Lecture 14 BIOAVAILABILITY Bioavailability Bioavailibility and Routes of

Lecture #14 BIOAVAILABILITY
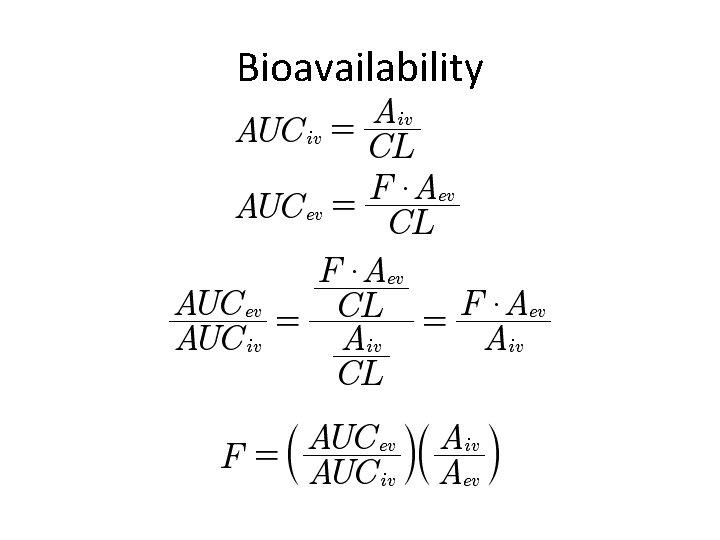
Bioavailability
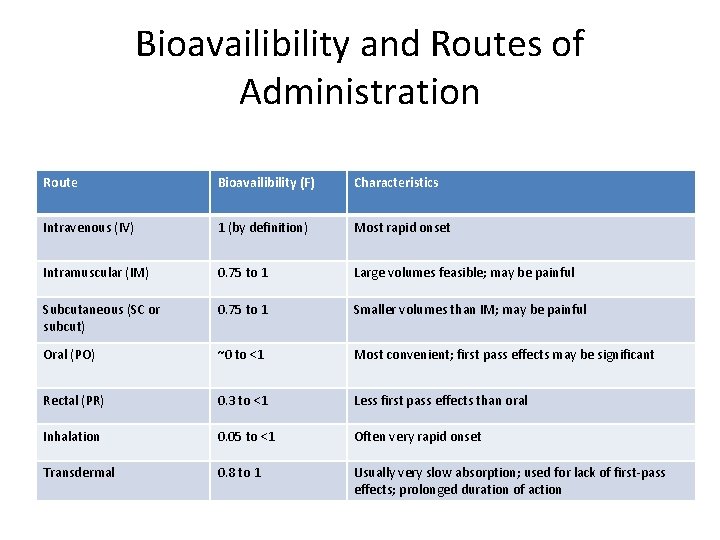
Bioavailibility and Routes of Administration Route Bioavailibility (F) Characteristics Intravenous (IV) 1 (by definition) Most rapid onset Intramuscular (IM) 0. 75 to 1 Large volumes feasible; may be painful Subcutaneous (SC or subcut) 0. 75 to 1 Smaller volumes than IM; may be painful Oral (PO) ~0 to <1 Most convenient; first pass effects may be significant Rectal (PR) 0. 3 to <1 Less first pass effects than oral Inhalation 0. 05 to <1 Often very rapid onset Transdermal 0. 8 to 1 Usually very slow absorption; used for lack of first-pass effects; prolonged duration of action
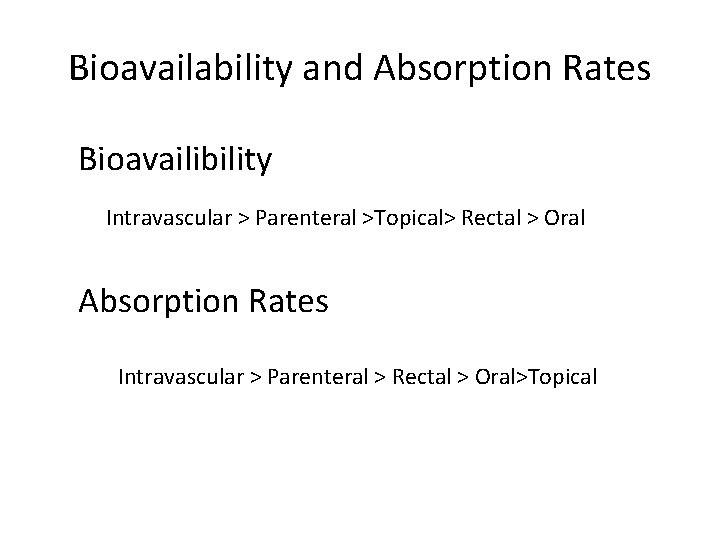
Bioavailability and Absorption Rates Bioavailibility Intravascular > Parenteral >Topical> Rectal > Oral Absorption Rates Intravascular > Parenteral > Rectal > Oral>Topical
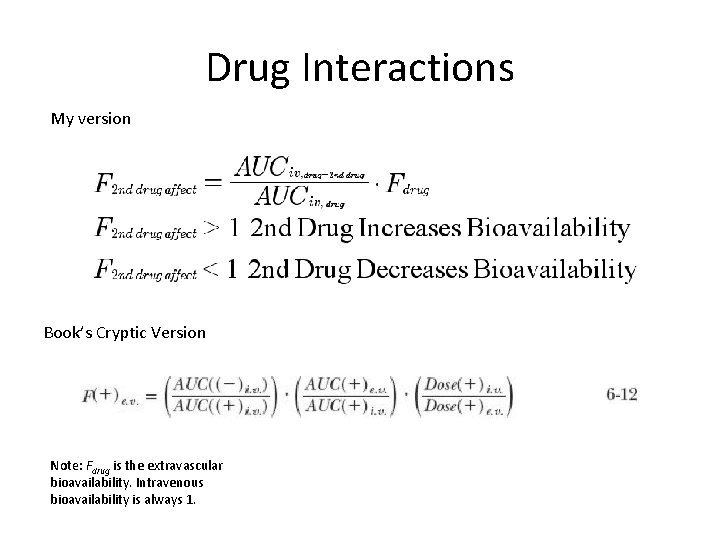
Drug Interactions My version Book’s Cryptic Version Note: Fdrug is the extravascular bioavailability. Intravenous bioavailability is always 1.
Relative Bioavailability • Dosage Forms • Different Extravascular Administration Routes • Different Conditions
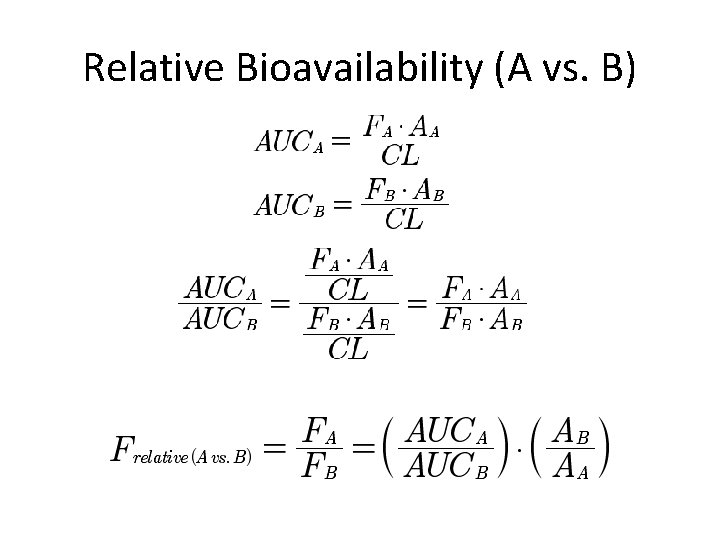
Relative Bioavailability (A vs. B)
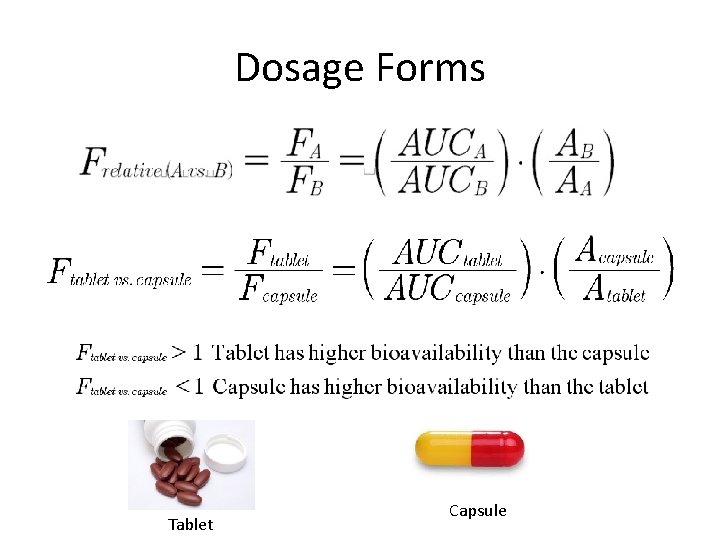
Dosage Forms Tablet Capsule
Dosage Forms Tablet Capsule
Extravascular Administration Routes Oral Intramuscular (I. M. )
Conditions Without food With food
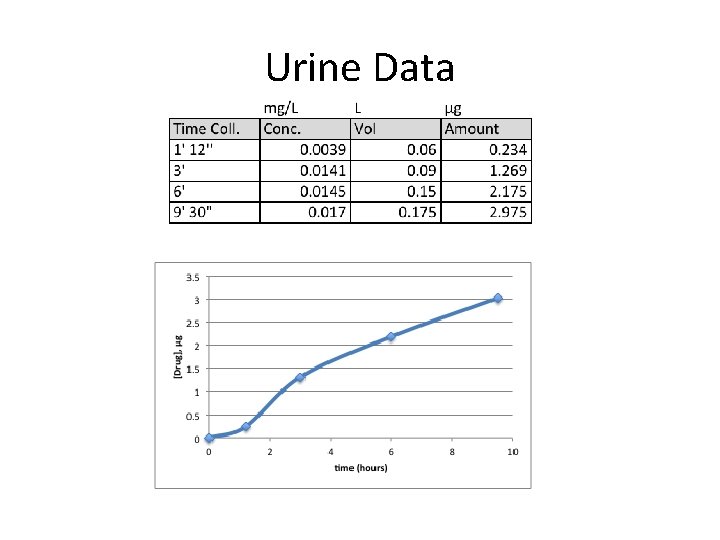
Urine Data
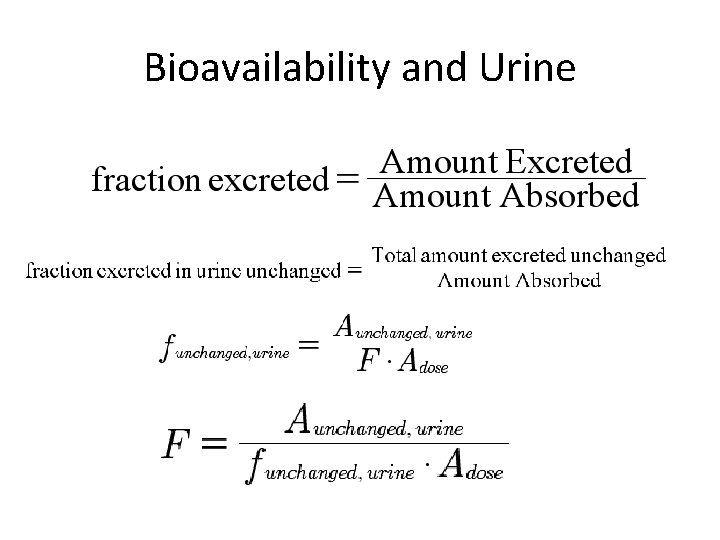
Bioavailability and Urine
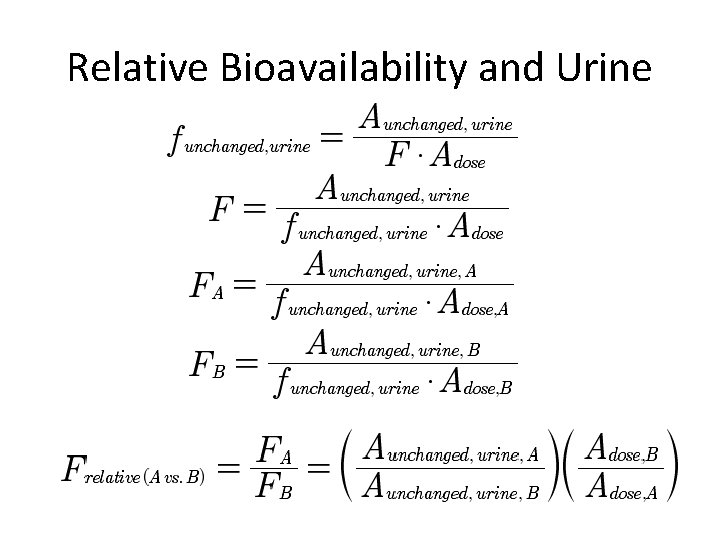
Relative Bioavailability and Urine
Assessment of Product Performance • Formulation – Biopharmaceutics – Excipients • Bioequivalence (BE) • Bioavailability (BA) and BE Testing
Biopharmaceutics • Physical/Chemical Drug Properties • Dosage Form (Tablet, Capsule) – Formulations (Industry) • Route of Administration • Rate/Extent of Systemic Drug Exposure

Excipients • Natural/Synthetic • inactive stuff that you put with the drug

Purposes of excipients 1. Bulking up the formulation “bulking agents”, “fillers”, “diluents” 2. Therapeutic Enhancement drug absorption solubility 3. Aid in handling the active pharmaceutical ingredient (API) 4. Provide stability and prevent denaturation.
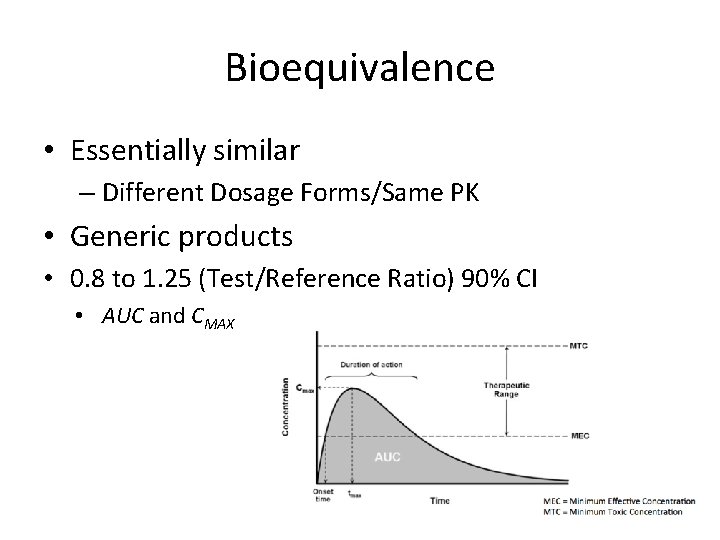
Bioequivalence • Essentially similar – Different Dosage Forms/Same PK • Generic products • 0. 8 to 1. 25 (Test/Reference Ratio) 90% CI • AUC and CMAX

On the http: //www. pharmwiki. org Not in Textbook NDA = New Drug Application IND = Investigational New Drug Application
BA and BE Testing: Study Types • Longitudinal Study – Same Subjects over a Period of Time – Cohort (Defined)/Panel (Cross-Section) – Retrospective • Clinical Trials – Randomized – Crossover – Blind, Double blind and Triple Blind
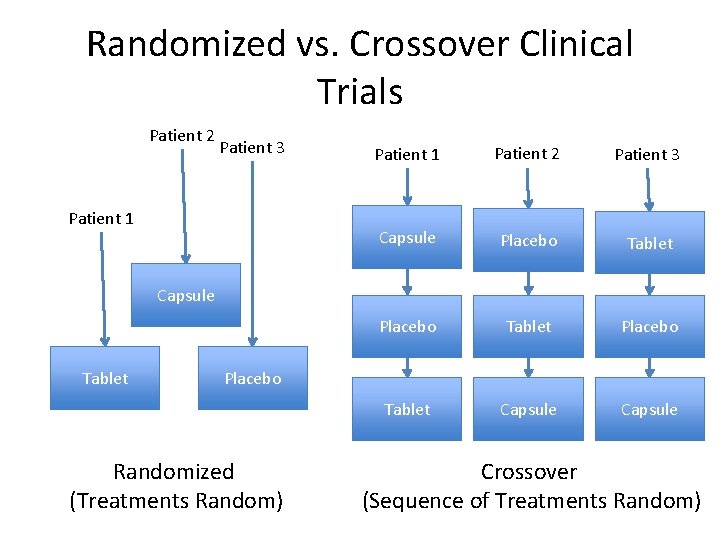
Randomized vs. Crossover Clinical Trials Patient 2 Patient 3 Patient 1 Patient 2 Patient 3 Capsule Placebo Tablet Capsule Tablet Placebo Randomized (Treatments Random) Crossover (Sequence of Treatments Random)
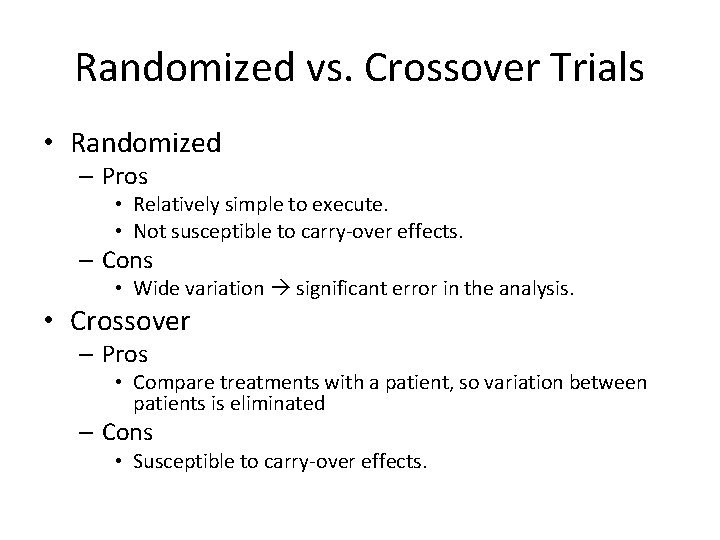
Randomized vs. Crossover Trials • Randomized – Pros • Relatively simple to execute. • Not susceptible to carry-over effects. – Cons • Wide variation significant error in the analysis. • Crossover – Pros • Compare treatments with a patient, so variation between patients is eliminated – Cons • Susceptible to carry-over effects.
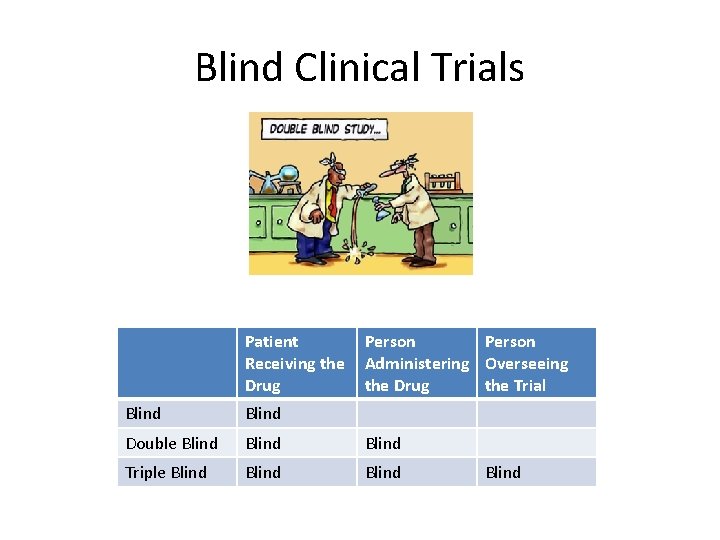
Blind Clinical Trials Patient Receiving the Drug Person Administering Overseeing the Drug the Trial Blind Double Blind Triple Blind

BA and BE: Considerations by the FDA • • • Study Type Dosing Frequency What is measured? PK? Other Documentation (Lots)

Study Type • Pilot Study – 8 -12 Subjects • Full-Scale – 24 -34 Subjects • >18 years old and healthy • Cohort or Panel – (gender? ) • Fasting conditions (>10 hours overnight)
Dosing Frequency • Single Dose – Preferred – Easier to assess PK parameters than multiple dose (steady state) • Multiple Dose – Differences absorption rate, not the extent of absorption. – Excessive variability in bioavailability (BA) – [drug]plasma too low with single dose – Extended release dosage form
Moieties to be Measured • Preferred: Active Ingredient or moiety • Active Metabolite – [Active ingredient or moiety] too low – Contributes to safety/efficacy
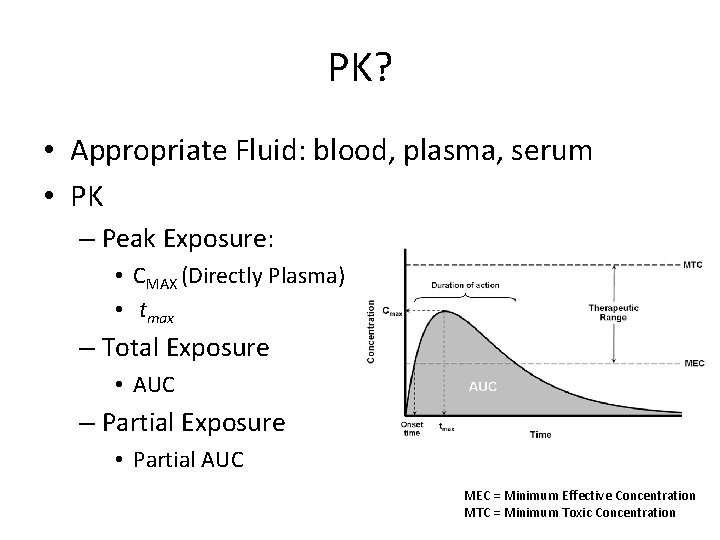
PK? • Appropriate Fluid: blood, plasma, serum • PK – Peak Exposure: • CMAX (Directly Plasma) • tmax – Total Exposure • AUC – Partial Exposure • Partial AUC MEC = Minimum Effective Concentration MTC = Minimum Toxic Concentration
Other • In vitro to human in vivo correlation (IVIVC) – in vitro dissolution/drug-release characterization • PD studies – not recommended for orally administered drugs – PK measurements preferred (More accurate/sensitive/reproducible) – only if PK fails • Clinical Endpoints (e. g. Cured) (Rare) • In vitro (e. g. dissolution testing)
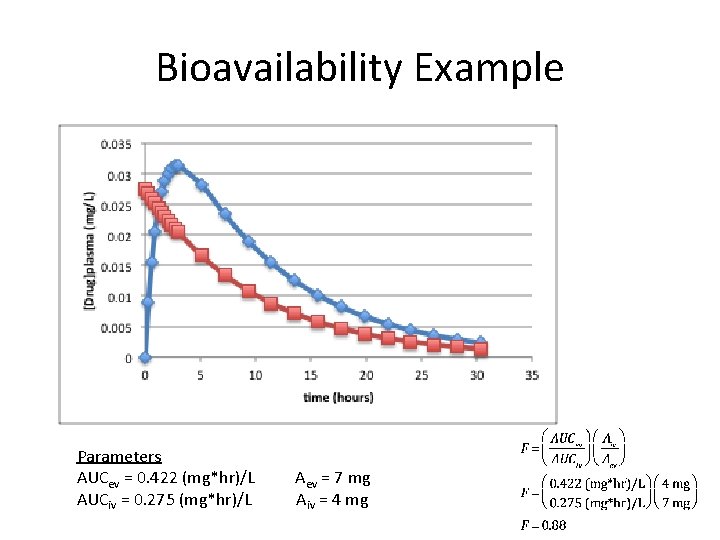
Bioavailability Example Parameters AUCev = 0. 422 (mg*hr)/L AUCiv = 0. 275 (mg*hr)/L Aev = 7 mg Aiv = 4 mg
- Slides: 32