Lecture 18 The Ocean Nitrogen Cycle Denitrification Reactions


![N* is defined as N* = [NO 3] – 16 x [PO 4] +2. N* is defined as N* = [NO 3] – 16 x [PO 4] +2.](https://slidetodoc.com/presentation_image_h2/7d4aee9c7042c37e6f9a77d39c96b5b3/image-11.jpg)

- Slides: 20

Lecture 18 The Ocean Nitrogen Cycle Denitrification Reactions Distributions Nitrogen Fixation Reactions Distributions

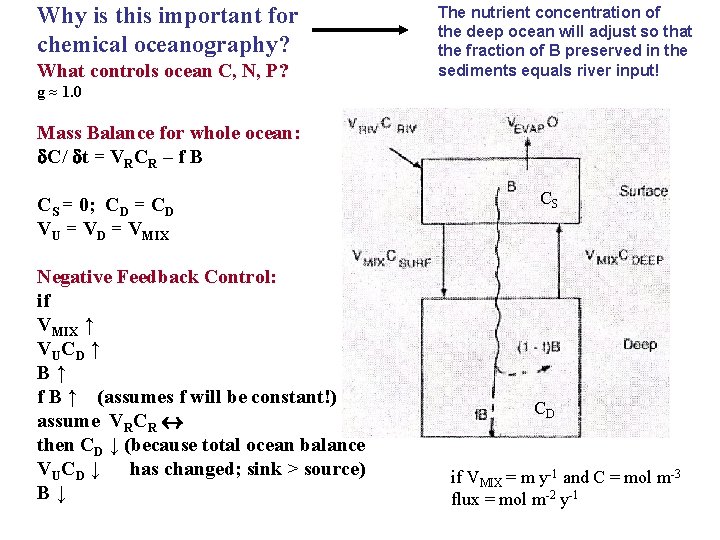
Why is this important for chemical oceanography? What controls ocean C, N, P? The nutrient concentration of the deep ocean will adjust so that the fraction of B preserved in the sediments equals river input! g ≈ 1. 0 Mass Balance for whole ocean: C/ t = VRCR – f B CS = 0; CD = CD VU = VD = VMIX Negative Feedback Control: if VMIX ↑ VUCD ↑ B↑ f B ↑ (assumes f will be constant!) assume VRCR then CD ↓ (because total ocean balance VUCD ↓ has changed; sink > source) B↓ CS CD if VMIX = m y-1 and C = mol m-3 flux = mol m-2 y-1

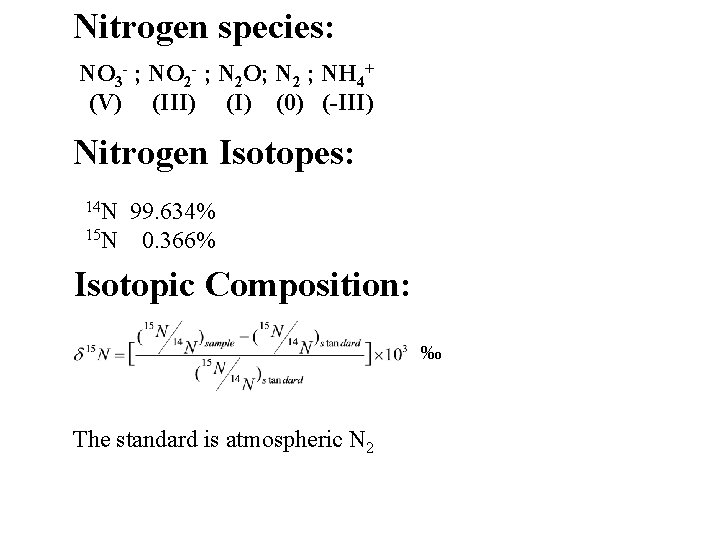
Nitrogen species: NO 3 - ; NO 2 - ; N 2 O; N 2 ; NH 4+ (V) (III) (0) (-III) Nitrogen Isotopes: 14 N 99. 634% 15 N 0. 366% Isotopic Composition: ‰ The standard is atmospheric N 2

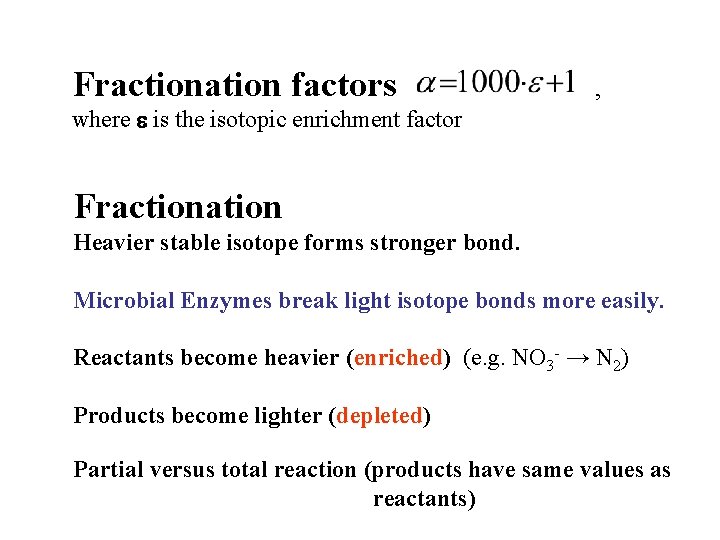
Fractionation factors , where e is the isotopic enrichment factor Fractionation Heavier stable isotope forms stronger bond. Microbial Enzymes break light isotope bonds more easily. Reactants become heavier (enriched) (e. g. NO 3 - → N 2) Products become lighter (depleted) Partial versus total reaction (products have same values as reactants)

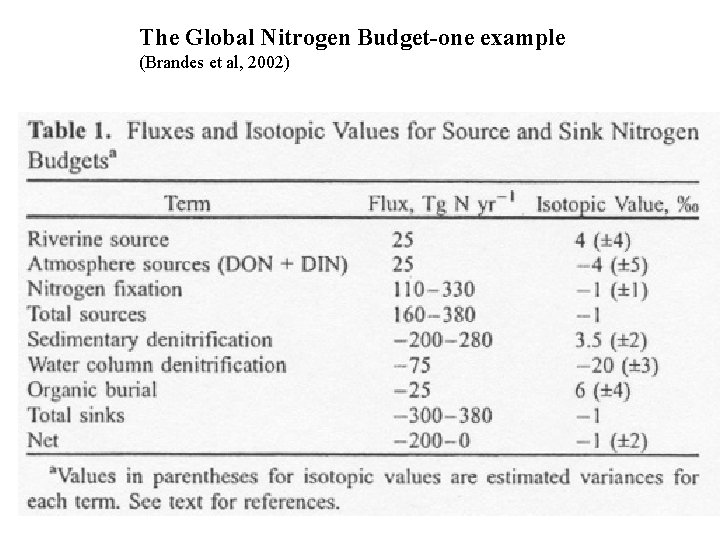
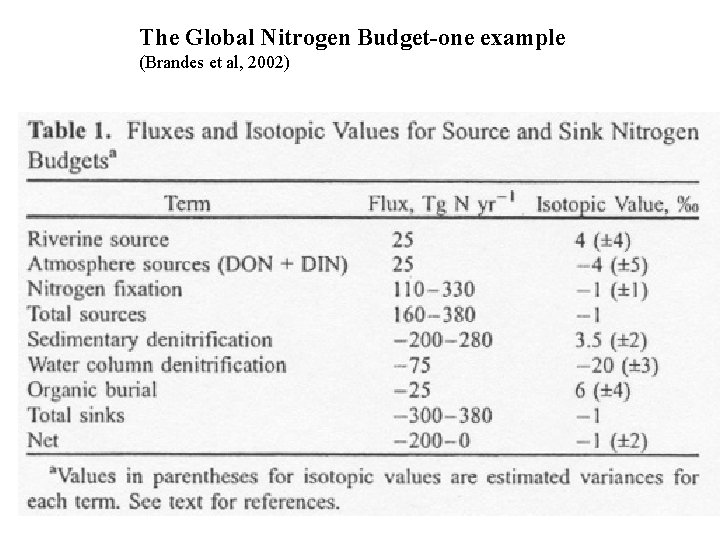
The Global Nitrogen Budget-one example (Brandes et al, 2002)

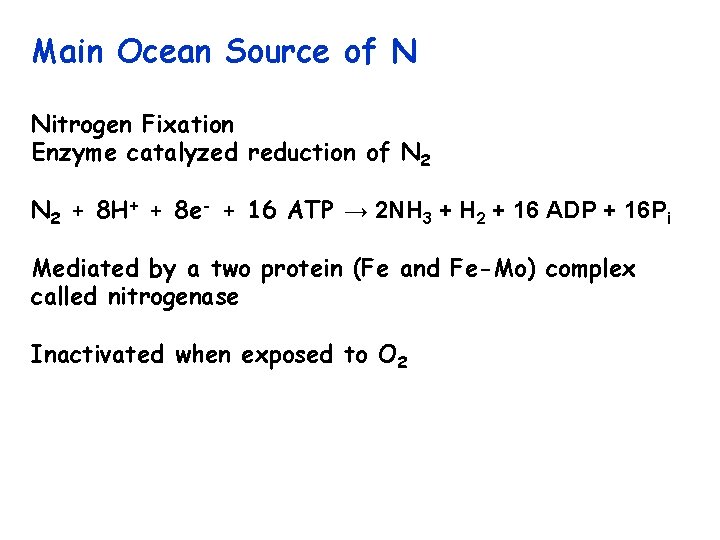
Main Ocean Source of N Nitrogen Fixation Enzyme catalyzed reduction of N 2 + 8 H+ + 8 e- + 16 ATP → 2 NH 3 + H 2 + 16 ADP + 16 Pi Mediated by a two protein (Fe and Fe-Mo) complex called nitrogenase Inactivated when exposed to O 2

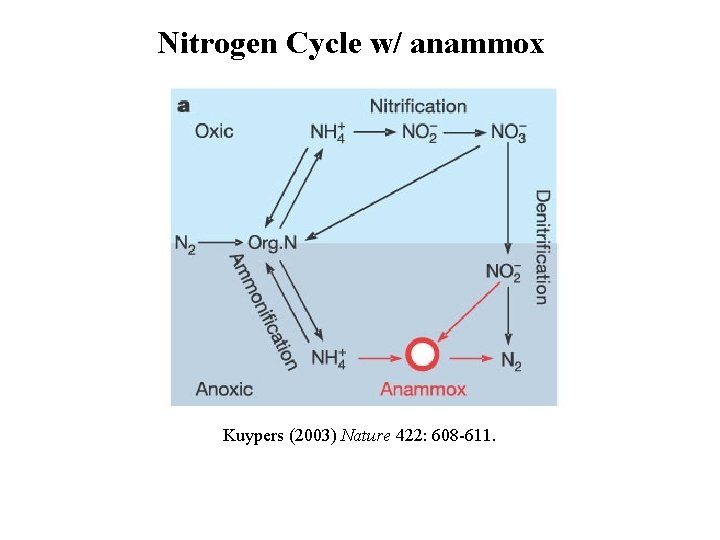
Main Ocean Sink of N Fixed Nitrogen (NO 3 -, NO 2 -, NH 4+) is converted to N 2 in low oxygen zones of the ocean Two Pathways Denitrification ( <2 to 10 m. M O 2): 2 NO 3 - + organic matter → N 2 Anammox (<2 m. M O 2) NH 4+ + NO 2 - → N 2 + H 2 O

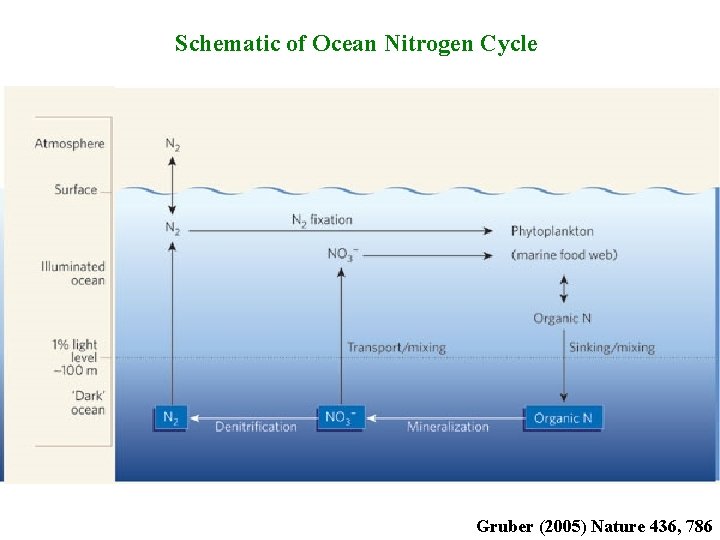
Schematic of Ocean Nitrogen Cycle Gruber (2005) Nature 436, 786

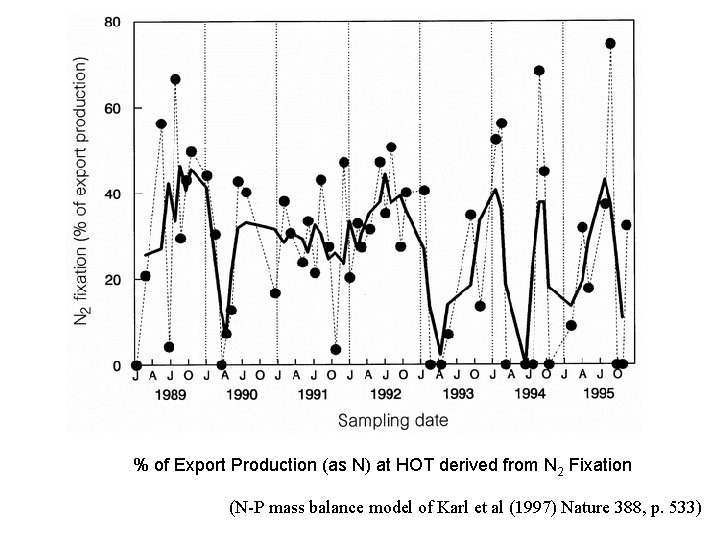
% of Export Production (as N) at HOT derived from N 2 Fixation (N-P mass balance model of Karl et al (1997) Nature 388, p. 533)

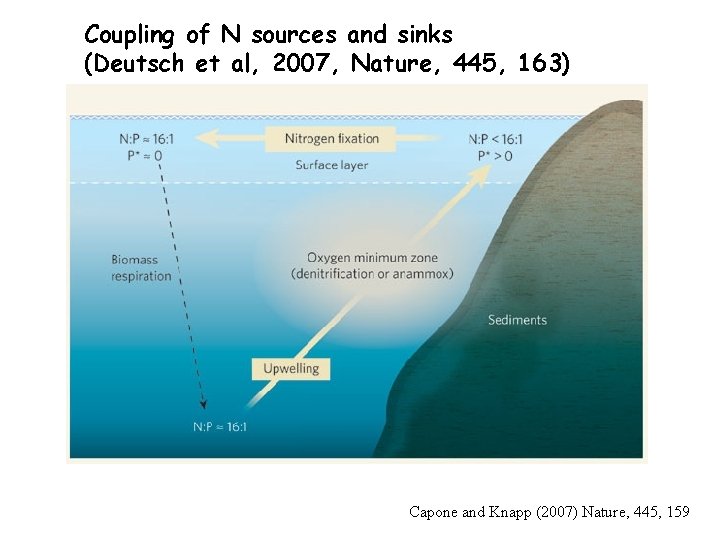
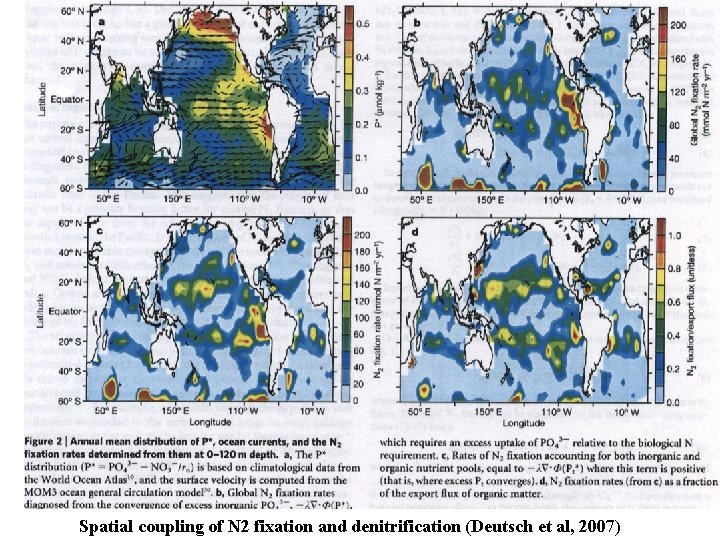
Coupling of N sources and sinks (Deutsch et al, 2007, Nature, 445, 163) Capone and Knapp (2007) Nature, 445, 159
![N is defined as N NO 3 16 x PO 4 2 N* is defined as N* = [NO 3] – 16 x [PO 4] +2.](https://slidetodoc.com/presentation_image_h2/7d4aee9c7042c37e6f9a77d39c96b5b3/image-11.jpg)
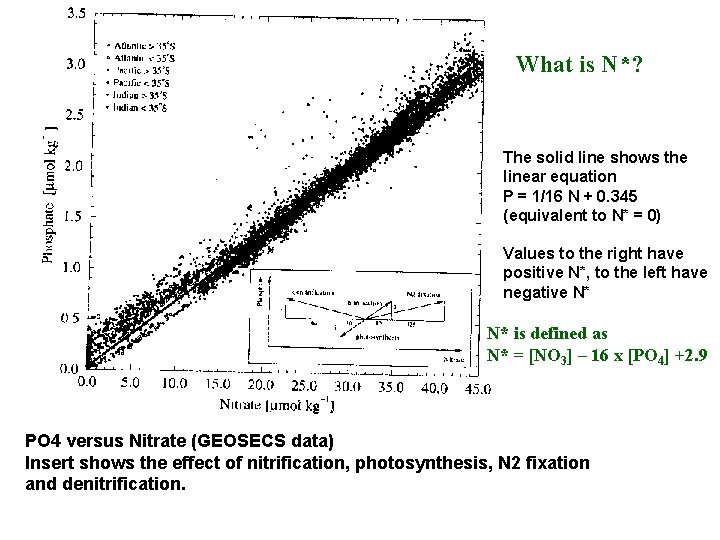
N* is defined as N* = [NO 3] – 16 x [PO 4] +2. 9

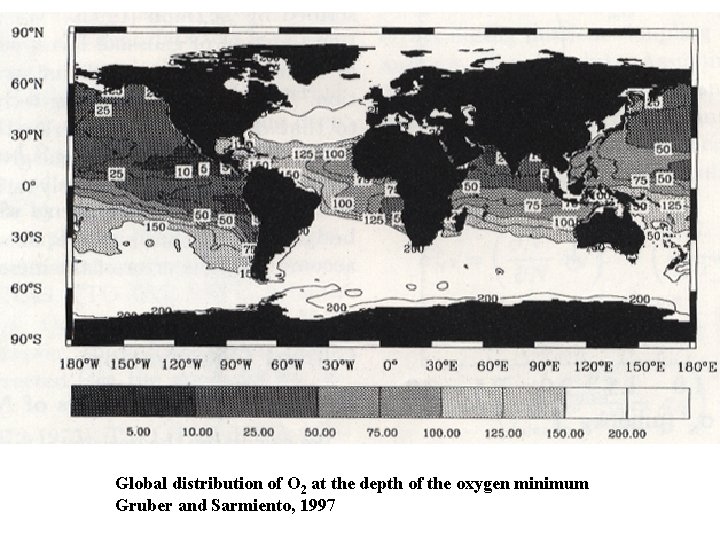
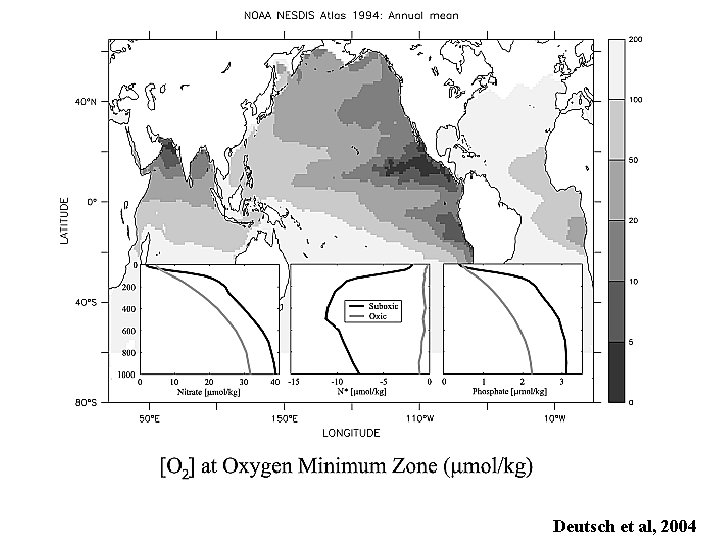
Global distribution of O 2 at the depth of the oxygen minimum Gruber and Sarmiento, 1997

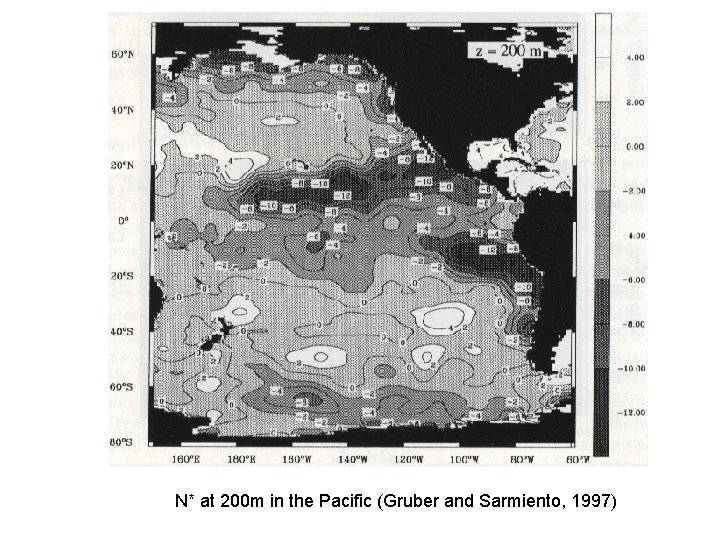
N* at 200 m in the Pacific (Gruber and Sarmiento, 1997)

Nitrogen Cycle w/ anammox Kuypers (2003) Nature 422: 608 -611.

What is N*? The solid line shows the linear equation P = 1/16 N + 0. 345 (equivalent to N* = 0) Values to the right have positive N*, to the left have negative N* N* is defined as N* = [NO 3] – 16 x [PO 4] +2. 9 PO 4 versus Nitrate (GEOSECS data) Insert shows the effect of nitrification, photosynthesis, N 2 fixation and denitrification.


The Global Nitrogen Budget-one example (Brandes et al, 2002)

Spatial coupling of N 2 fixation and denitrification (Deutsch et al, 2007)

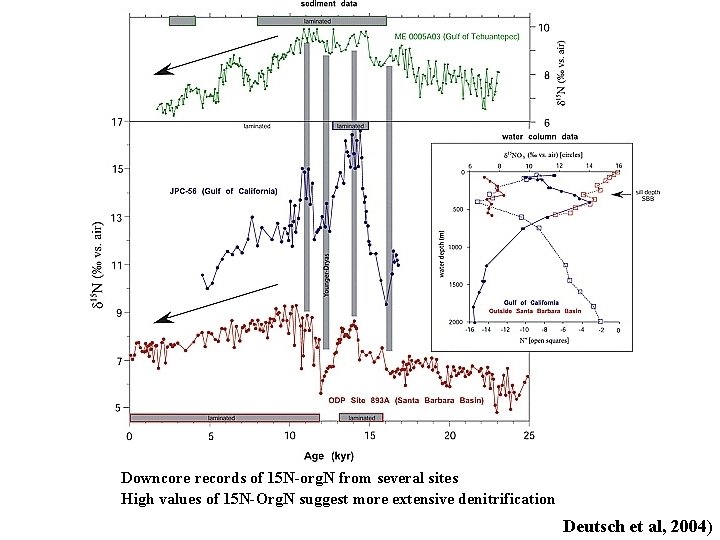
Downcore records of 15 N-org. N from several sites High values of 15 N-Org. N suggest more extensive denitrification Deutsch et al, 2004)

Deutsch et al, 2004