changes to biological evolution and ocean chemistry through geologic time. The five major biological innovations are indicated by different vertical profiles of ocean chemistry. Rates of oceanic primary production (mol C year− 1) also increased as they changed in response to the evolving biosphere and the oxygenation of the oceans.

A negative feedback linking nitrogen and carbon cycles?

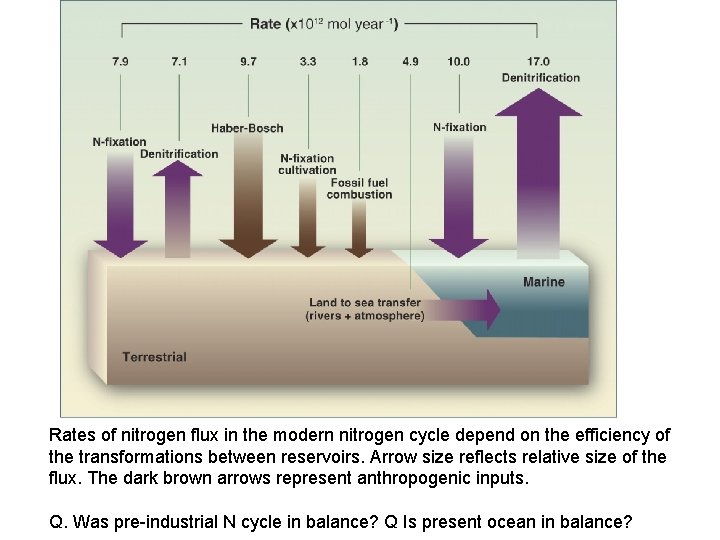
Rates of nitrogen flux in the modern nitrogen cycle depend on the efficiency of the transformations between reservoirs. Arrow size reflects relative size of the flux. The dark brown arrows represent anthropogenic inputs. Q. Was pre-industrial N cycle in balance? Q Is present ocean in balance?

Canfield et al (2010) Student Questions 1 Could you explain the Haber-Bausch process and why is it a flux into terrestrial terrain? 2 Is there anyway changes could be made to the composition of nitrogen fertilizers themselves so that they can have a better nitrogen use efficiency by crops? The paper says that it is currently less than 40% efficient. 3. How does nitrogen contributed to net oxygen accumulation being retarded? 4. Since N 2 O has such a high warming factor, and seems to contribute more warming than all CO 2, why doesn't there seem to be a greater focus on cutting N 2 O? 5. Can you confirm what the grey dashed lines represent in figure 3? 6. How can lightning produce NO, and how can we know that the rate of production was met by a stoichiometric rate of NO subspecies production by lightning during the earliest biosphere? 7. Are there places where anthropogenically attributable anoxia has essentially stopped the biological cycle? Is this likely in the future? 8. Nitrogenase is a tetramer composed of 2 alpha and 2 beta monomer components. Can you clarify the structure of the alpha Mo. Fe 7 S 9 cluster? Does it acquire electrons from organic C nearby? What are the beta monomers composed of? 9. Have any technologies been developed for N sequestration/immobilization? How relevant is N 2 O as a greenhouse gas? 10. Why is NO 3 more abundant than NH 4? 11. During nitrification, are these reactions happening freely in the external environment, or is it occurring within the cell membrane? Do some of the intermediate molecules to escape in a similar manner to how N 2 O escapes during nitrogen reduction? 12. Do all meteorite strikes result in NO formation? Also, does the heat shock have to be very sudden, or can it be a rapid but prolonged temperature change?

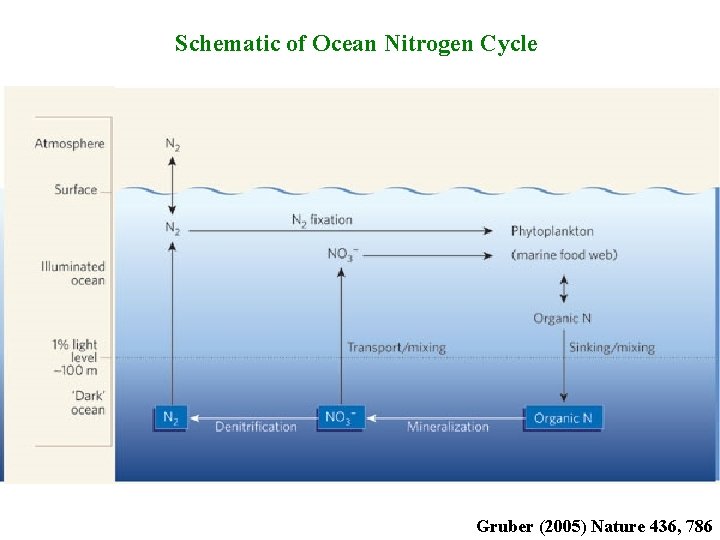
Schematic of Ocean Nitrogen Cycle Gruber (2005) Nature 436, 786

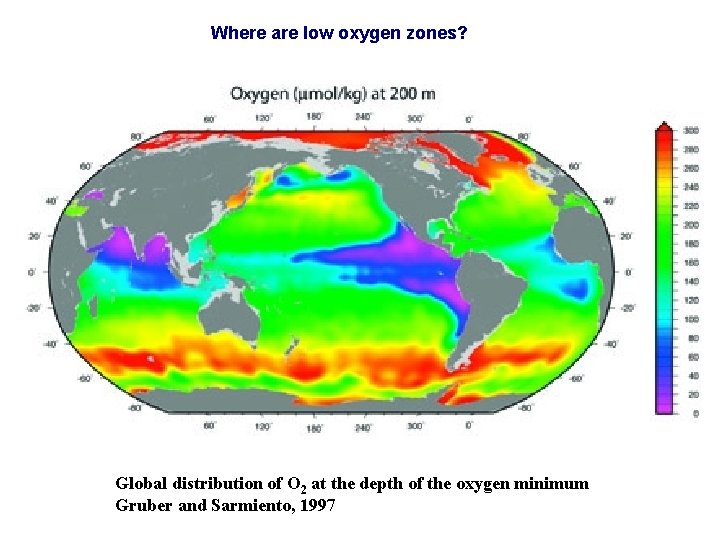
Where are low oxygen zones? Global distribution of O 2 at the depth of the oxygen minimum Gruber and Sarmiento, 1997

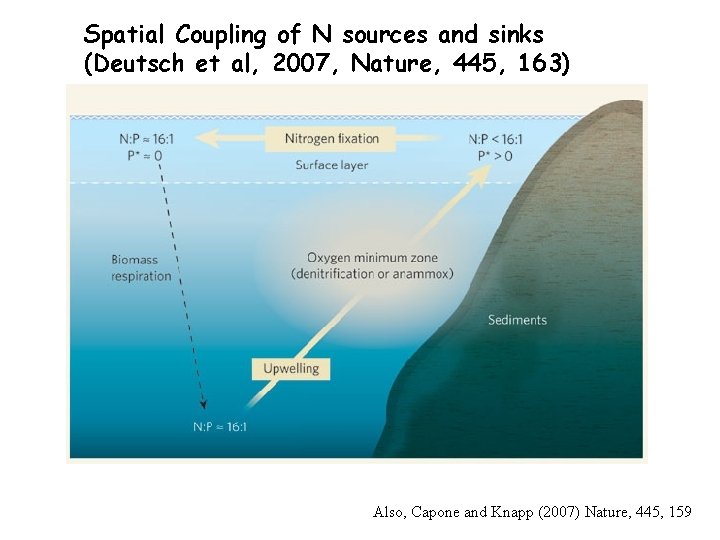
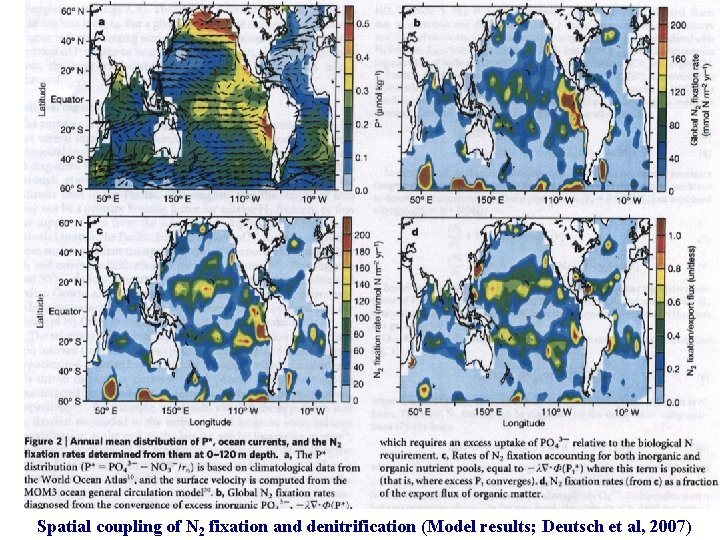
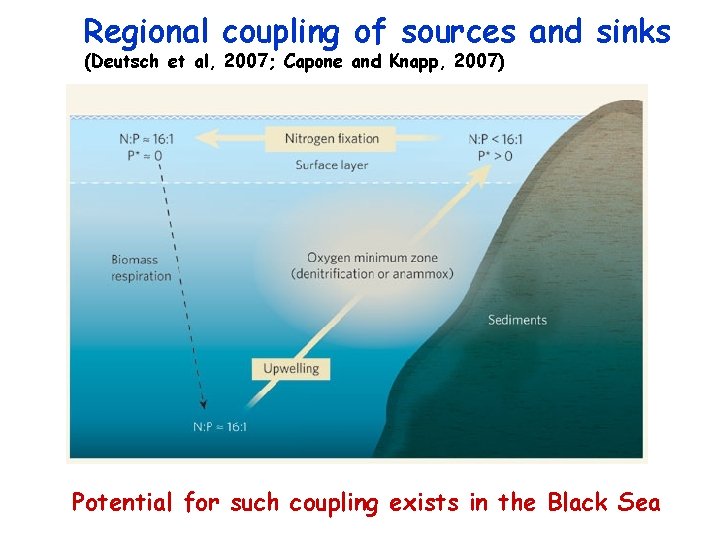
Spatial Coupling of N sources and sinks (Deutsch et al, 2007, Nature, 445, 163) Also, Capone and Knapp (2007) Nature, 445, 159

Spatial coupling of N 2 fixation and denitrification (Model results; Deutsch et al, 2007)

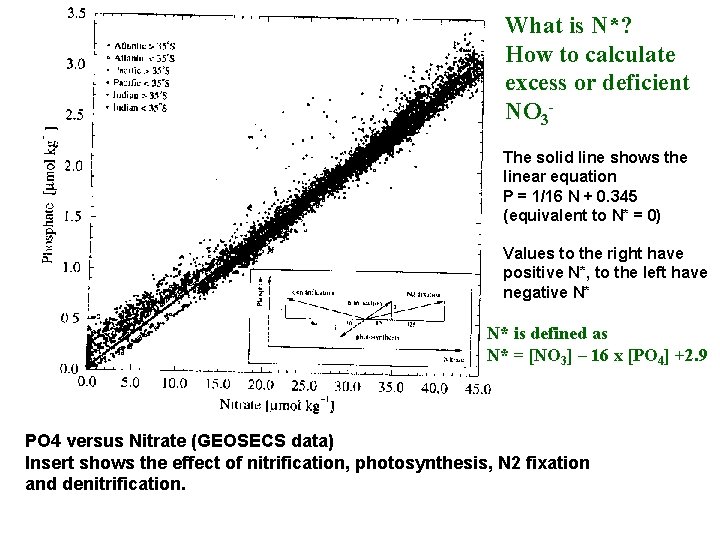
What is N*? How to calculate excess or deficient NO 3 The solid line shows the linear equation P = 1/16 N + 0. 345 (equivalent to N* = 0) Values to the right have positive N*, to the left have negative N* N* is defined as N* = [NO 3] – 16 x [PO 4] +2. 9 PO 4 versus Nitrate (GEOSECS data) Insert shows the effect of nitrification, photosynthesis, N 2 fixation and denitrification.

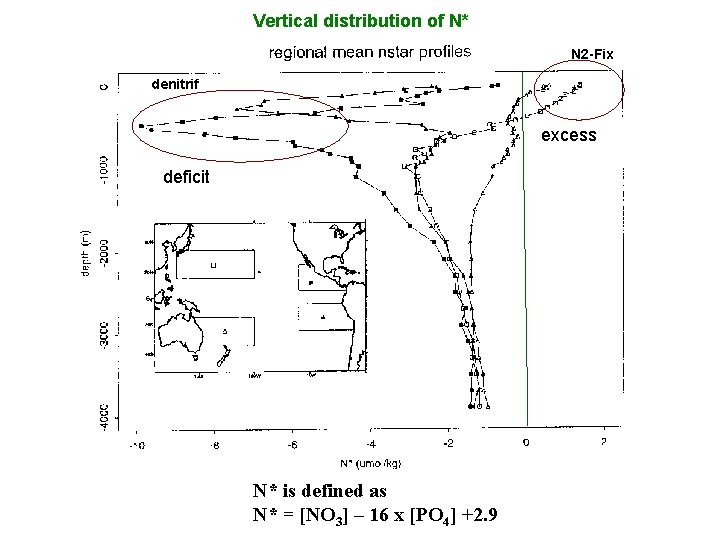
Vertical distribution of N* N 2 -Fix denitrif excess deficit N* is defined as N* = [NO 3] – 16 x [PO 4] +2. 9

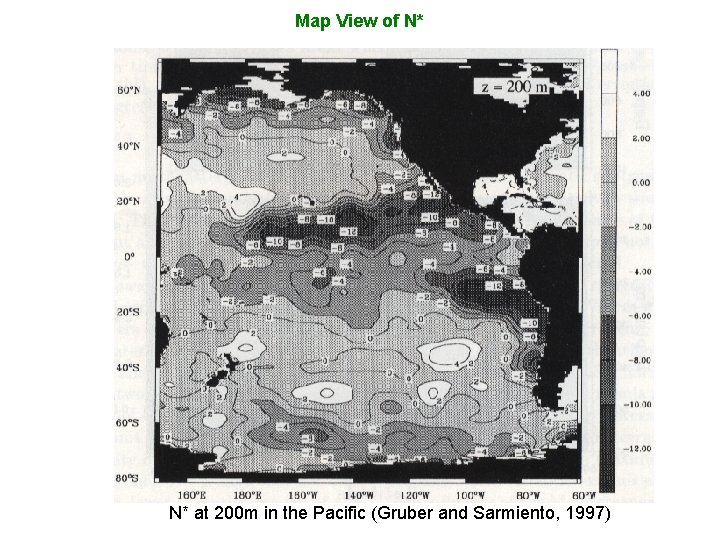
Map View of N* N* at 200 m in the Pacific (Gruber and Sarmiento, 1997)

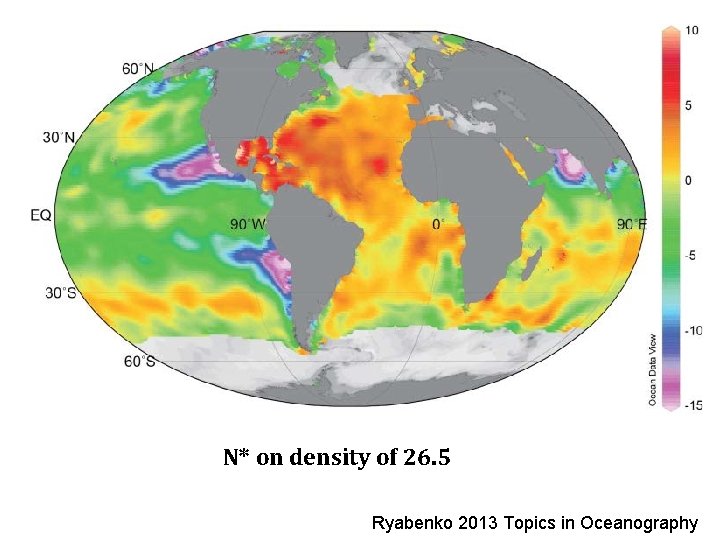
N* on density of 26. 5 Ryabenko 2013 Topics in Oceanography

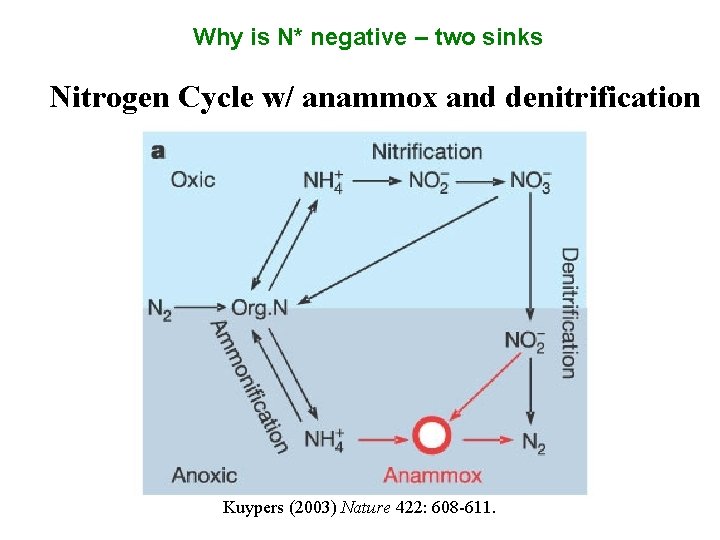
Why is N* negative – two sinks Nitrogen Cycle w/ anammox and denitrification Kuypers (2003) Nature 422: 608 -611.

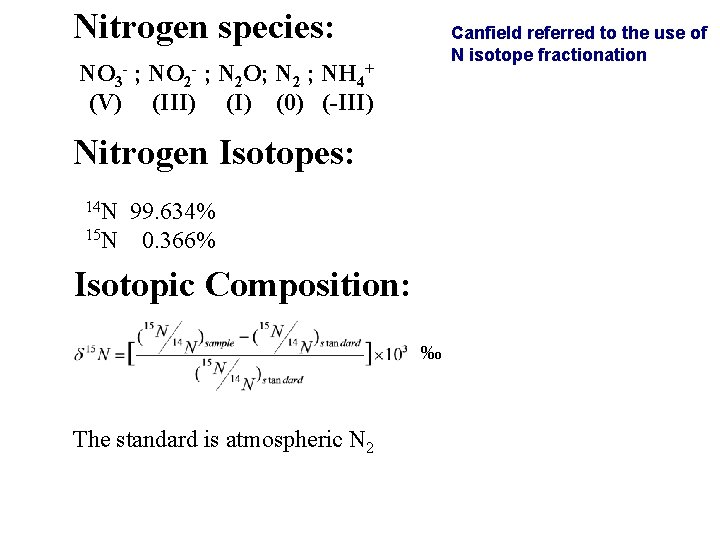
Nitrogen species: Canfield referred to the use of N isotope fractionation NO 3 - ; NO 2 - ; N 2 O; N 2 ; NH 4+ (V) (III) (0) (-III) Nitrogen Isotopes: 14 N 99. 634% 15 N 0. 366% Isotopic Composition: ‰ The standard is atmospheric N 2

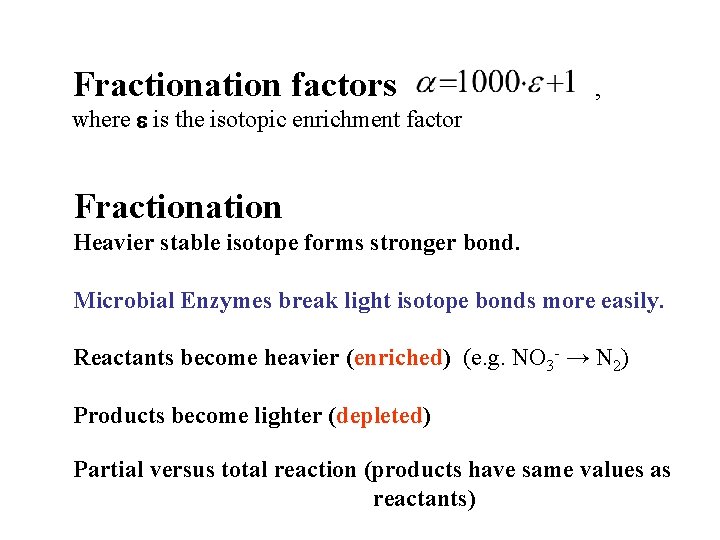
Fractionation factors , where e is the isotopic enrichment factor Fractionation Heavier stable isotope forms stronger bond. Microbial Enzymes break light isotope bonds more easily. Reactants become heavier (enriched) (e. g. NO 3 - → N 2) Products become lighter (depleted) Partial versus total reaction (products have same values as reactants)

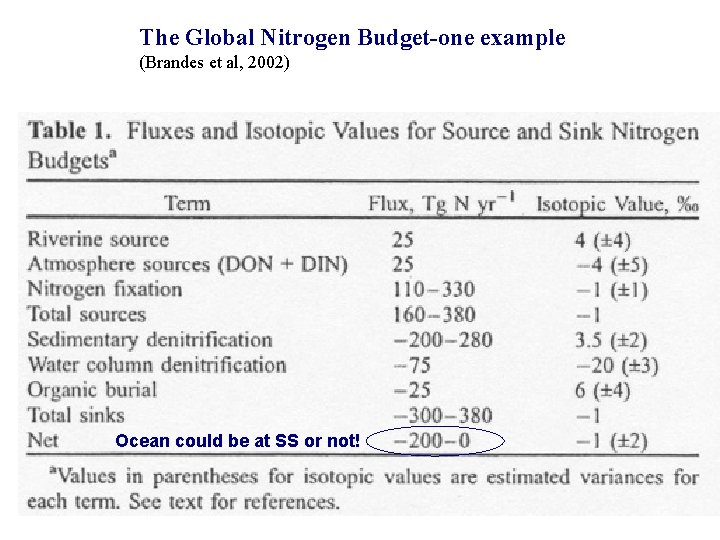
The Global Nitrogen Budget-one example (Brandes et al, 2002) Ocean could be at SS or not!

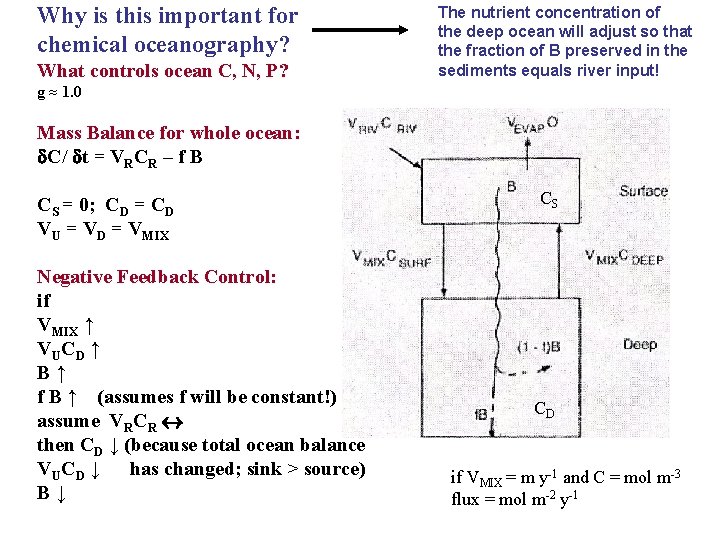
Why is this important for chemical oceanography? What controls ocean C, N, P? The nutrient concentration of the deep ocean will adjust so that the fraction of B preserved in the sediments equals river input! g ≈ 1. 0 Mass Balance for whole ocean: C/ t = VRCR – f B CS = 0; CD = CD VU = VD = VMIX Negative Feedback Control: if VMIX ↑ VUCD ↑ B↑ f B ↑ (assumes f will be constant!) assume VRCR then CD ↓ (because total ocean balance VUCD ↓ has changed; sink > source) B↓ CS CD if VMIX = m y-1 and C = mol m-3 flux = mol m-2 y-1

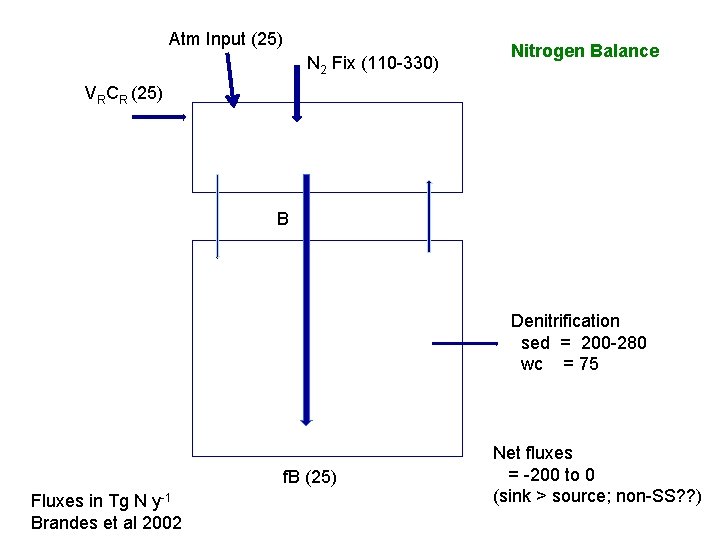
Atm Input (25) N 2 Fix (110 -330) Nitrogen Balance VRCR (25) B Denitrification sed = 200 -280 wc = 75 f. B (25) Fluxes in Tg N y-1 Brandes et al 2002 Net fluxes = -200 to 0 (sink > source; non-SS? ? )

The Black Sea - Istanbul

Why is the Black Sea Interesting to Oceanographers? 1. The classic anoxic basin. Oxic layer over sulfidic layer. 2. Model for modern and ancient anoxic environments. 3. Well developed transition or suboxic zone. Model for world’s organic rich sediments. 4. Suboxic reactions easy to study here because of predictable depth locations. 5. An ideal location to study effect of climate forcing on ocean distributions. Climate Physical Chemical Biological

Rush hour on the Bosphorus

Rumelihisarı Fall of Constantinople in 1453

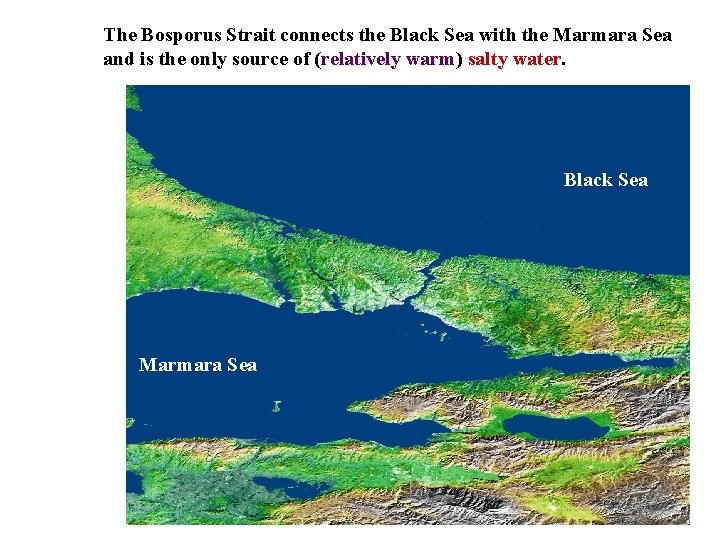
The Bosporus Strait connects the Black Sea with the Marmara Sea and is the only source of (relatively warm) salty water. Black Sea Marmara Sea

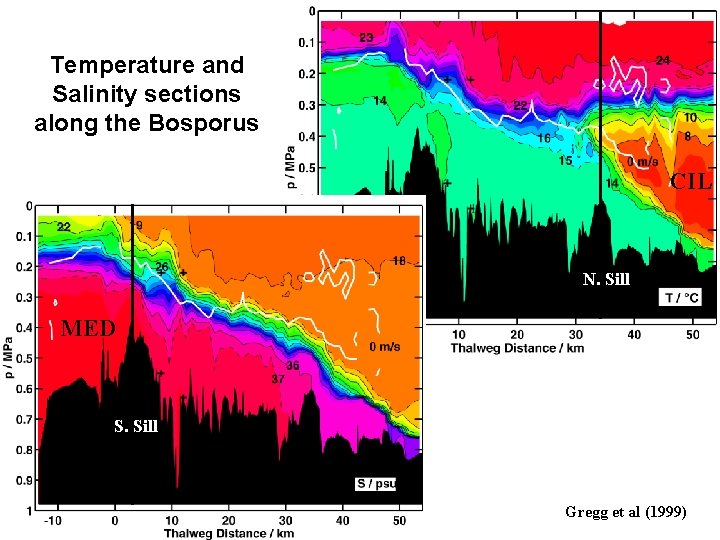
Temperature and Salinity sections along the Bosporus CIL N. Sill MED S. Sill Gregg et al (1999)

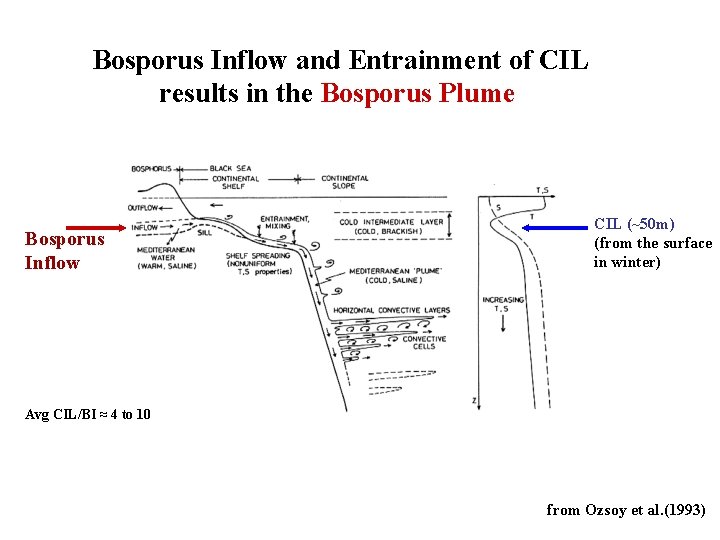
Bosporus Inflow and Entrainment of CIL results in the Bosporus Plume Bosporus Inflow CIL (~50 m) (from the surface in winter) Avg CIL/BI ≈ 4 to 10 from Ozsoy et al. (1993)

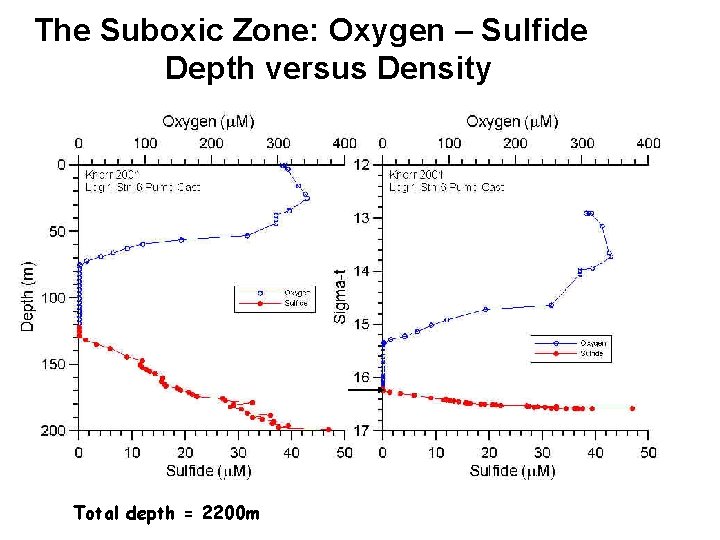
The Suboxic Zone: Oxygen – Sulfide Depth versus Density Total depth = 2200 m

Regional coupling of sources and sinks (Deutsch et al, 2007; Capone and Knapp, 2007) Potential for such coupling exists in the Black Sea

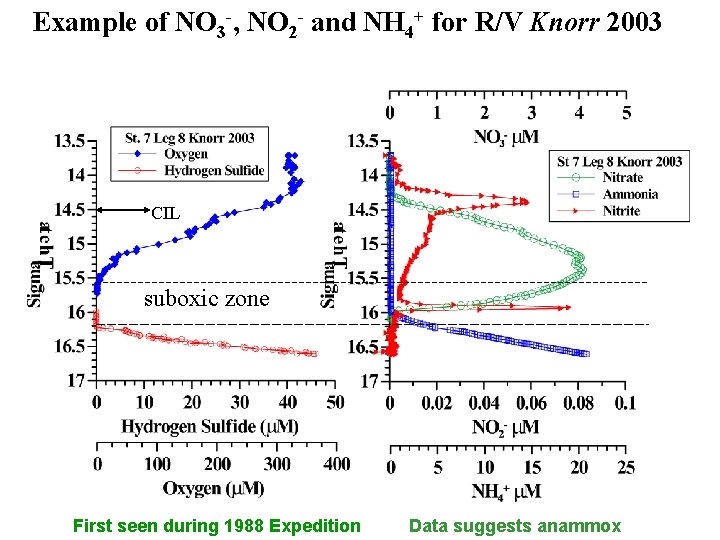
Example of NO 3 -, NO 2 - and NH 4+ for R/V Knorr 2003 CIL suboxic zone First seen during 1988 Expedition Data suggests anammox

A combined geochemical and microbiological research approach consists of four approaches. 1) Determine net reactions using in situ distributions of nitrogen species and their 15 N signatures 2) Measure specific rates of reactions using labeled nutrient spikes and selective inhibitors 3) Culture organisms of interest 4) Use DNA sequencing to determine the distributions of attached and free living microorganisms 5) Conduct m. RNA analyses to determine which bacteria are functionally active.

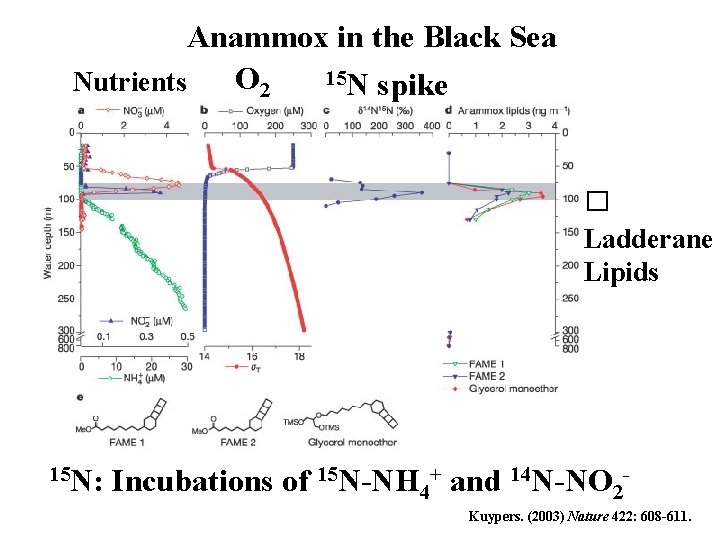
Anammox in the Black Sea 15 N spike O 2 Nutrients � Ladderane Lipids 15 N: Incubations of 15 N-NH 4+ and 14 N-NO 2 Kuypers. (2003) Nature 422: 608 -611.

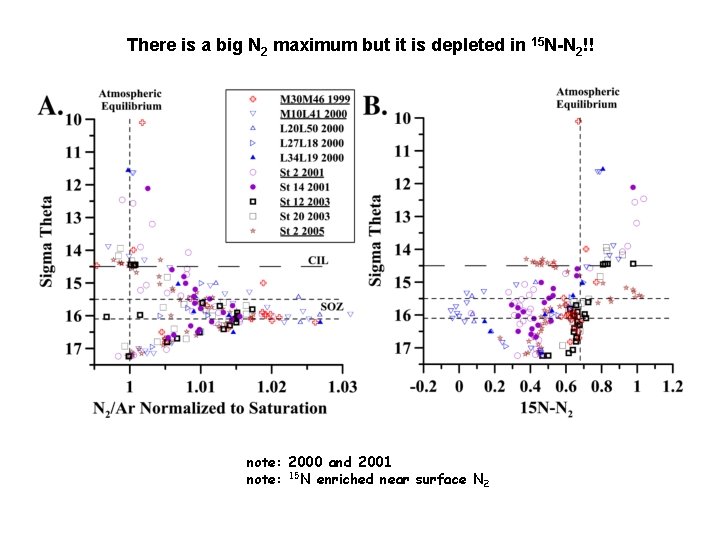
There is a big N 2 maximum but it is depleted in 15 N-N 2!! note: 2000 and 2001 note: 15 N enriched near surface N 2

So The distributions of NO 3 - + NH 4+ suggest anammox at sө = 15. 95 There is a big maximum in N 2/Ar at the right depth But 15 N 2 is quite variable and can be very depleted. If N 2 was made by total consumption of NO 3 + NH 4 it’s 15 N would be +6 to +8‰ Clearly there additional fluxes or processes we need to understand.

Conclusions from distributions 1. Temporal variability in 15 N-N 2 2. Anammox bacteria free-living 3. Denitrifiers particle attached 4. Our Hypothesis that variability seen in anammox / denitrification is due to variability in organic rich particulate aggregates. The redox state inside particles is more reducing than outside


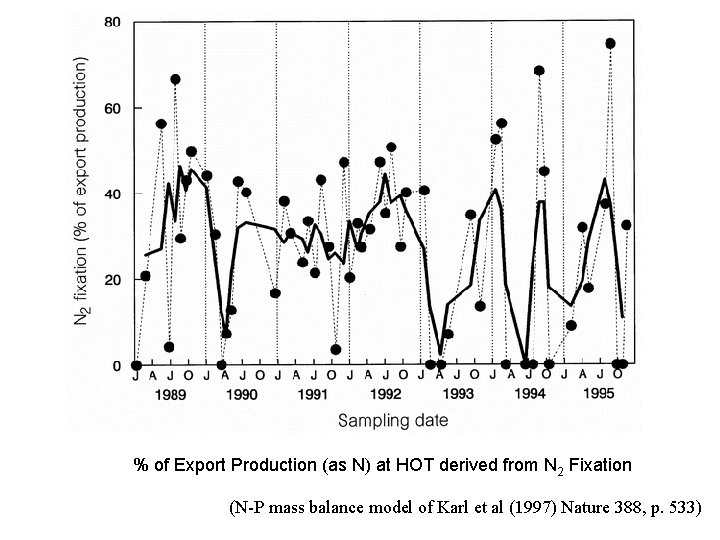
% of Export Production (as N) at HOT derived from N 2 Fixation (N-P mass balance model of Karl et al (1997) Nature 388, p. 533)

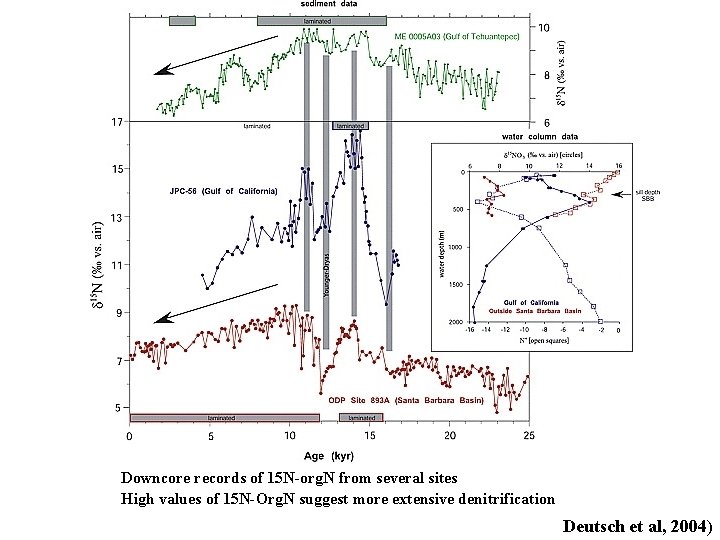
Downcore records of 15 N-org. N from several sites High values of 15 N-Org. N suggest more extensive denitrification Deutsch et al, 2004)