Lecture 15 a Metal Carbonyl Compounds Introduction The

Lecture 15 a Metal Carbonyl Compounds
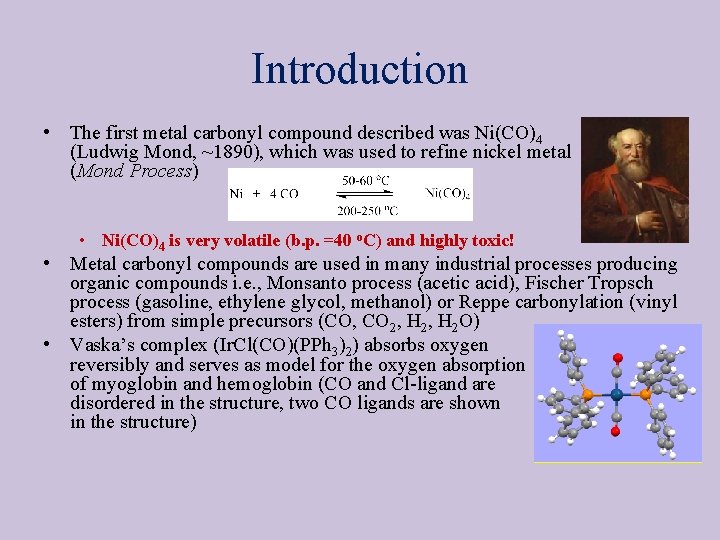
Introduction • The first metal carbonyl compound described was Ni(CO)4 (Ludwig Mond, ~1890), which was used to refine nickel metal (Mond Process) • Ni(CO)4 is very volatile (b. p. =40 o. C) and highly toxic! • Metal carbonyl compounds are used in many industrial processes producing organic compounds i. e. , Monsanto process (acetic acid), Fischer Tropsch process (gasoline, ethylene glycol, methanol) or Reppe carbonylation (vinyl esters) from simple precursors (CO, CO 2, H 2 O) • Vaska’s complex (Ir. Cl(CO)(PPh 3)2) absorbs oxygen reversibly and serves as model for the oxygen absorption of myoglobin and hemoglobin (CO and Cl-ligand are disordered in the structure, two CO ligands are shown in the structure)
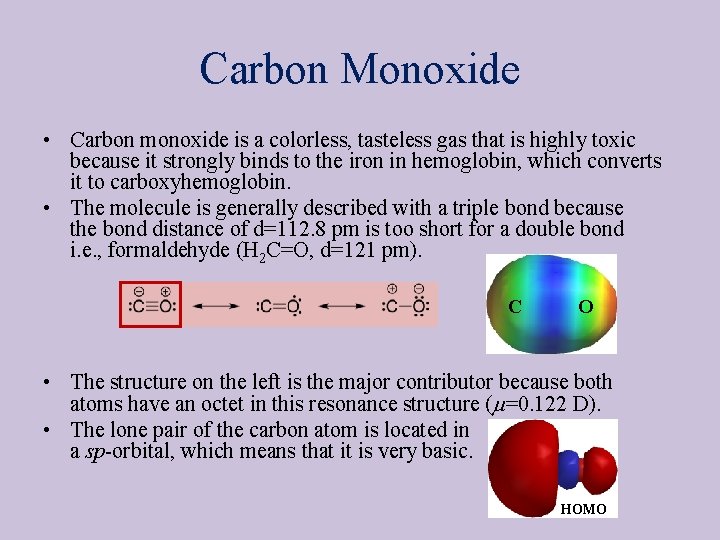
Carbon Monoxide • Carbon monoxide is a colorless, tasteless gas that is highly toxic because it strongly binds to the iron in hemoglobin, which converts it to carboxyhemoglobin. • The molecule is generally described with a triple bond because the bond distance of d=112. 8 pm is too short for a double bond i. e. , formaldehyde (H 2 C=O, d=121 pm). C O • The structure on the left is the major contributor because both atoms have an octet in this resonance structure (m=0. 122 D). • The lone pair of the carbon atom is located in a sp-orbital, which means that it is very basic. HOMO
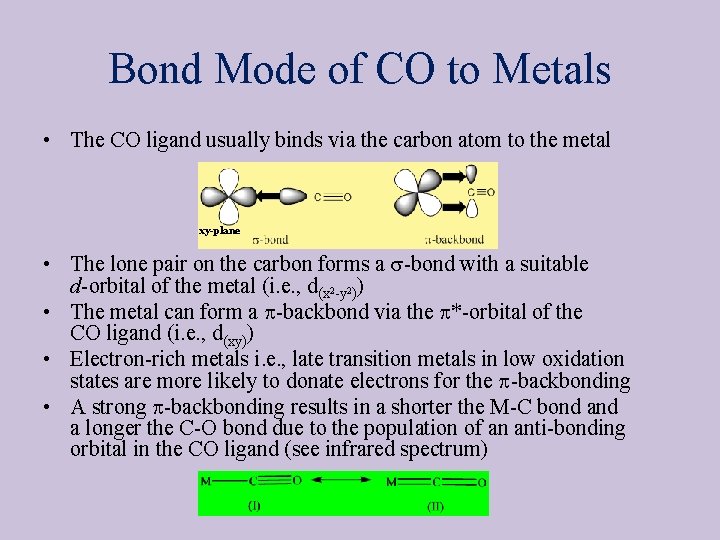
Bond Mode of CO to Metals • The CO ligand usually binds via the carbon atom to the metal xy-plane • The lone pair on the carbon forms a s-bond with a suitable d-orbital of the metal (i. e. , d(x 2 -y 2)) • The metal can form a p-backbond via the p*-orbital of the CO ligand (i. e. , d(xy)) • Electron-rich metals i. e. , late transition metals in low oxidation states are more likely to donate electrons for the p-backbonding • A strong p-backbonding results in a shorter the M-C bond a longer the C-O bond due to the population of an anti-bonding orbital in the CO ligand (see infrared spectrum)
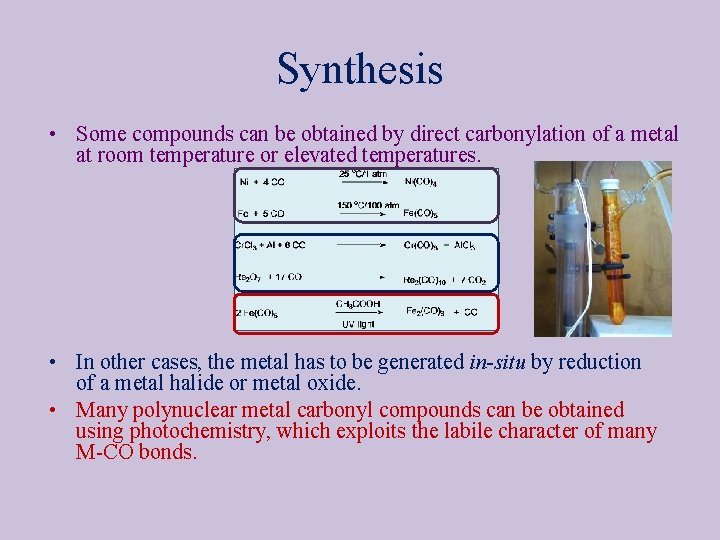
Synthesis • Some compounds can be obtained by direct carbonylation of a metal at room temperature or elevated temperatures. • In other cases, the metal has to be generated in-situ by reduction of a metal halide or metal oxide. • Many polynuclear metal carbonyl compounds can be obtained using photochemistry, which exploits the labile character of many M-CO bonds.
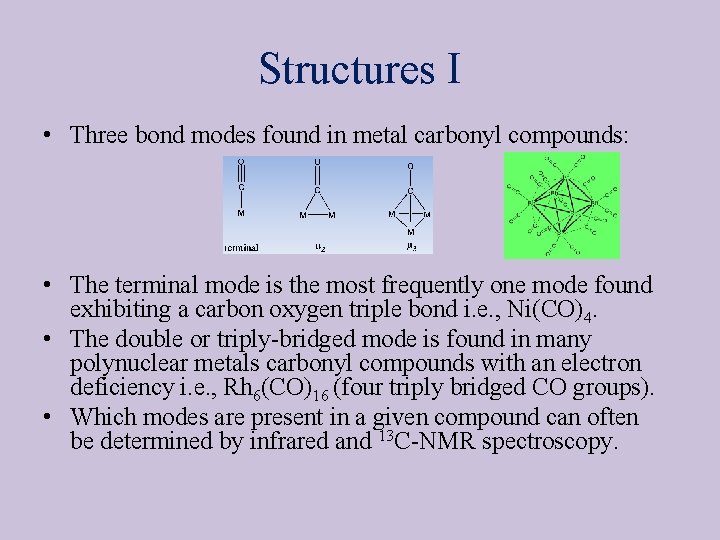
Structures I • Three bond modes found in metal carbonyl compounds: • The terminal mode is the most frequently one mode found exhibiting a carbon oxygen triple bond i. e. , Ni(CO)4. • The double or triply-bridged mode is found in many polynuclear metals carbonyl compounds with an electron deficiency i. e. , Rh 6(CO)16 (four triply bridged CO groups). • Which modes are present in a given compound can often be determined by infrared and 13 C-NMR spectroscopy.
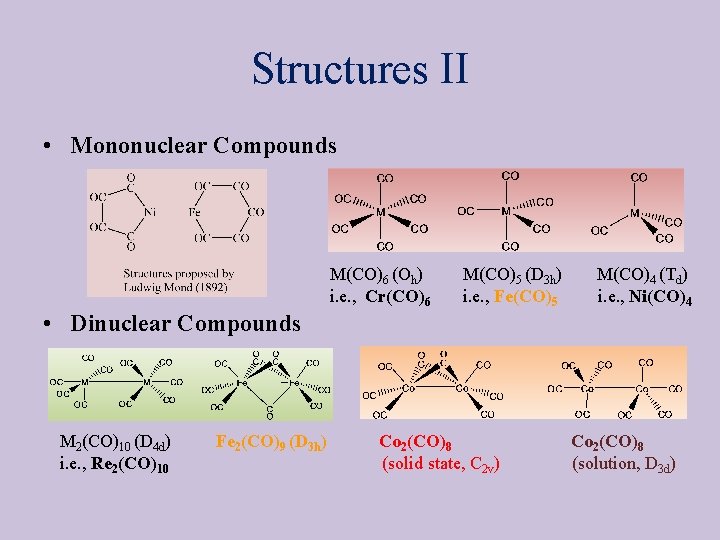
Structures II • Mononuclear Compounds M(CO)6 (Oh) i. e. , Cr(CO)6 M(CO)5 (D 3 h) i. e. , Fe(CO)5 M(CO)4 (Td) i. e. , Ni(CO)4 • Dinuclear Compounds M 2(CO)10 (D 4 d) i. e. , Re 2(CO)10 Fe 2(CO)9 (D 3 h) Co 2(CO)8 (solid state, C 2 v) Co 2(CO)8 (solution, D 3 d)
Infrared Spectroscopy • • • Free CO: 2143 cm-1 Terminal CO groups: 1850 -2125 cm-1 m 2 -brigding CO groups: 1750 -1850 cm-1 m 3 -bridging CO groups: 1620 -1730 cm-1 Compound n(CO) [cm-1] d(CO) [pm] Ni(CO)4 2057 112. 6 Fe(CO)5 2013, 2034 112. 2, 114. 6 Cr(CO)6 2000 114. 0 Re 2(CO)10 1976, 2014, 2070 112 -113, 114. 7 Fe 2(CO)9 1829, 2019, 2082 112. 6, 116. 0 Rh 6(CO)16 1800, 2026, 2073 115. 5, 120. 1 Ag(CO)+ 2204 107. 7 Cu(CO)2+ 2164 111. 0 Non-classical metal carbonyl compounds can have n(CO) greater than the one observed in free CO.

13 C-NMR Spectroscopy • Terminal CO: 180 -220 ppm • Bridging CO: 230 -280 ppm • Examples: • M(CO)6: Cr: 211 ppm, Mo: 201. 2 ppm, W: 193. 1 ppm • Fe(CO)5 • Solid state: 208. 1 ppm (equatorial) and 216 ppm (axial) in a 3: 2 -ratio • Solution: 211. 6 ppm (due to rapid axial-equatorial exchange) • Fe 2(CO)9 (solid state): 204. 2 ppm (terminal), 236. 4 ppm (bridging) • Co 2(CO)8 • Solid state: 182 ppm (terminal), 234 ppm (bridging) • Solution: 205. 3 ppm

Collman’s Reagent • This reagent is obtained from iron pentacarbonyl and sodium hydroxide in an ether i. e. , 1, 4 -dioxane. • It exploits the labile character of the Fe-C bond of alkyl iron compounds, which allows for the insertion of a CO ligand generating a “RC=O-”. • Advantages: high degree of chemoselectivity, produces high yields (70 -90 %), bears low cost and is relatively environmental friendly
Fischer Tropsch Reaction/Process • The reaction was discovered in 1923 • The reaction employs hydrogen, carbon monoxide and a “metal carbonyl catalyst” to form alkanes, alcohols, etc. • Ruhrchemie A. G. (1936) • Used this process to convert synthesis gas into gasoline using a catalyst Co/Th. O 2/Mg. O/Silica gel at 170 -200 o. C at 1 atm • The yield of gasoline was only ~50 % while about 25 % diesel oil and 25 % waxes were formed • An improved process (Sasol) using iron oxides as catalyst, 320 -340 o. C and 25 atm pressure affords 70 % gasoline
![Fischer Tropsch Reaction/Process • Second generation catalyst are homogeneous i. e. , [Rh 6(CO)34]2 Fischer Tropsch Reaction/Process • Second generation catalyst are homogeneous i. e. , [Rh 6(CO)34]2](http://slidetodoc.com/presentation_image_h2/ba4a492bd5a31ba826d70480713c78cd/image-12.jpg)
Fischer Tropsch Reaction/Process • Second generation catalyst are homogeneous i. e. , [Rh 6(CO)34]2 • Union Carbide: ethylene glycol (antifreeze) is obtain at high pressures (3000 atm, 250 o. C) Gasolines • Production of long-chain alkanes is favored at a temperature around 220 o. C and pressures of 1 -30 atm
![Monsanto Process (Acetic Acid) • This process uses cis-[(CO)2 Rh. I 2]- as catalyst Monsanto Process (Acetic Acid) • This process uses cis-[(CO)2 Rh. I 2]- as catalyst](http://slidetodoc.com/presentation_image_h2/ba4a492bd5a31ba826d70480713c78cd/image-13.jpg)
Monsanto Process (Acetic Acid) • This process uses cis-[(CO)2 Rh. I 2]- as catalyst to convert methanol and carbon dioxide to acetic acid • The reaction is carried out at 180 o. C and 30 atm pressure Oxidative Addition (+I to +III) Reductive Elimination (+III to +I) CO Insertion CO Addition • Two separate cycles that are combined with each other • The BP Captiva Process uses cis-[(CO)2 Ir. I 2]- as catalyst
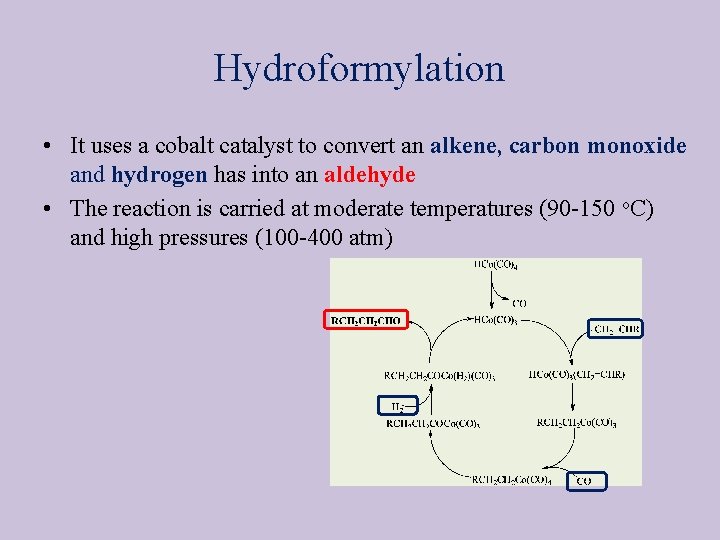
Hydroformylation • It uses a cobalt catalyst to convert an alkene, carbon monoxide and hydrogen has into an aldehyde • The reaction is carried at moderate temperatures (90 -150 o. C) and high pressures (100 -400 atm)
![Pauson–Khand Reaction • The Pauson–Khand reaction is a [2+2+1] cycloaddition reaction between an alkene, Pauson–Khand Reaction • The Pauson–Khand reaction is a [2+2+1] cycloaddition reaction between an alkene,](http://slidetodoc.com/presentation_image_h2/ba4a492bd5a31ba826d70480713c78cd/image-15.jpg)
Pauson–Khand Reaction • The Pauson–Khand reaction is a [2+2+1] cycloaddition reaction between an alkene, alkyne and carbon monoxide to form an α, β-cyclopentenone • Originally it was catalyzed by dicobalt octacarbonyl, more recently also by Rh-complexes (i. e. , Wilkinson’s complex with silver triflate as co-catalyst)
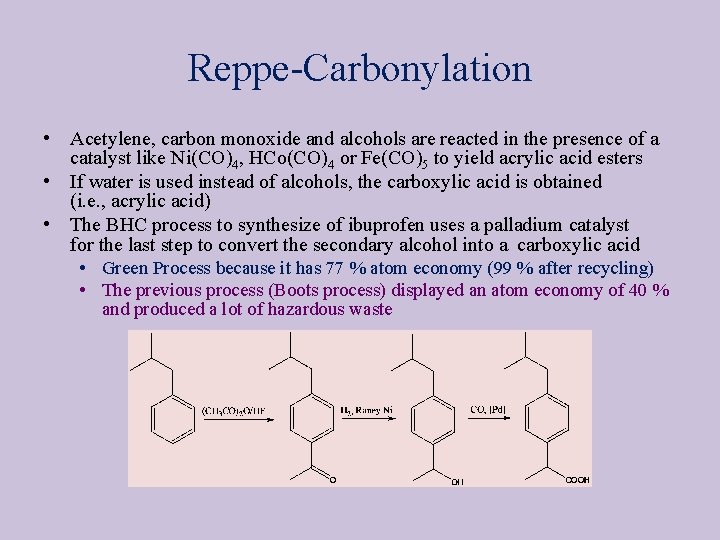
Reppe-Carbonylation • Acetylene, carbon monoxide and alcohols are reacted in the presence of a catalyst like Ni(CO)4, HCo(CO)4 or Fe(CO)5 to yield acrylic acid esters • If water is used instead of alcohols, the carboxylic acid is obtained (i. e. , acrylic acid) • The BHC process to synthesize of ibuprofen uses a palladium catalyst for the last step to convert the secondary alcohol into a carboxylic acid • Green Process because it has 77 % atom economy (99 % after recycling) • The previous process (Boots process) displayed an atom economy of 40 % and produced a lot of hazardous waste
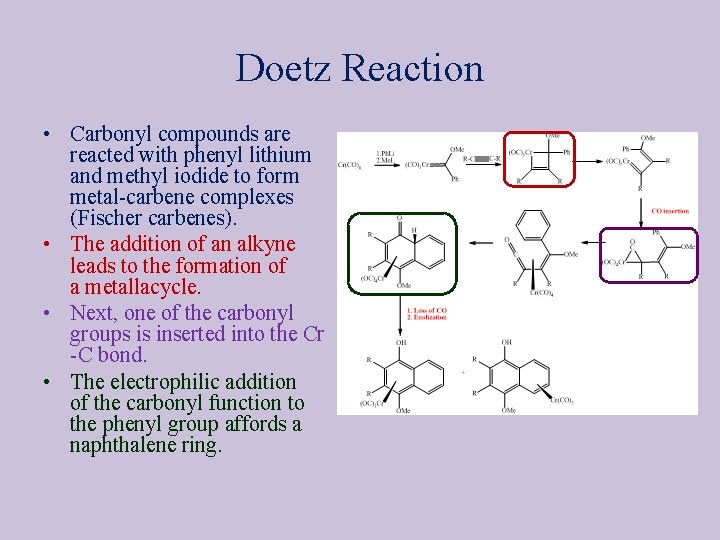
Doetz Reaction • Carbonyl compounds are reacted with phenyl lithium and methyl iodide to form metal-carbene complexes (Fischer carbenes). • The addition of an alkyne leads to the formation of a metallacycle. • Next, one of the carbonyl groups is inserted into the Cr -C bond. • The electrophilic addition of the carbonyl function to the phenyl group affords a naphthalene ring.

Further Reading • Werner, H. : Landmarks in Organo-Transition Metal Chemistry, Springer, 2009
- Slides: 18