Lecture 13 Applications of Nuclear Physics Fission Reactors










- Slides: 43

Lecture 13 Applications of Nuclear Physics Fission Reactors and Bombs Dec 2006, Lecture 13 Nuclear Physics Lectures, Dr. Armin Reichold 1

12. 1 Overview n 12. 1 Induced fission n n n Fissile nuclei Time scales of the fission process Crossections for neutrons on U and Pu Neutron economy Energy balance A simple bomb 12. 2 Fission reactors n Reactor basics n n n n Thermal vs. fast Light water vs. heavy water Pressurised vs. Boiling water Enrichment off syllabus, only in notes at end of slides 12. 3 Fission Bombs n n Dec 2006, Lecture 13 Moderation Control Thermal stability Fission bomb fuels Suspicious behaviour Nuclear Physics Lectures, Dr. Armin Reichold 2

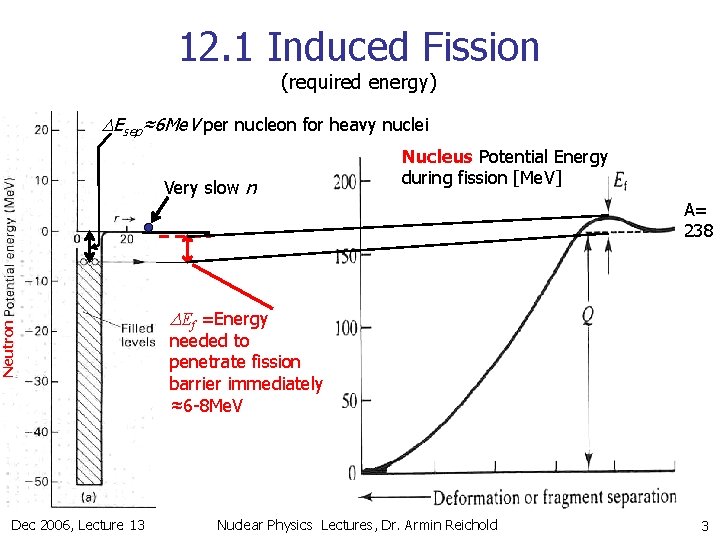
12. 1 Induced Fission (required energy) DEsep≈6 Me. V per nucleon for heavy nuclei Very slow n Nucleus Potential Energy during fission [Me. V] A= 238 Neutron DEf =Energy needed to penetrate fission barrier immediately ≈6 -8 Me. V Neutrons Dec 2006, Lecture 13 Nuclear Physics Lectures, Dr. Armin Reichold 3

12. 1 Induced Fission (required energy & thermal fission) n n n n Spontaneous fission rates low due to high coulomb barrier (6 -8 Me. V @ A≈240) Slow neutron releases DEsep as excitation into nucleus Excited nucleus has enough energy for immediate fission if Ef - DEsep >0 We call this “thermal fission” (slow, thermal neutron needed) But due to pairing term … even N nuclei have low DEsep for additional n odd N nuclei have high DEsep for additional n Fission yield in n -absorption varies dramatically between odd and even N Dec 2006, Lecture 13 Nuclear Physics Lectures, Dr. Armin Reichold 4

12. 1 Induced Fission (fast fission & fissile nuclei) n n DEsep(n, 23892 U) = 4. 78 Me. V only Fission of 238 U needs additional kinetic energy from neutron En, kin>Ef-DEsep≈1. 4 Me. V We call this “fast fission” (fast neutrons needed) Thermally fissile nuclei, En, kinthermal=0. 1 e. V @ 1160 K n n n 235 U, 239 Pu, 241 Pu U, 92 92 94 94 Fast fissile nuclei En, kin=O(Me. V) 238 U, 240 Pu, 242 Pu Th, 90 92 94 94 Note: all Pu isotopes on earth are man made Note: only 0. 72% of natural U is 235 U n n 233 232 Dec 2006, Lecture 13 Nuclear Physics Lectures, Dr. Armin Reichold 5

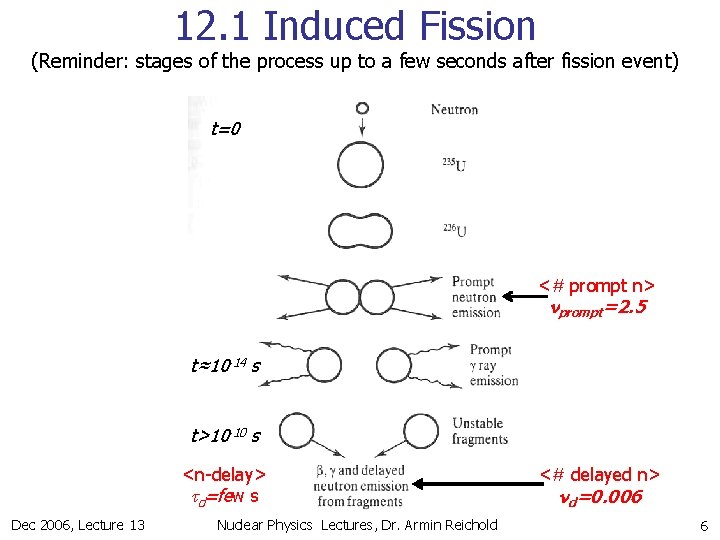
12. 1 Induced Fission (Reminder: stages of the process up to a few seconds after fission event) t=0 <# prompt n> nprompt=2. 5 t≈10 -14 s t>10 -10 s <n-delay> td=few s Dec 2006, Lecture 13 Nuclear Physics Lectures, Dr. Armin Reichold <# delayed n> nd=0. 006 6

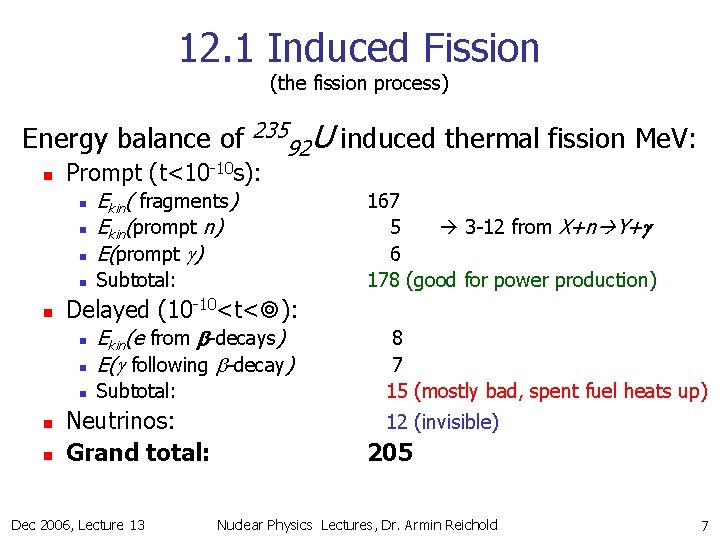
12. 1 Induced Fission (the fission process) Energy balance of n Prompt (t<10 -10 s): n Ekin( fragments) Ekin(prompt n) E(prompt g) n Subtotal: n n 92 U induced thermal fission Me. V: 167 5 3 -12 from X+n Y+g 6 178 (good for power production) Delayed (10 -10<t< ): n Ekin(e from b-decays) E(g following b-decay) n Subtotal: n n 235 Neutrinos: Grand total: Dec 2006, Lecture 13 8 7 15 (mostly bad, spent fuel heats up) 12 (invisible) 205 Nuclear Physics Lectures, Dr. Armin Reichold 7

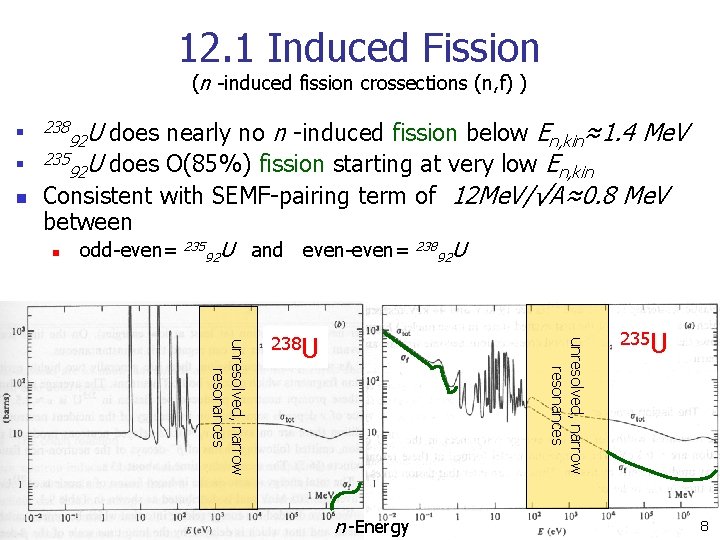
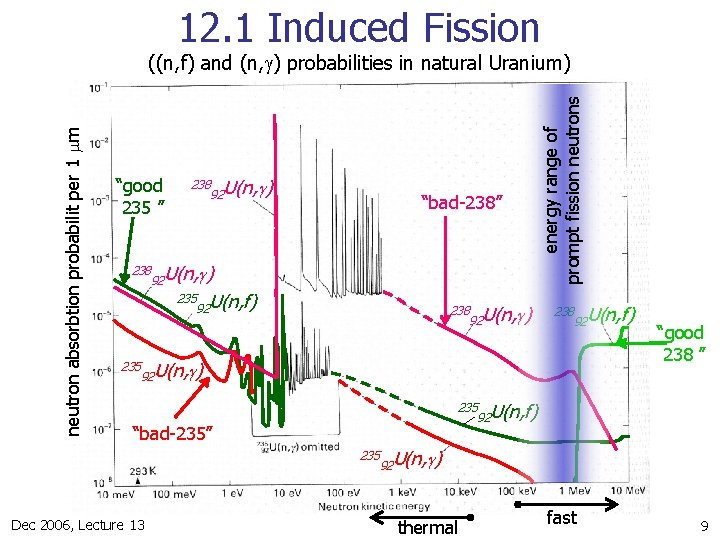
12. 1 Induced Fission (n -induced fission crossections (n, f) ) n n n does nearly no n -induced fission below En, kin≈1. 4 Me. V 235 U does O(85%) fission starting at very low E 92 n, kin Consistent with SEMF-pairing term of 12 Me. V/√A≈0. 8 Me. V between 238 n 92 U odd-even= 235 92 U and even-even= 92 U unresolved, narrow resonances 238 U 238 n -Energy 235 U 8

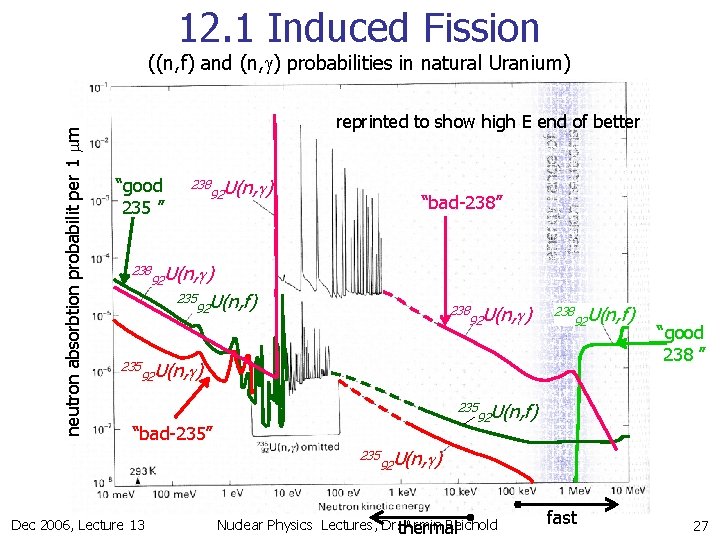
12. 1 Induced Fission “good 235 ” 238 235 238 92 U(n, g) “bad-238” 92 U(n, g) 235 U(n, f) 92 238 92 U(n, g) energy range of prompt fission neutrons neutron absorbtion probabilit per 1 mm ((n, f) and (n, g) probabilities in natural Uranium) 238 92 U(n, f) 92 U(n, g) 235 “bad-235” 235 Dec 2006, Lecture 13 “good 238 ” 92 U(n, f) 92 U(n, g) thermal fast 9

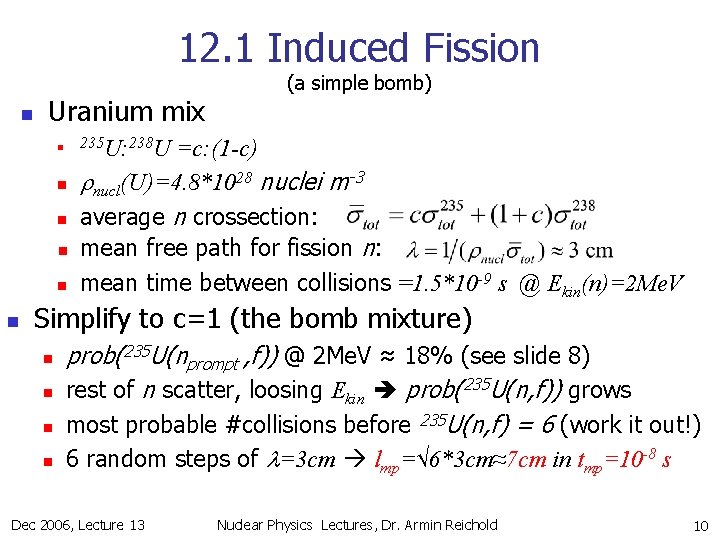
12. 1 Induced Fission n Uranium mix 235 U: 238 U n n n (a simple bomb) =c: (1 -c) rnucl(U)=4. 8*1028 nuclei m-3 average n crossection: mean free path for fission n: mean time between collisions =1. 5*10 -9 s @ Ekin(n)=2 Me. V Simplify to c=1 (the bomb mixture) n n prob(235 U(nprompt , f)) @ 2 Me. V ≈ 18% (see slide 8) rest of n scatter, loosing Ekin prob(235 U(n, f)) grows most probable #collisions before 235 U(n, f) = 6 (work it out!) 6 random steps of l=3 cm lmp=√ 6*3 cm≈7 cm in tmp=10 -8 s Dec 2006, Lecture 13 Nuclear Physics Lectures, Dr. Armin Reichold 10

12. 1 Induced Fission (a simple bomb) n n n After 10 -8 s 1 n is replaced with n=2. 5 n, n=average prompt neutron yield of this fission process Let probability of new n inducing fission before it is lost = q (others escape or give radiative capture) Each n produces on average (nq-1) new such n in tmp=10 -8 s (ignoring delayed n as bombs don’t last for seconds!) if nq>1 exponential growths of neutron number For 235 U, n=2. 5 if q>0. 4 you get a bomb Dec 2006, Lecture 13 Nuclear Physics Lectures, Dr. Armin Reichold 11

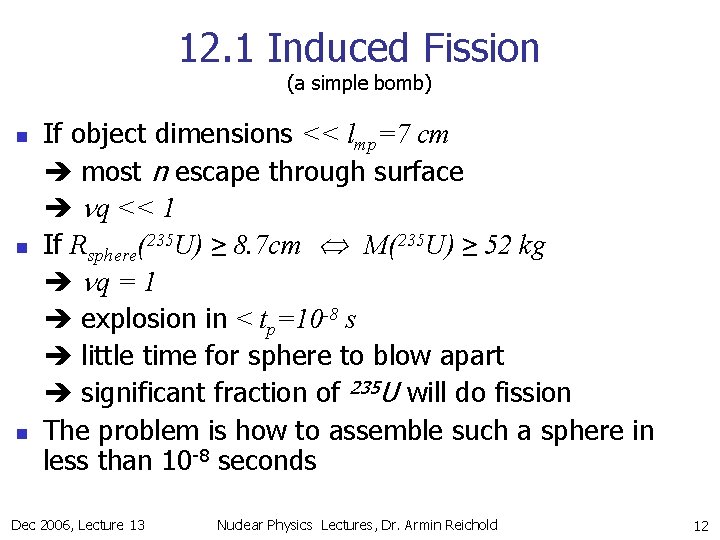
12. 1 Induced Fission (a simple bomb) n n n If object dimensions << lmp=7 cm most n escape through surface nq << 1 If Rsphere(235 U) ≥ 8. 7 cm M(235 U) ≥ 52 kg nq = 1 explosion in < tp=10 -8 s little time for sphere to blow apart significant fraction of 235 U will do fission The problem is how to assemble such a sphere in less than 10 -8 seconds Dec 2006, Lecture 13 Nuclear Physics Lectures, Dr. Armin Reichold 12

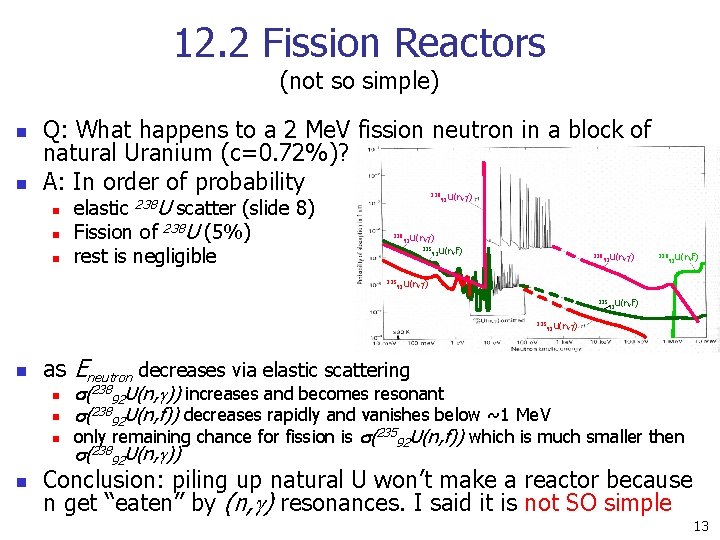
12. 2 Fission Reactors (not so simple) n n Q: What happens to a 2 Me. V fission neutron in a block of natural Uranium (c=0. 72%)? A: In order of probability n n n 238 U elastic scatter (slide 8) Fission of 238 U (5%) rest is negligible 238 235 92 U(n, g) 235 U(n, f) 92 238 92 U(n, g) 235 92 U(n, f) 92 U(n, g) as Eneutron decreases via elastic scattering n n 92 U(n, f) 92 U(n, g) 235 n 238 s(23892 U(n, g)) increases and becomes resonant s(23892 U(n, f)) decreases rapidly and vanishes below ~1 Me. V only remaining chance for fission is s(23592 U(n, f)) which is much smaller then s(23892 U(n, g)) Conclusion: piling up natural U won’t make a reactor because n get “eaten” by (n, g) resonances. I said it is not SO simple 13

12. 2 Fission Reactors (two ways out) n Way 1: Thermal Reactors n n bring neutrons to thermal energies without absorbing them = moderate them use low mass nuclei with low n-capture crossection as moderator. (Why low mass? ) sandwich fuel rods with moderator and coolant layers when n returns from moderator its energy is so low that it will predominantly cause fission in 235 U Dec 2006, Lecture 13 Nuclear Physics Lectures, Dr. Armin Reichold 14

12. 2 Fission Reactors (two ways out) n Way 2: Fast Reactors n n n Use fast neutrons for fission Use higher fraction of fissile material, typically 20% of 239 Pu + 80% 238 U This is self refuelling (fast breeding) via: n 238 92 U+n n 23992 U + g 23993 Np + e- + ne 23994 Pu + e¯ + ne Details about fast reactors later Dec 2006, Lecture 13 Nuclear Physics Lectures, Dr. Armin Reichold 15

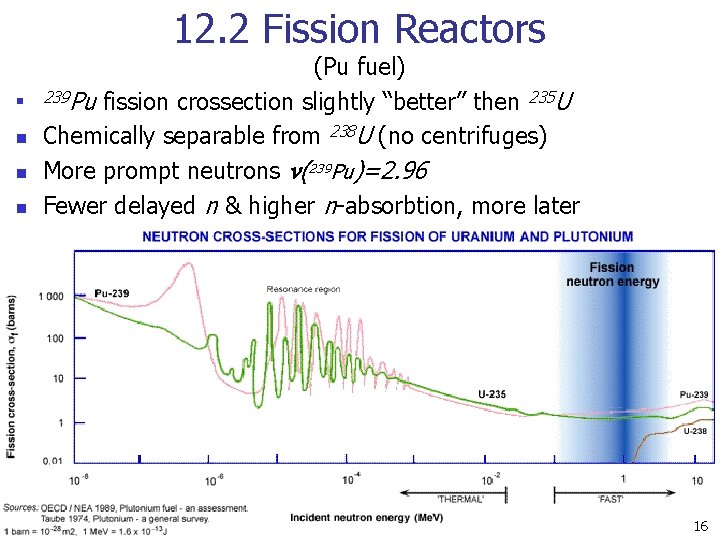
12. 2 Fission Reactors n n (Pu fuel) 239 Pu fission crossection slightly “better” then 235 U Chemically separable from 238 U (no centrifuges) More prompt neutrons n(239 Pu)=2. 96 Fewer delayed n & higher n-absorbtion, more later 16

12. 2 Fission Reactors (Reactor control) n For bomb we found: n n Reactors use control rods with large n-capture crossection snc like B or Cd to regulate q Lifetime of prompt n: n n “boom” if: nq > 1 where n was number of prompt n we don’t want “boom” need to get rid of most prompt n O(10 -8 s) in pure 235 U O(10 -3 s) in thermal reactor (“long” time in moderator) not “long” enough Far too fast to control … but there also delayed neutrons Dec 2006, Lecture 13 Nuclear Physics Lectures, Dr. Armin Reichold 17

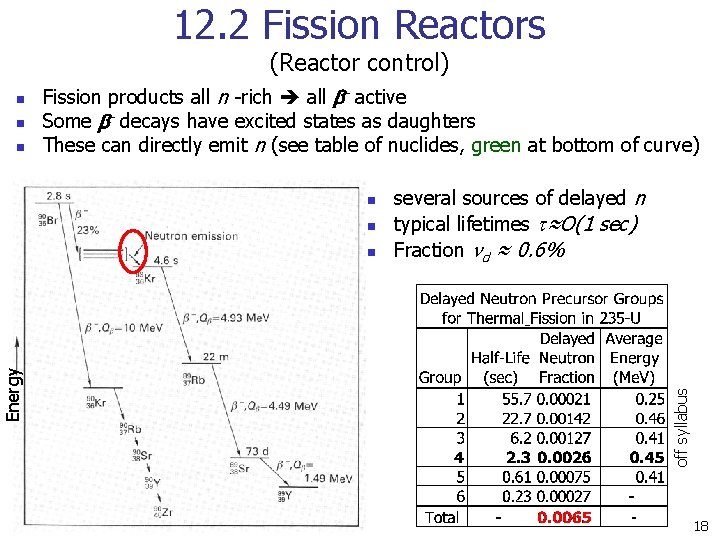
12. 2 Fission Reactors (Reactor control) n n Fission products all n -rich all b- active Some b- decays have excited states as daughters These can directly emit n (see table of nuclides, green at bottom of curve) n n Energy n several sources of delayed n typical lifetimes t≈O(1 sec) Fraction nd ≈ 0. 6% off syllabus n 18

12. 2 Fission Reactors (Reactor control) n n Since fuel rods “hopefully” remain in reactor longer then 10 -2 s must include delayed n fraction nd into our calculations New control problem: n n keep (n+nd)q = 1 to accuracy of < 0. 6% at time scale of a few seconds Doable with mechanical systems but not easy Dec 2006, Lecture 13 Nuclear Physics Lectures, Dr. Armin Reichold 19

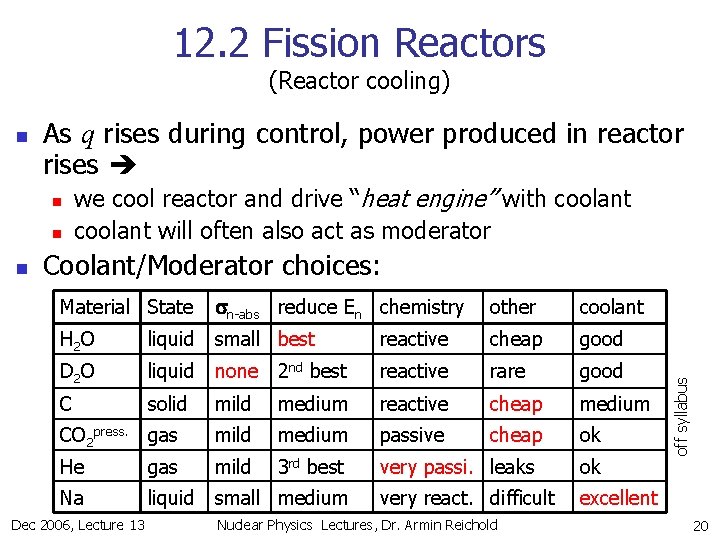
12. 2 Fission Reactors (Reactor cooling) As q rises during control, power produced in reactor rises n n n we cool reactor and drive “heat engine” with coolant will often also act as moderator Coolant/Moderator choices: Material State sn-abs reduce En chemistry other coolant H 2 O liquid small best reactive cheap good D 2 O liquid none 2 nd best reactive rare good C solid mild medium reactive cheap medium CO 2 press. gas mild medium passive cheap ok He gas mild 3 rd best very passi. leaks ok Na liquid small medium very react. difficult excellent Dec 2006, Lecture 13 Nuclear Physics Lectures, Dr. Armin Reichold off syllabus n 20

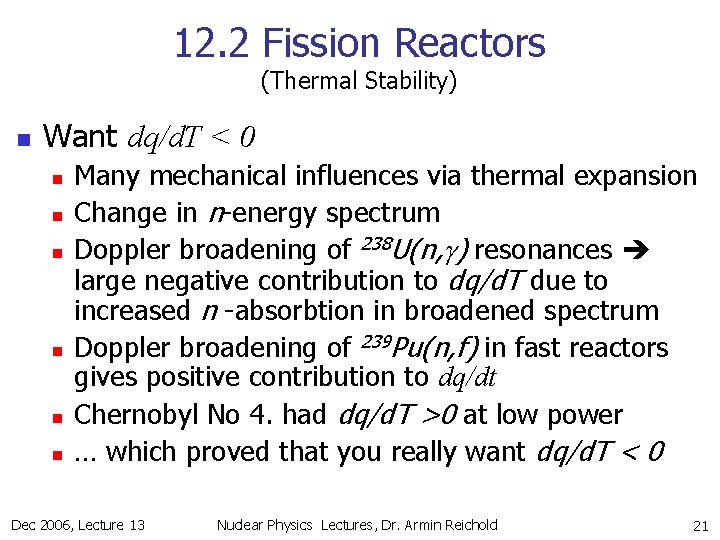
12. 2 Fission Reactors (Thermal Stability) n Want dq/d. T < 0 n n n Many mechanical influences via thermal expansion Change in n-energy spectrum Doppler broadening of 238 U(n, g) resonances large negative contribution to dq/d. T due to increased n -absorbtion in broadened spectrum Doppler broadening of 239 Pu(n, f) in fast reactors gives positive contribution to dq/dt Chernobyl No 4. had dq/d. T >0 at low power … which proved that you really want dq/d. T < 0 Dec 2006, Lecture 13 Nuclear Physics Lectures, Dr. Armin Reichold 21

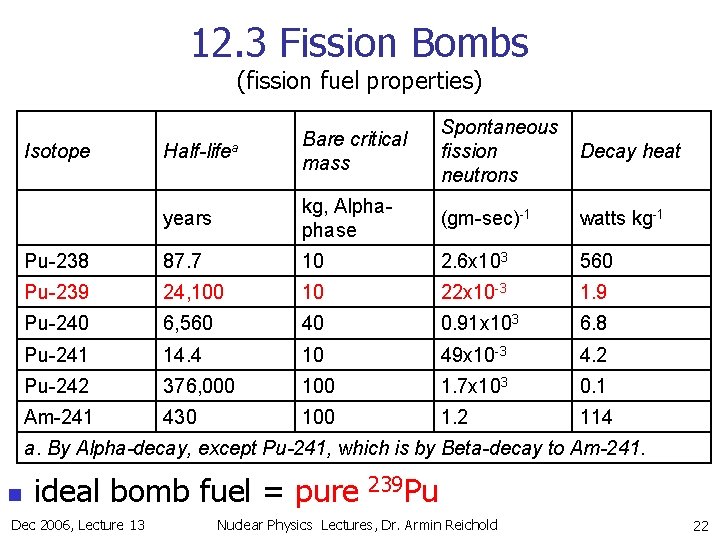
12. 3 Fission Bombs (fission fuel properties) Half-lifea Bare critical mass Spontaneous fission neutrons Decay heat years kg, Alphaphase (gm-sec)-1 watts kg-1 Pu-238 87. 7 10 2. 6 x 103 560 Pu-239 24, 100 10 22 x 10 -3 1. 9 Pu-240 6, 560 40 0. 91 x 103 6. 8 Pu-241 14. 4 10 49 x 10 -3 4. 2 Pu-242 376, 000 1. 7 x 103 0. 1 Am-241 430 100 1. 2 114 Isotope a. By Alpha-decay, except Pu-241, which is by Beta-decay to Am-241. n ideal bomb fuel = pure Dec 2006, Lecture 13 239 Pu Nuclear Physics Lectures, Dr. Armin Reichold 22

12. 3 Fission Bombs (drawbacks of various Pu isotopes) n n n 241 Pu : decays to 241 Am which gives very high energy g-rays shielding problem 240 Pu : lots of n from spontaneous fission 238 Pu : a-decays quickly (t 1/2 = 88 years) lots of heat conventional ignition explosives don’t like that! in pure 239 Pu bomb, the nuclear ignition is timed optimally during compression using a burst of external n maximum explosion yield … but using reactor grade Pu, n from 240 Pu decays can ignite bomb prematurely lower explosion yield but still very bad if you are holding it in your hand Reactor grade Pu mix has “drawbacks” but could be made into a bomb. Dec 2006, Lecture 13 Nuclear Physics Lectures, Dr. Armin Reichold 23

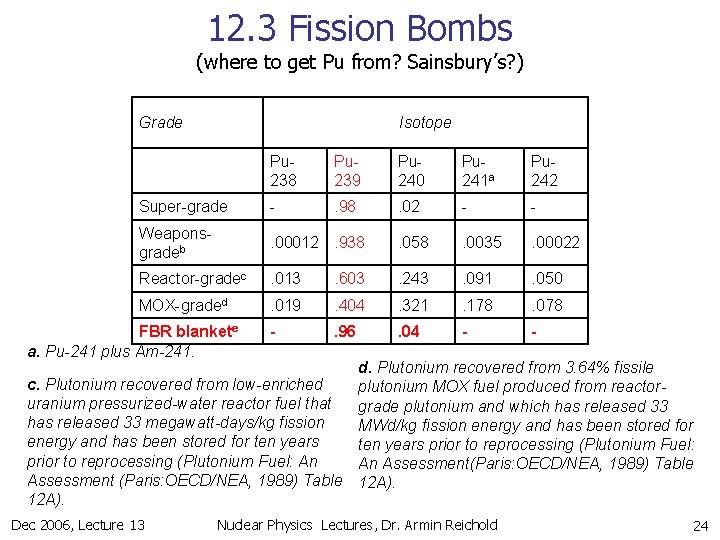
12. 3 Fission Bombs (where to get Pu from? Sainsbury’s? ) Grade Isotope Pu 238 Pu 239 Pu 240 Pu 241 a Pu 242 Super-grade - . 98 . 02 - - Weaponsgradeb . 00012. 938 . 058 . 0035 . 00022 Reactor-gradec . 013 . 603 . 243 . 091 . 050 MOX-graded . 019 . 404 . 321 . 178 . 078 - . 96 . 04 - - FBR blankete a. Pu-241 plus Am-241. d. Plutonium recovered from 3. 64% fissile c. Plutonium recovered from low-enriched plutonium MOX fuel produced from reactoruranium pressurized-water reactor fuel that grade plutonium and which has released 33 megawatt-days/kg fission MWd/kg fission energy and has been stored for ten years prior to reprocessing (Plutonium Fuel: An An Assessment(Paris: OECD/NEA, 1989) Table Assessment (Paris: OECD/NEA, 1989) Table 12 A). Dec 2006, Lecture 13 Nuclear Physics Lectures, Dr. Armin Reichold 24

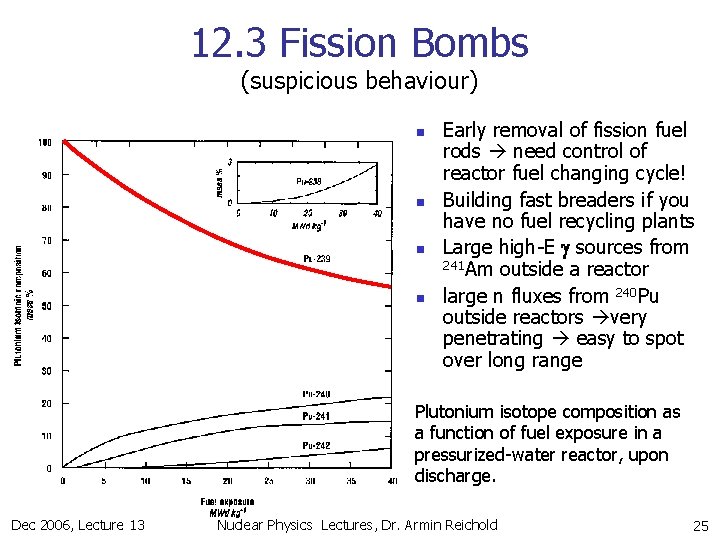
12. 3 Fission Bombs (suspicious behaviour) n n Early removal of fission fuel rods need control of reactor fuel changing cycle! Building fast breaders if you have no fuel recycling plants Large high-E g sources from 241 Am outside a reactor large n fluxes from 240 Pu outside reactors very penetrating easy to spot over long range Plutonium isotope composition as a function of fuel exposure in a pressurized-water reactor, upon discharge. Dec 2006, Lecture 13 Nuclear Physics Lectures, Dr. Armin Reichold 25

End of Lecture 13 even more energetic fusion and radioactive dating can be found in Dr. Weidberg’s notes for lecture 14 Dec 2006, Lecture 13 Nuclear Physics Lectures, Dr. Armin Reichold 26

12. 1 Induced Fission reprinted to show high E end of better “good 235 ” 238 235 238 92 U(n, g) “bad-238” 92 U(n, g) 235 U(n, f) 92 238 92 U(n, g) energy range of fission neutrons neutron absorbtion probabilit per 1 mm ((n, f) and (n, g) probabilities in natural Uranium) 238 92 U(n, f) 92 U(n, g) 235 “bad-235” 235 Dec 2006, Lecture 13 “good 238 ” 92 U(n, f) 92 U(n, g) Nuclear Physics Lectures, Dr. thermal Armin Reichold fast 27

Appendix to lecture 13 n Dec 2006, Lecture 13 More on various reactors n Uranium enrichment Off Syllabus Nuclear Physics Lectures, Dr. Armin Reichold 28

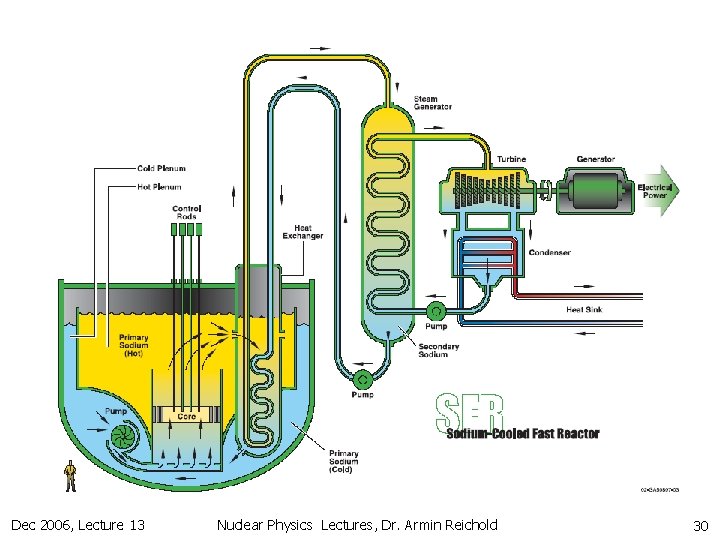
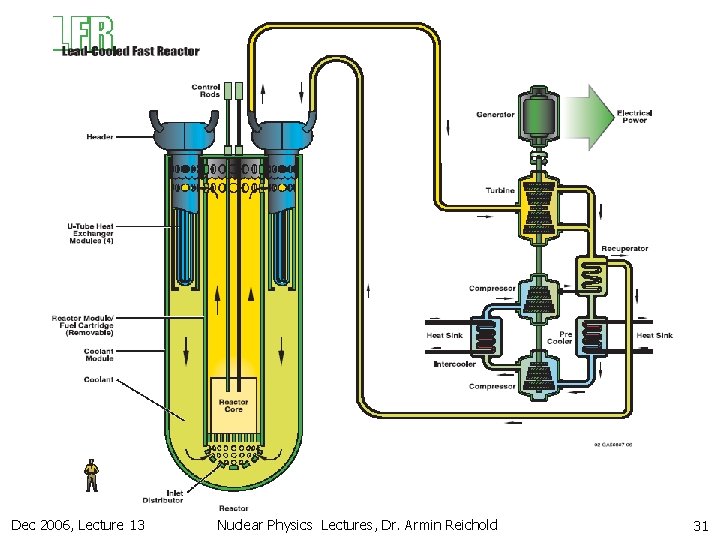
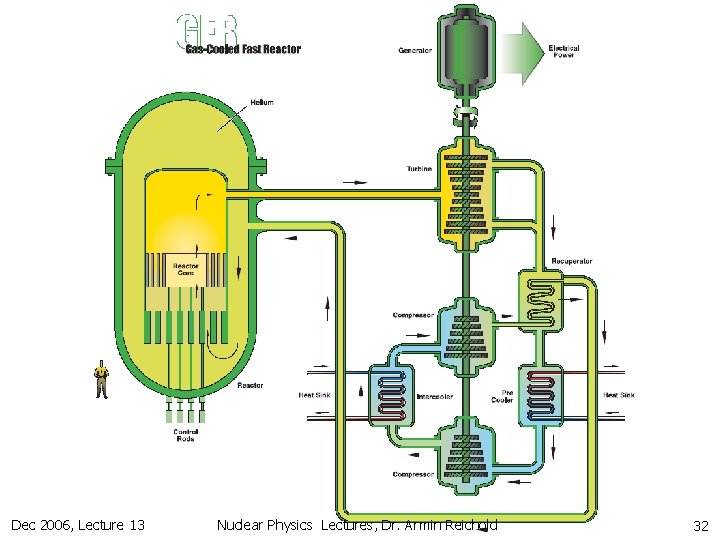
12. 2 Fission Reactors (Thermal vs. Fast) n Fast reactors n n n need very high 239 Pu concentration Bombs very compact core hard to cool need high Cp coolant like liq. Na or liq. Na. K-mix don’t like water & air & must keep coolant circuit molten & high activation of Na High coolant temperature (550 C) good thermal efficiency Low pressure in vessel better safety can utilise all 238 U via breeding 141 times more fuel High fuel concentration + breading Can operate for long time without rod changes Designs for 4 th generation molten Pb or gas cooled fast reactors exist. Could overcome the Na problems Dec 2006, Lecture 13 Nuclear Physics Lectures, Dr. Armin Reichold 29

Dec 2006, Lecture 13 Nuclear Physics Lectures, Dr. Armin Reichold 30

Dec 2006, Lecture 13 Nuclear Physics Lectures, Dr. Armin Reichold 31

Dec 2006, Lecture 13 Nuclear Physics Lectures, Dr. Armin Reichold 32

12. 2 Fission Reactors (Thermal vs. Fast) n Thermal Reactors n Many different types exist n n n BWR = Boiling Water Reactor PWR = Pressure Water Reactor BWP/PWR exist as n n n LWR = Light Water Reactors (H 2 O) HWR = Heavy Water Reactors (D 2 O) (HT)GCR = (High Temperature) Gas Cooled Reactor exist as n n PBR = Pebble Bed Reactor other more conventional geometries Dec 2006, Lecture 13 Nuclear Physics Lectures, Dr. Armin Reichold 33

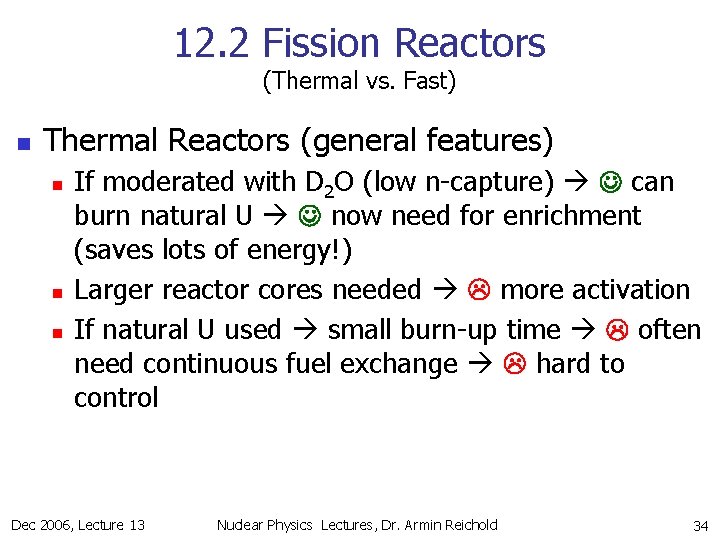
12. 2 Fission Reactors (Thermal vs. Fast) n Thermal Reactors (general features) n n n If moderated with D 2 O (low n-capture) can burn natural U now need for enrichment (saves lots of energy!) Larger reactor cores needed more activation If natural U used small burn-up time often need continuous fuel exchange hard to control Dec 2006, Lecture 13 Nuclear Physics Lectures, Dr. Armin Reichold 34

12. 2 Fission Reactors (Light vs. Heavy water thermal reactors) n Light Water n n n it is cheap very well understood chemistry compatible with steam part of plant can not use natural uranium (too much n-capture) must have enrichment plant bombs need larger moderator volume larger core with more activation enriched U has bigger n-margin easier to control Dec 2006, Lecture 13 Nuclear Physics Lectures, Dr. Armin Reichold 35

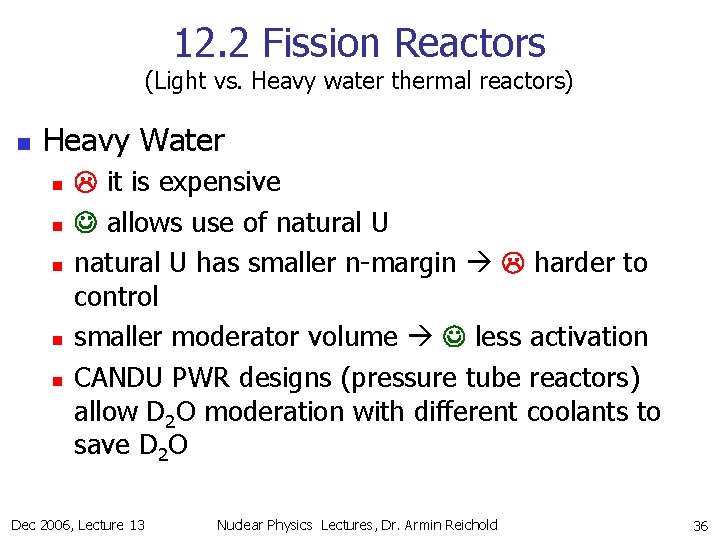
12. 2 Fission Reactors (Light vs. Heavy water thermal reactors) n Heavy Water n n n it is expensive allows use of natural U has smaller n-margin harder to control smaller moderator volume less activation CANDU PWR designs (pressure tube reactors) allow D 2 O moderation with different coolants to save D 2 O Dec 2006, Lecture 13 Nuclear Physics Lectures, Dr. Armin Reichold 36

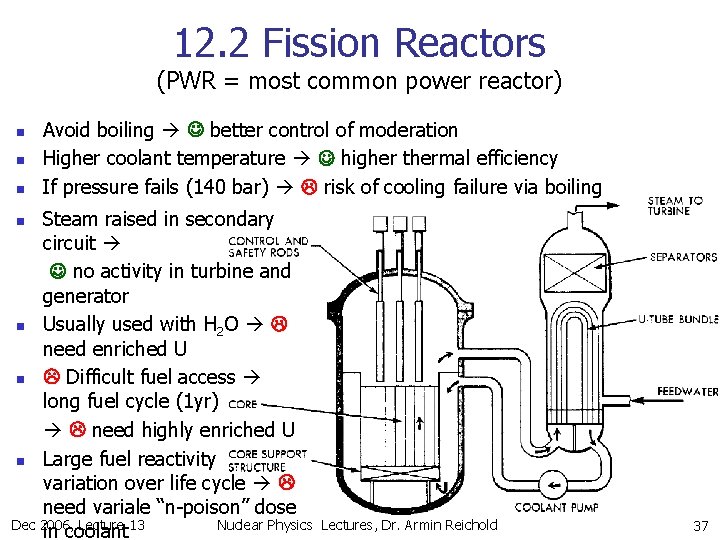
12. 2 Fission Reactors (PWR = most common power reactor) n n n Avoid boiling better control of moderation Higher coolant temperature higher thermal efficiency If pressure fails (140 bar) risk of cooling failure via boiling Steam raised in secondary circuit no activity in turbine and generator n Usually used with H 2 O need enriched U n Difficult fuel access long fuel cycle (1 yr) need highly enriched U n Large fuel reactivity variation over life cycle need variale “n-poison” dose Dec 2006, Lecture 13 Nuclear Physics in coolant n Lectures, Dr. Armin Reichold 37

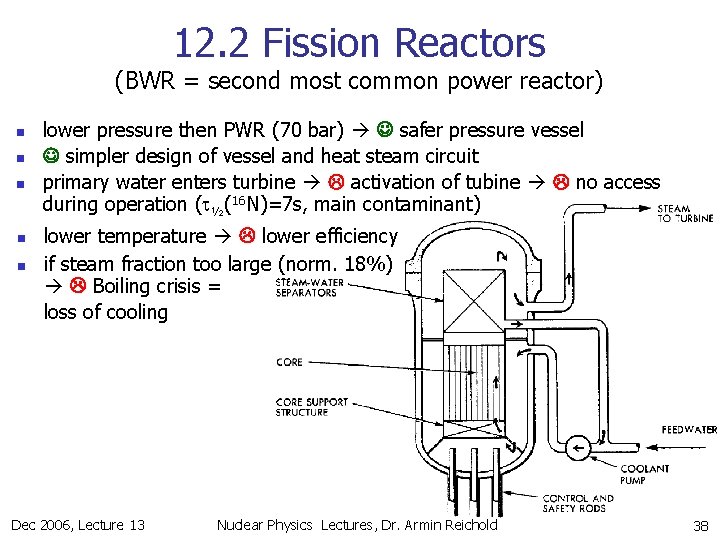
12. 2 Fission Reactors (BWR = second most common power reactor) n n n lower pressure then PWR (70 bar) safer pressure vessel simpler design of vessel and heat steam circuit primary water enters turbine activation of tubine no access during operation (t½(16 N)=7 s, main contaminant) lower temperature lower efficiency if steam fraction too large (norm. 18%) Boiling crisis = loss of cooling Dec 2006, Lecture 13 Nuclear Physics Lectures, Dr. Armin Reichold 38

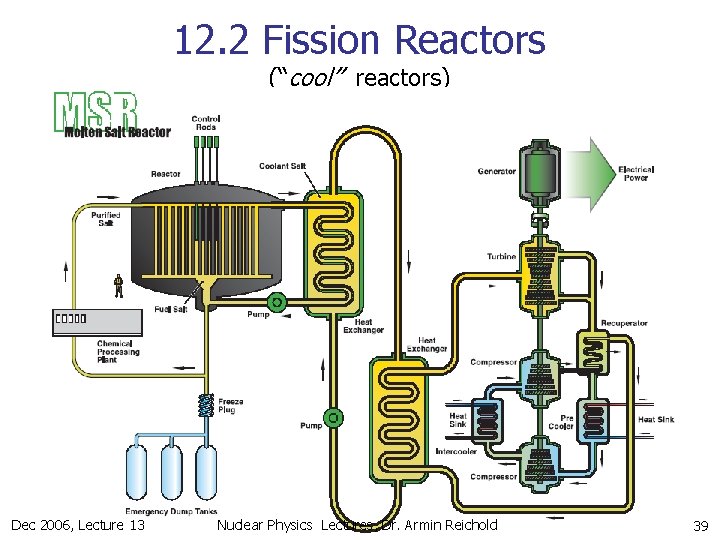
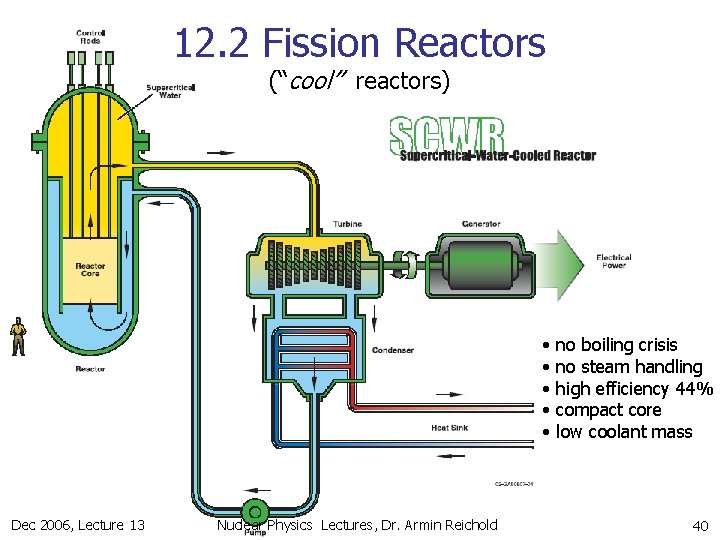
12. 2 Fission Reactors (“cool” reactors) Dec 2006, Lecture 13 Nuclear Physics Lectures, Dr. Armin Reichold 39

12. 2 Fission Reactors (“cool” reactors) • • • Dec 2006, Lecture 13 Nuclear Physics Lectures, Dr. Armin Reichold no boiling crisis no steam handling high efficiency 44% compact core low coolant mass 40

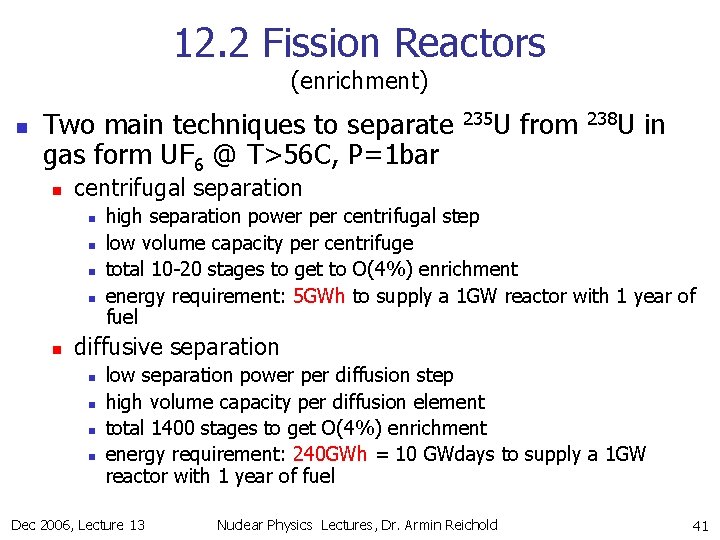
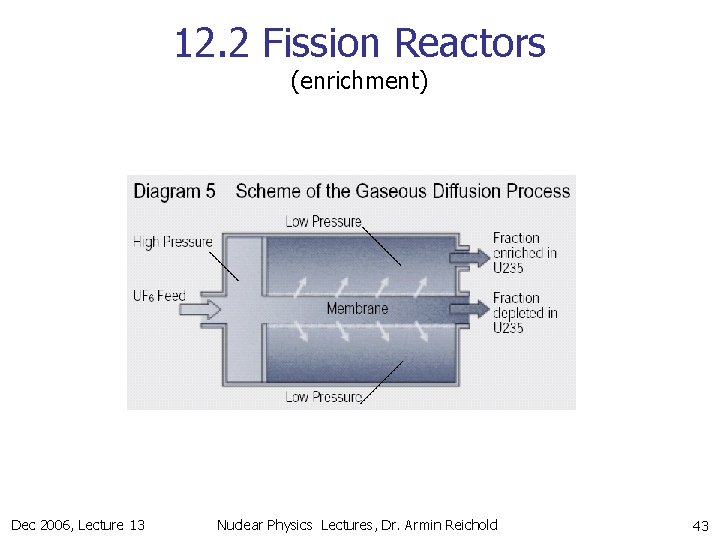
12. 2 Fission Reactors (enrichment) n Two main techniques to separate gas form UF 6 @ T>56 C, P=1 bar n from 238 U in centrifugal separation n n 235 U high separation power per centrifugal step low volume capacity per centrifuge total 10 -20 stages to get to O(4%) enrichment energy requirement: 5 GWh to supply a 1 GW reactor with 1 year of fuel diffusive separation n n low separation power per diffusion step high volume capacity per diffusion element total 1400 stages to get O(4%) enrichment energy requirement: 240 GWh = 10 GWdays to supply a 1 GW reactor with 1 year of fuel Dec 2006, Lecture 13 Nuclear Physics Lectures, Dr. Armin Reichold 41

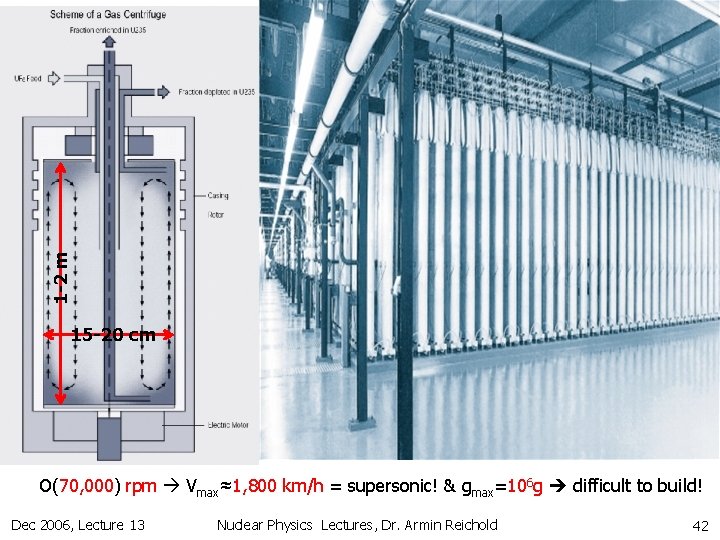
1 -2 m 15 -20 cm O(70, 000) rpm Vmax≈1, 800 km/h = supersonic! & gmax=106 g difficult to build! Dec 2006, Lecture 13 Nuclear Physics Lectures, Dr. Armin Reichold 42

12. 2 Fission Reactors (enrichment) Dec 2006, Lecture 13 Nuclear Physics Lectures, Dr. Armin Reichold 43