Lecture 14 Chemical Kinetics Dr A K M

![Example 5 Time, min [A], M log [A] 1/[A] 0 1. 00 0. 00 Example 5 Time, min [A], M log [A] 1/[A] 0 1. 00 0. 00](https://slidetodoc.com/presentation_image_h/01b5ffe0697f5339f87149185df48397/image-7.jpg)
![The Hydrogen-Iodine Reaction 1 st step: [Fast] 2 nd step: [Slow] Net: �If the The Hydrogen-Iodine Reaction 1 st step: [Fast] 2 nd step: [Slow] Net: �If the](https://slidetodoc.com/presentation_image_h/01b5ffe0697f5339f87149185df48397/image-18.jpg)
![Enzymatic catalysis �Rate of Catalysis - At a fixed enzyme concentration [E], the initial Enzymatic catalysis �Rate of Catalysis - At a fixed enzyme concentration [E], the initial](https://slidetodoc.com/presentation_image_h/01b5ffe0697f5339f87149185df48397/image-24.jpg)
![Enzymatic catalysis Effect of enzyme concentration [E] on velocity (v) In fixed, saturating [S], Enzymatic catalysis Effect of enzyme concentration [E] on velocity (v) In fixed, saturating [S],](https://slidetodoc.com/presentation_image_h/01b5ffe0697f5339f87149185df48397/image-26.jpg)
- Slides: 34

Lecture – 14 Chemical Kinetics Dr. A. K. M. Shafiqul Islam Dept. of Chemical Engineering Technology Industrial Biotechnology University Malaysia Perlis 24/04/2015 2/19/2021

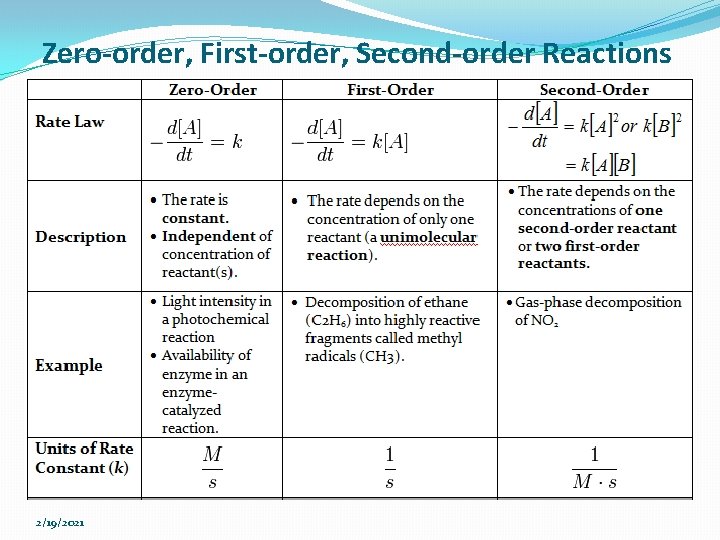
Zero-order, First-order, Second-order Reactions 2/19/2021

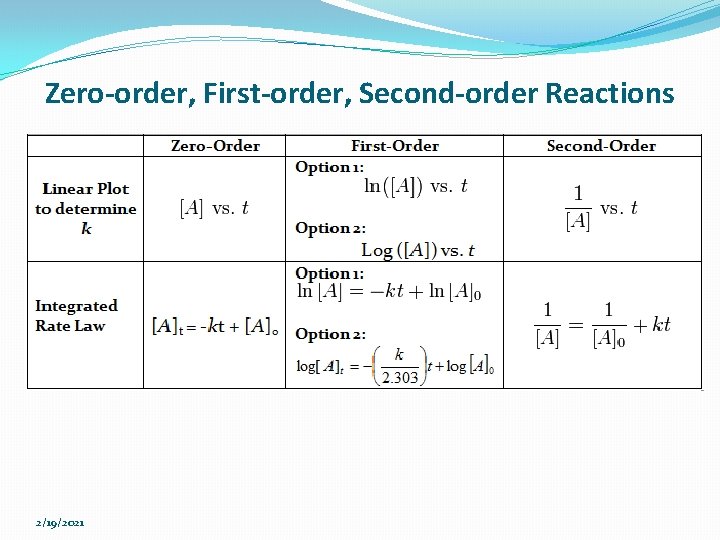
Zero-order, First-order, Second-order Reactions 2/19/2021

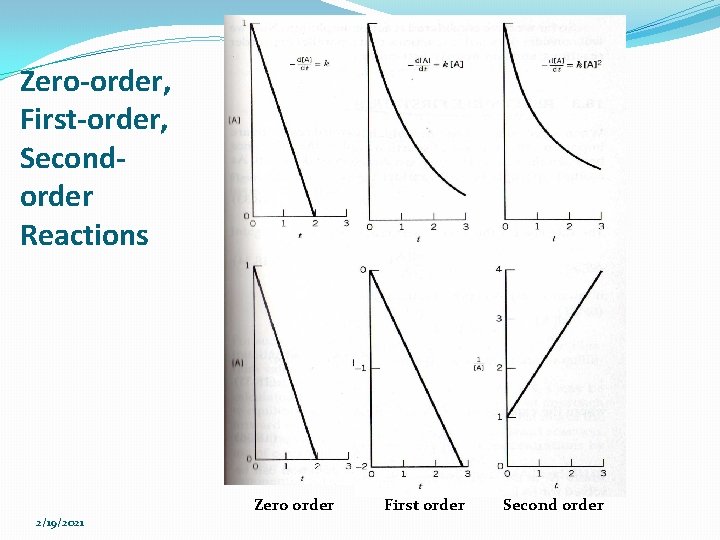
Zero-order, First-order, Secondorder Reactions Zero order 2/19/2021 First order Second order

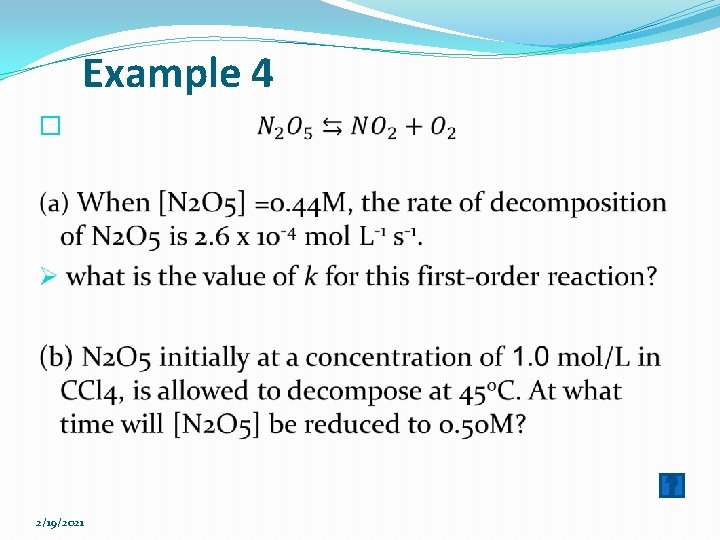
Example 4 � 2/19/2021

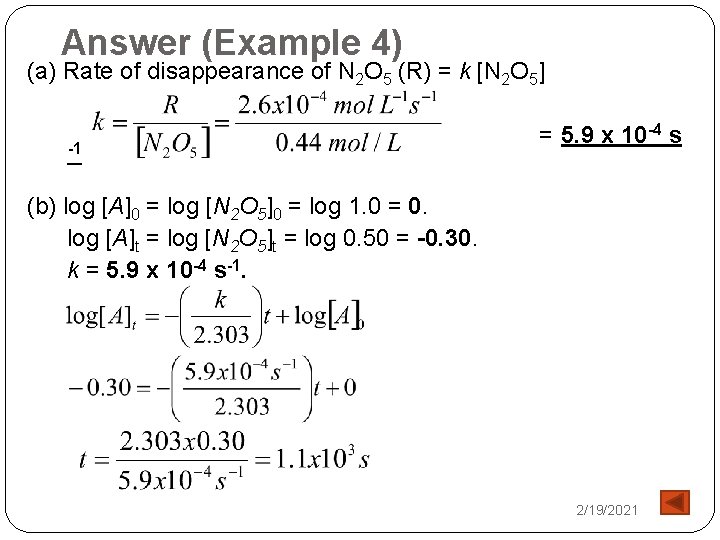
Answer (Example 4) (a) Rate of disappearance of N 2 O 5 (R) = k [N 2 O 5] -1 = 5. 9 x 10 -4 s (b) log [A]0 = log [N 2 O 5]0 = log 1. 0 = 0. log [A]t = log [N 2 O 5]t = log 0. 50 = -0. 30. k = 5. 9 x 10 -4 s-1. 2/19/2021
![Example 5 Time min A M log A 1A 0 1 00 0 00 Example 5 Time, min [A], M log [A] 1/[A] 0 1. 00 0. 00](https://slidetodoc.com/presentation_image_h/01b5ffe0697f5339f87149185df48397/image-7.jpg)
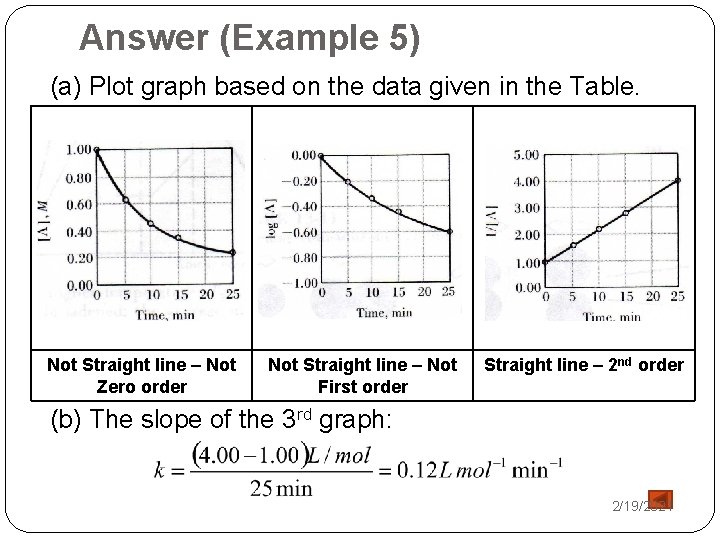
Example 5 Time, min [A], M log [A] 1/[A] 0 1. 00 0. 00 1. 00 5 0. 63 -0. 20 1. 59 10 0. 46 -0. 34 2. 17 15 0. 36 -0. 44 2. 78 25 0. 25 -0. 60 4. 00 �The data of the above table were obtained for the decomposition reaction: A → 2 B + C. (a) Establish the order of the reaction. (b) What is the rate constant, k? 2/19/2021

Answer (Example 5) (a) Plot graph based on the data given in the Table. Not Straight line – Not Zero order (b) The slope of Not Straight line – Not First order the 3 rd graph: Straight line – 2 nd order 2/19/2021

Reaction rates: Effect of temperature �Chemical reactions tend to go faster at higher temperature. Ø slow down some reactions by lowering the temperature. �Increasing the temperature increases the fraction of the molecules that have energies in excess of the activation energy. Ø this factor is so important that for many chemical reactions it can lead to a doubling or tripling of the reaction rate for a temperature increase of only 100 C. 2/19/2021

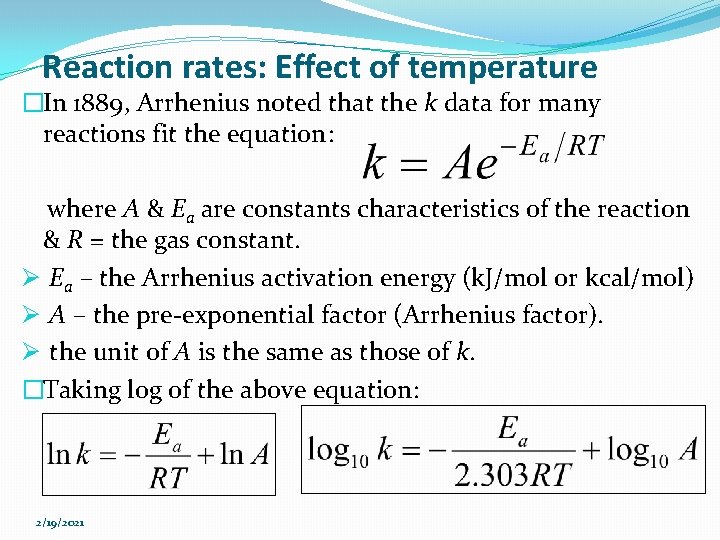
Reaction rates: Effect of temperature �In 1889, Arrhenius noted that the k data for many reactions fit the equation: where A & Ea are constants characteristics of the reaction & R = the gas constant. Ø Ea – the Arrhenius activation energy (k. J/mol or kcal/mol) Ø A – the pre-exponential factor (Arrhenius factor). Ø the unit of A is the same as those of k. �Taking log of the above equation: 2/19/2021

Reaction rates: Effect of temperature �If the Arrhenius equation is obeyed: Ø a plot of log 10 k versus 1/T is a straight line with slope: -Ea/2. 303 R and intercept log 10 A. Ø This enables Ea and A to be found. • Another useful equation: (eliminate the constant A). Ø T 2 and T 1 - two kelvin temperatures. Ø k 2 and k 1 - the rate constants at these temperatures. Ø Ea – the activation energy (J/mol) Ø R – the gas constant (8. 314 Jmol-1 K-1). 2/19/2021

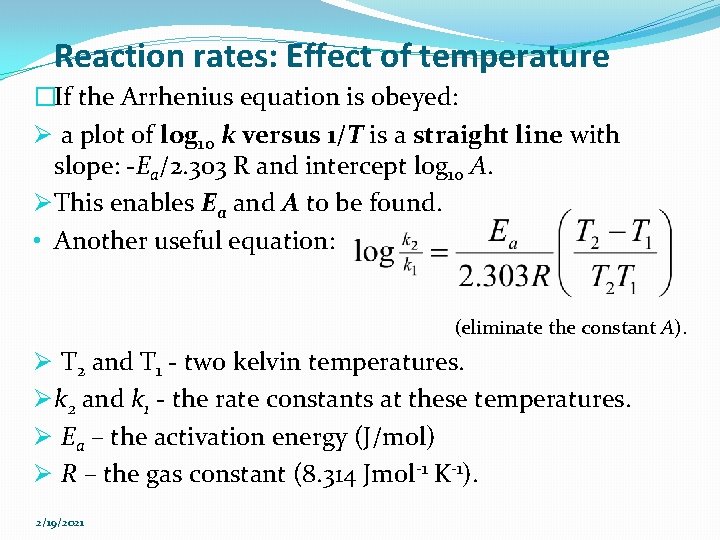
Example 6 (a) Use the figure given to find A and Ea for: (b) Calculate Ea for a reaction where rate constant at room temperature is doubled by a 100 C increase in T. 2/19/2021 Figure: Rate constant versus temperature for the gas-phase first order decomposition reaction

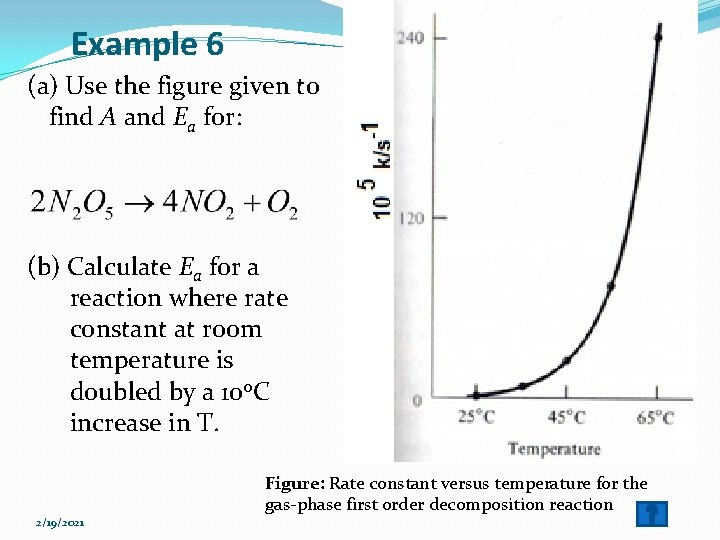
Answer (Example 6 a) �Tabulate the data as follows. Temp, 0 C Temp, K 1/Temp, 1/K k, s-1 log 10 k 25 298 0. 0034 0. 001 -3 �Construct the Arrhenius plot of log 10 k versus 1/T for the reaction. Ø Intercept (log 10 A)=13. 5 A = 3 x 1013 s-1 Ø Slope=-5500 K, Ea=25 kcal/mol =105 k. J/mol Figure: Arrhenius plot of log 10 k versus 1/T for this 2/19/2021 reaction. Note: the long extrapolation needed to find A.

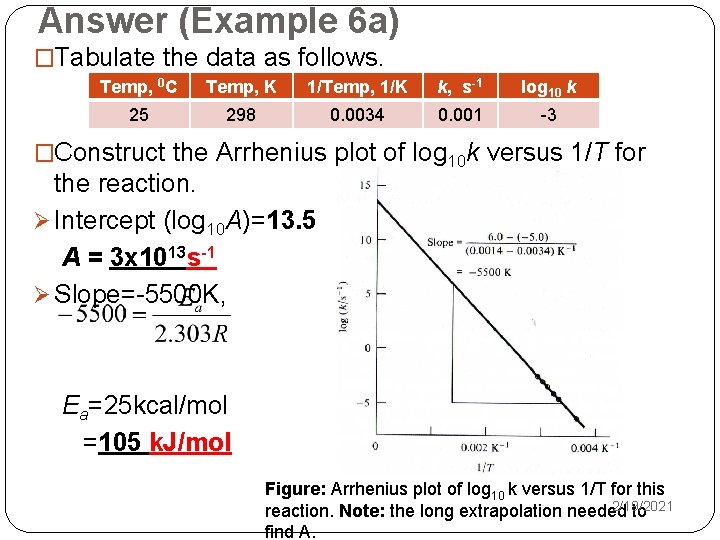
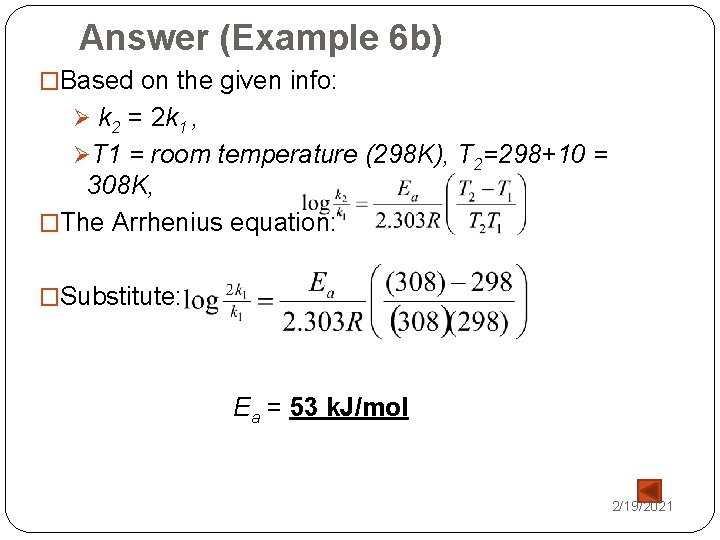
Answer (Example 6 b) �Based on the given info: Ø k 2 = 2 k 1 , ØT 1 = room temperature (298 K), T 2=298+10 = 308 K, �The Arrhenius equation: �Substitute: Ea = 53 k. J/mol 2/19/2021

Reaction Mechanisms �Each molecular event that significantly alters a molecule’s energy or geometry is called an elementary process (reaction). �The mechanism of a reaction: Ø the sequence of elementary reactions that add up to give the overall reaction. �A mechanism is a hypothesis about the elementary steps through which chemical change occurs. 2/19/2021

Reaction Mechanisms �Elementary processes in which a single molecule dissociates (unimolecular) or two molecules collide (bimolecular) much more probable than a process requiring the simultaneous collision of three bodies (termolecular). �All elementary processes are reversible and may reach a steady-state condition. In the steady state the rates of the forward & reverse processes become equal. The concentration of some intermediate becomes constant with time. �One elementary process may occur much more slower than all the others. In this case, it determines the rate at which the overall reaction proceeds & is called the ratedetermining step. 2/19/2021

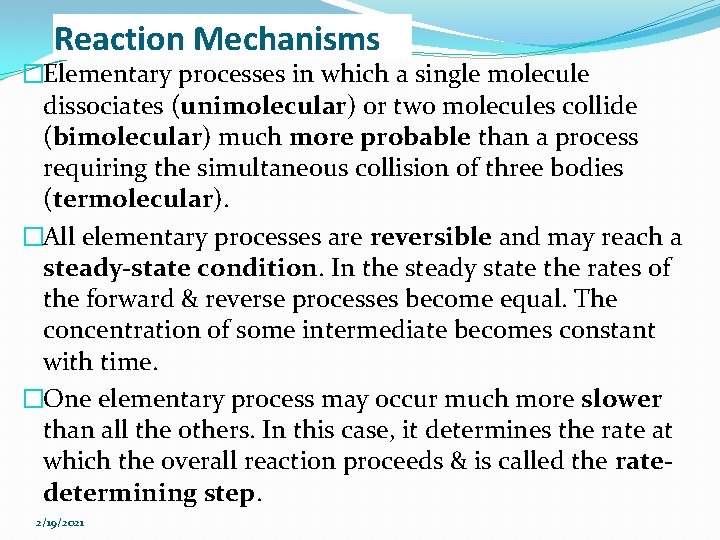
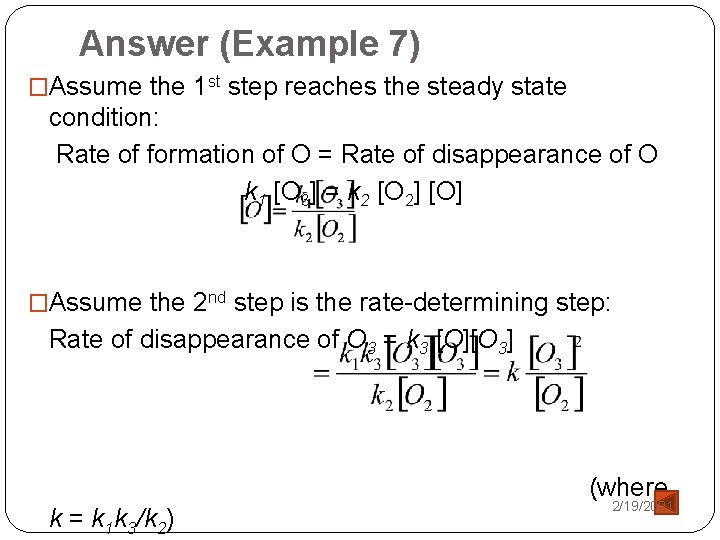
The Hydrogen-Iodine Reaction H 2 (g) + I 2 (g) → 2 HI (g) �Rate of formation of HI = k [H 2][I 2] �The hydrogen-iodine reaction is proposed to be a twostep mechanism [Sullivan J. (1967). J. Chem. Phys. 46: 73]. Ø 1 st step: iodine molecules are believed to dissociate into iodine atoms. Ø 2 nd step: simultaneous collision of two iodine atoms and a hydrogen molecule. (this termolecular step is expected to occur much more slowly – the rate-determining step). 2/19/2021
![The HydrogenIodine Reaction 1 st step Fast 2 nd step Slow Net If the The Hydrogen-Iodine Reaction 1 st step: [Fast] 2 nd step: [Slow] Net: �If the](https://slidetodoc.com/presentation_image_h/01b5ffe0697f5339f87149185df48397/image-18.jpg)
The Hydrogen-Iodine Reaction 1 st step: [Fast] 2 nd step: [Slow] Net: �If the reversible step reaches a steady state condition: Ø rate of disappearance of I 2 = rate of formation of I 2 2/19/2021

The Hydrogen-Iodine Reaction �For the rate-determining step: Rate of formation of HI = k 3 [I]2[H 2] = K[H 2][I 2] (K=k 1 k 3/k 2) 2/19/2021

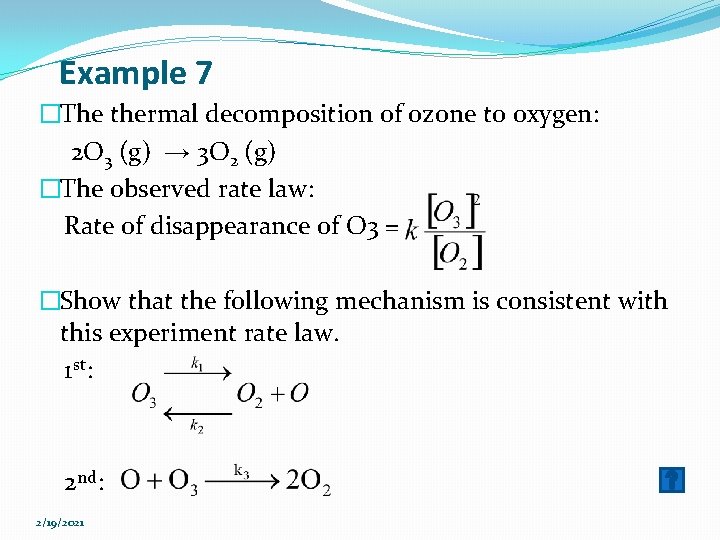
Example 7 �The thermal decomposition of ozone to oxygen: 2 O 3 (g) → 3 O 2 (g) �The observed rate law: Rate of disappearance of O 3 = �Show that the following mechanism is consistent with this experiment rate law. 1 st: 2 nd: 2/19/2021

Answer (Example 7) �Assume the 1 st step reaches the steady state condition: Rate of formation of O = Rate of disappearance of O k 1 [O 3] = k 2 [O 2] [O] �Assume the 2 nd step is the rate-determining step: Rate of disappearance of O 3 = k 3 [O][O 3] (where k = k 1 k 3/k 2) 2/19/2021

Enzymatic catalysis • An enzyme is a protein molecule that is a biological catalyst that catalyzes chemical reaction. • A catalyst is a substance that increasing the rate (speed) of a chemical reaction itself being consumed or transformed. • It participates in reactions but is neither a chemical reactant nor 2/19/2021

Enzymatic catalysis � During reaction enzyme molecules, E, and substrate molecules, S, combine in a reversible step to form an intermediate enzyme-substrate (ES) complex E + S k 1 k-1 ES k 2 E + P k-2 k 1, k-1, k 2, k-2 - rate constant - indicate the speed or efficiency of a reaction 2/19/2021
![Enzymatic catalysis Rate of Catalysis At a fixed enzyme concentration E the initial Enzymatic catalysis �Rate of Catalysis - At a fixed enzyme concentration [E], the initial](https://slidetodoc.com/presentation_image_h/01b5ffe0697f5339f87149185df48397/image-24.jpg)
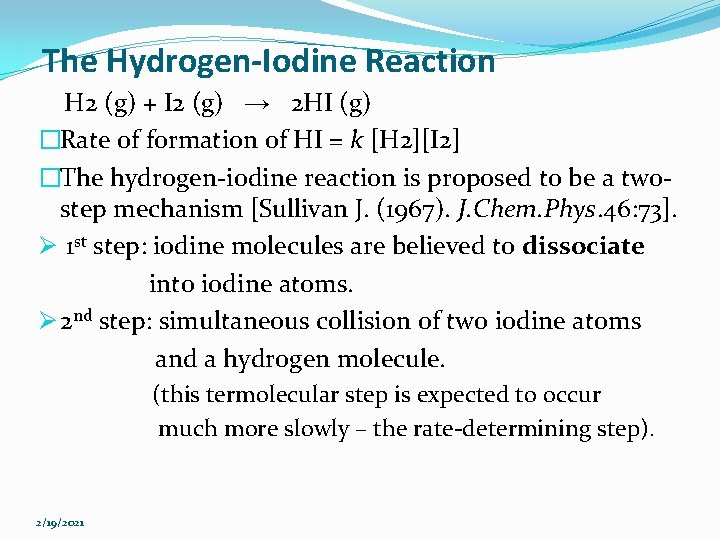
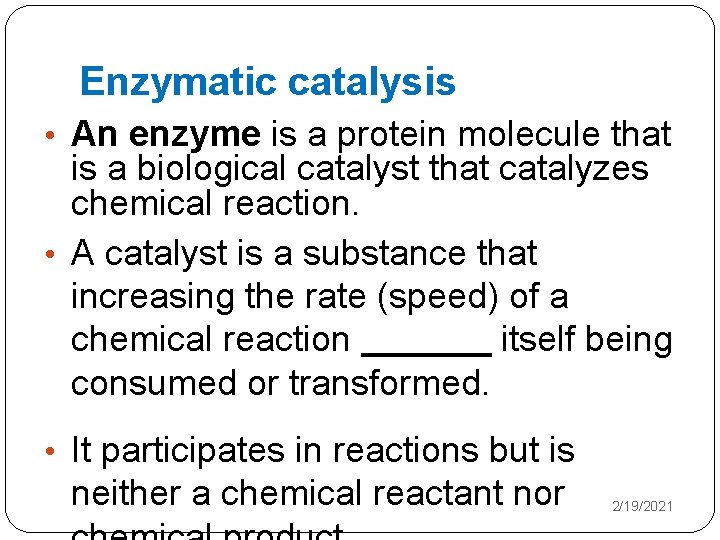
Enzymatic catalysis �Rate of Catalysis - At a fixed enzyme concentration [E], the initial velocity Vo is almost linearly proportional to substrate concentration [S] when [S] is small but is nearly independent of [S] when [S] is large - Rate rises linearly as [S] increases and then levels off at high [S] (saturated) 2/19/2021

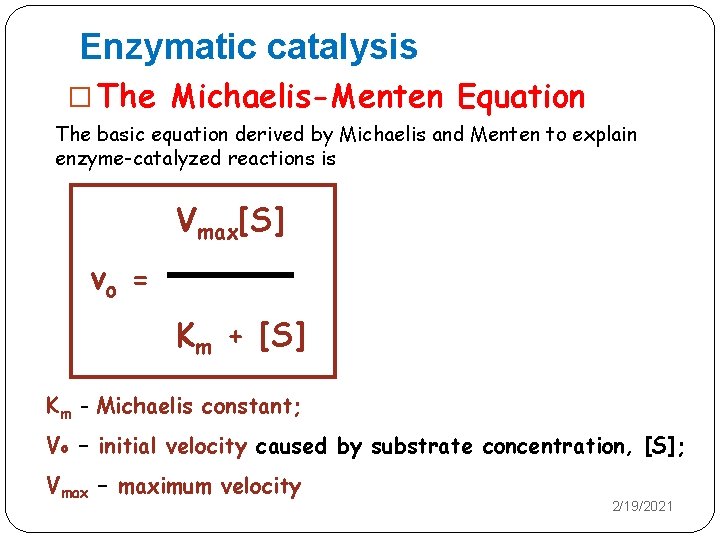
Enzymatic catalysis � The Michaelis-Menten Equation The basic equation derived by Michaelis and Menten to explain enzyme-catalyzed reactions is Vmax[S] vo = Km + [S] Km - Michaelis constant; Vo – initial velocity caused by substrate concentration, [S]; Vmax – maximum velocity 2/19/2021
![Enzymatic catalysis Effect of enzyme concentration E on velocity v In fixed saturating S Enzymatic catalysis Effect of enzyme concentration [E] on velocity (v) In fixed, saturating [S],](https://slidetodoc.com/presentation_image_h/01b5ffe0697f5339f87149185df48397/image-26.jpg)
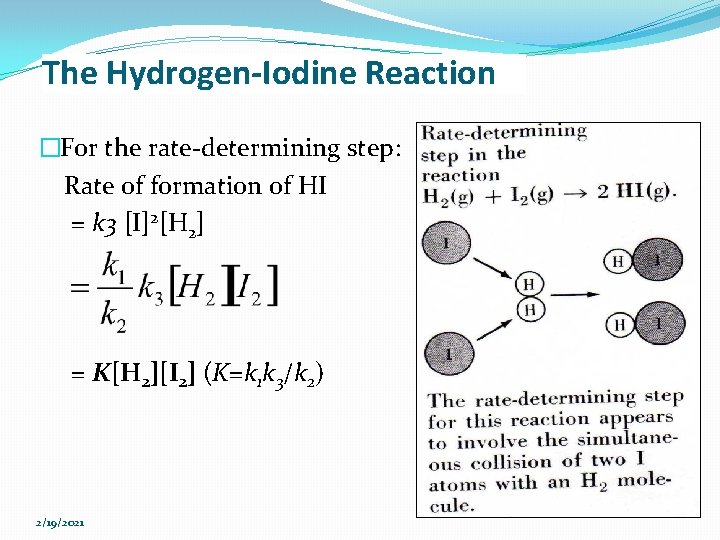
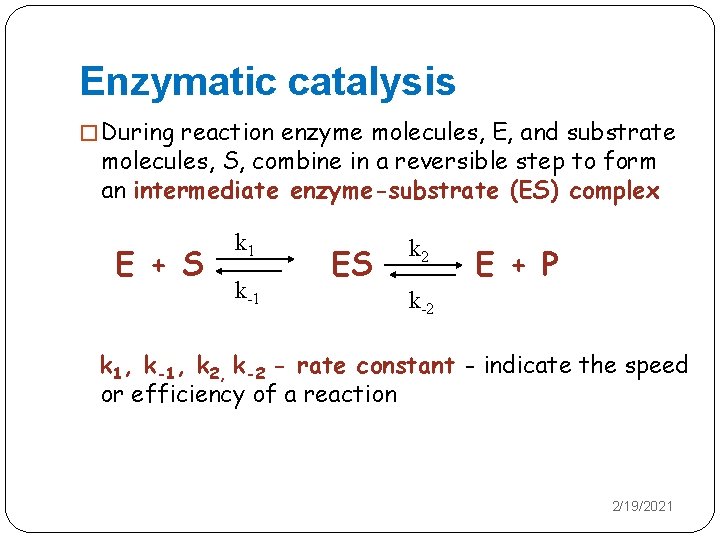
Enzymatic catalysis Effect of enzyme concentration [E] on velocity (v) In fixed, saturating [S], the higher the concentration of enzyme, the greater the initial reaction rate This relationship will hold as long as there is enough substrate present 2/19/2021

Experimental methods for fast reactions �Many reactions are too fast to follow by the classical methods. �Several ways to study fast reactions : 1. Rapid flow methods: (i) Continuous flow (ii) Stopped flow 2. Relaxation methods: (i) Temperature jump (T-jump) method (ii) Pressure jump method (iii)Electric field jump method 3. Flash photolysis 4. Shock tube 5. Nuclear-magnetic-resonance (NMR) spectroscopy 2/19/2021

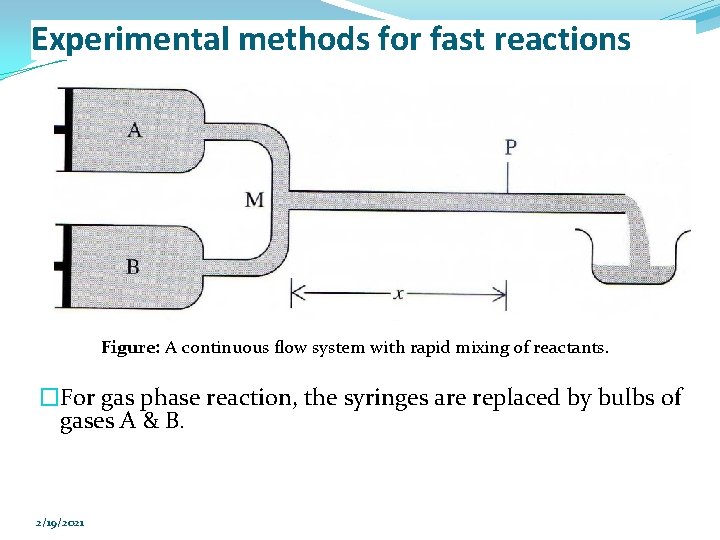
Experimental methods for fast reactions �Continuous flow system �Liquid phase: Ø Reactant A & B are rapidly drive into the mixing chamber M by pushing in the plungers of the syringes. Ø Mixing occurs in 0. 5 – 1 ms. Ø The reaction mixture then flows through the narrow observation tube, where one measures the light absorption at a wavelength (at which one species absorbs to determine the concentration of that species). 2/19/2021

Experimental methods for fast reactions Figure: A continuous flow system with rapid mixing of reactants. �For gas phase reaction, the syringes are replaced by bulbs of gases A & B. 2/19/2021

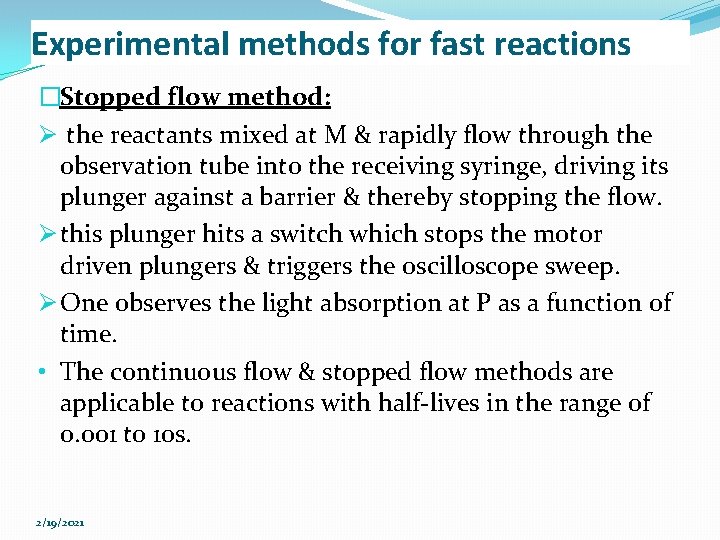
Experimental methods for fast reactions �Stopped flow method: Ø the reactants mixed at M & rapidly flow through the observation tube into the receiving syringe, driving its plunger against a barrier & thereby stopping the flow. Ø this plunger hits a switch which stops the motor driven plungers & triggers the oscilloscope sweep. Ø One observes the light absorption at P as a function of time. • The continuous flow & stopped flow methods are applicable to reactions with half-lives in the range of 0. 001 to 10 s. 2/19/2021

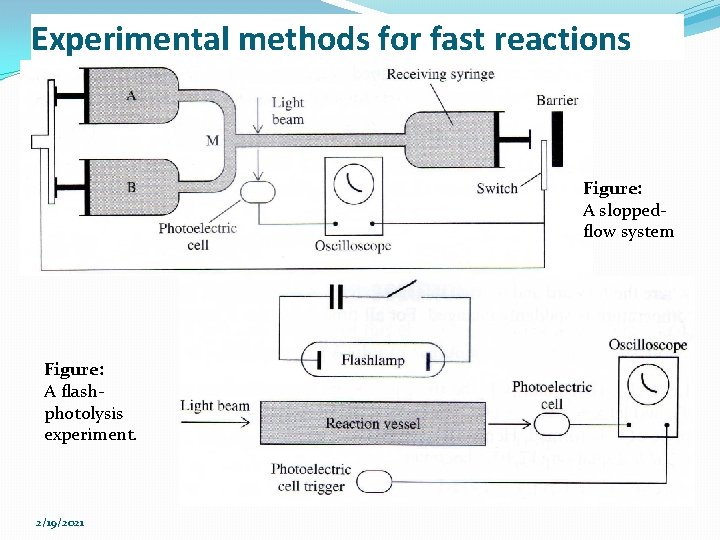
Experimental methods for fast reactions Figure: A sloppedflow system Figure: A flashphotolysis experiment. 2/19/2021

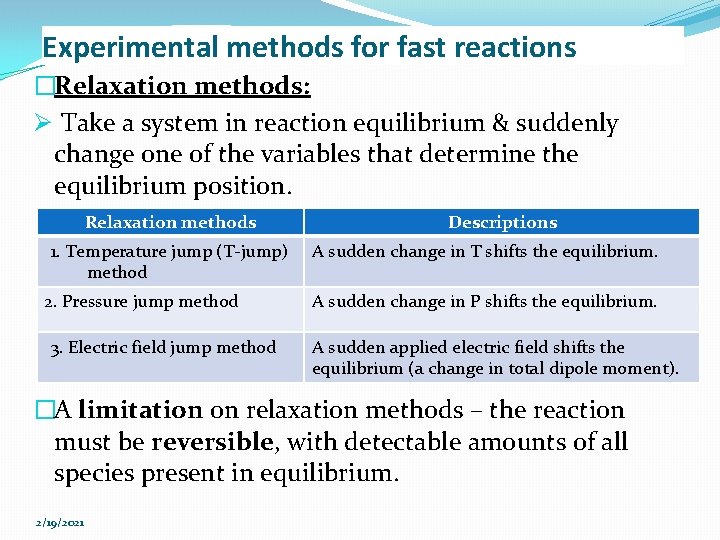
Experimental methods for fast reactions �Relaxation methods: Ø Take a system in reaction equilibrium & suddenly change one of the variables that determine the equilibrium position. Relaxation methods 1. Temperature jump (T-jump) method 2. Pressure jump method 3. Electric field jump method Descriptions A sudden change in T shifts the equilibrium. A sudden change in P shifts the equilibrium. A sudden applied electric field shifts the equilibrium (a change in total dipole moment). �A limitation on relaxation methods – the reaction must be reversible, with detectable amounts of all species present in equilibrium. 2/19/2021

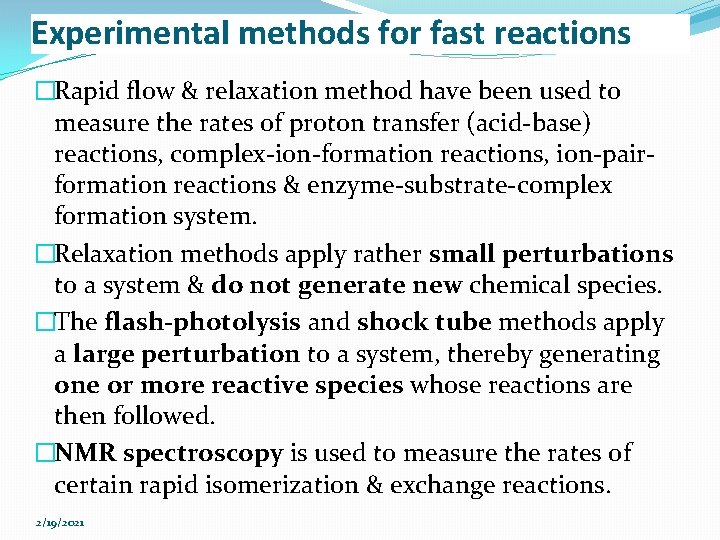
Experimental methods for fast reactions �Rapid flow & relaxation method have been used to measure the rates of proton transfer (acid-base) reactions, complex-ion-formation reactions, ion-pairformation reactions & enzyme-substrate-complex formation system. �Relaxation methods apply rather small perturbations to a system & do not generate new chemical species. �The flash-photolysis and shock tube methods apply a large perturbation to a system, thereby generating one or more reactive species whose reactions are then followed. �NMR spectroscopy is used to measure the rates of certain rapid isomerization & exchange reactions. 2/19/2021

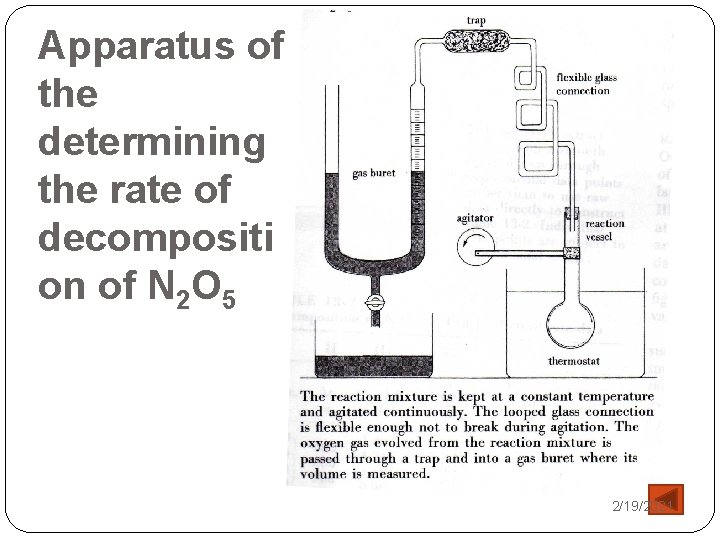
Apparatus of the determining the rate of decompositi on of N 2 O 5 2/19/2021