Lecture 12 a Phosphine complexes Introduction I In









- Slides: 21

Lecture 12 a Phosphine complexes

Introduction I �In order to understand what a ligand does, one has to look at its electronic and its steric properties �The reaction conditions (kinetic and thermodynamic control) during the reaction determine the configuration is observed in the product (cis-trans, fac-mer) �In many cases, there is an equilibrium in solution, which can be detected by NMR or infrared spectroscopy �The polarity of the solvent determines which product precipitates i. e. , Sn. Cl 4(THT)2: dichloromethane (trans), pentane (cis)

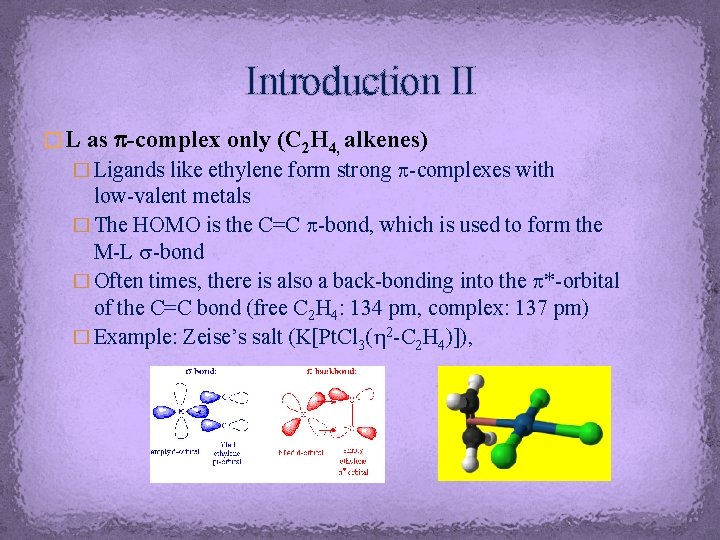
Introduction II � L as p-complex only (C 2 H 4, alkenes) � Ligands like ethylene form strong p-complexes with low-valent metals � The HOMO is the C=C p-bond, which is used to form the M-L s-bond � Often times, there is also a back-bonding into the p*-orbital of the C=C bond (free C 2 H 4: 134 pm, complex: 137 pm) � Example: Zeise’s salt (K[Pt. Cl 3(h 2 -C 2 H 4)]),

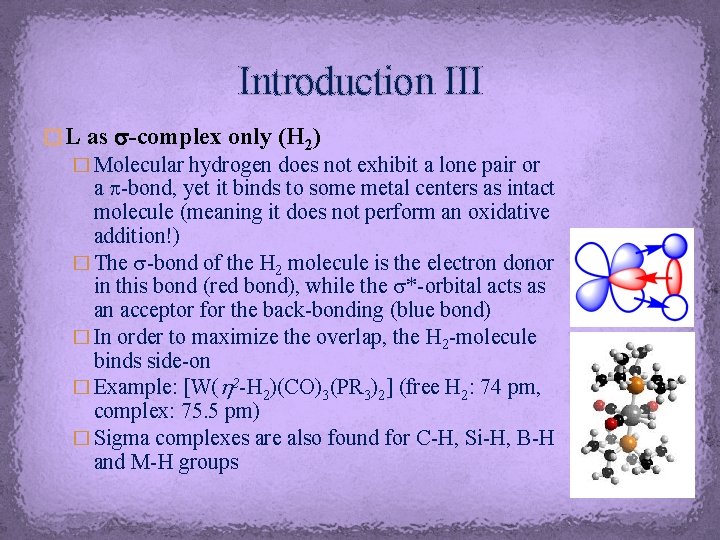
Introduction III � L as s-complex only (H 2) � Molecular hydrogen does not exhibit a lone pair or a p-bond, yet it binds to some metal centers as intact molecule (meaning it does not perform an oxidative addition!) � The s-bond of the H 2 molecule is the electron donor in this bond (red bond), while the s*-orbital acts as an acceptor for the back-bonding (blue bond) � In order to maximize the overlap, the H 2 -molecule binds side-on � Example: [W(h 2 -H 2)(CO)3(PR 3)2] (free H 2: 74 pm, complex: 75. 5 pm) � Sigma complexes are also found for C-H, Si-H, B-H and M-H groups

Introduction IV � L as s-donor only (NH 3, NR 3) � The metal has to exhibit a medium or high oxidation state in order for these complexes to be stable � Metal acts as a hard acid and the ligand as a hard base � Examples: [M(NH 3)4]2+ (M=Cu, Zn), [M(NH 3)6]2+ (M=Co, Ni)

Introduction V � L as s- and p-donor (H 2 O, OH-, OR-, NR 2 -, F-) � The metal has to exhibit a medium or high oxidation state in order for these complexes to be stable � The ligand acts as very hard base and the metal as hard acid � Examples: [Ni(H 2 O)6]2+, [Co. F 6]3 -, [Sn(OH)6]2 -

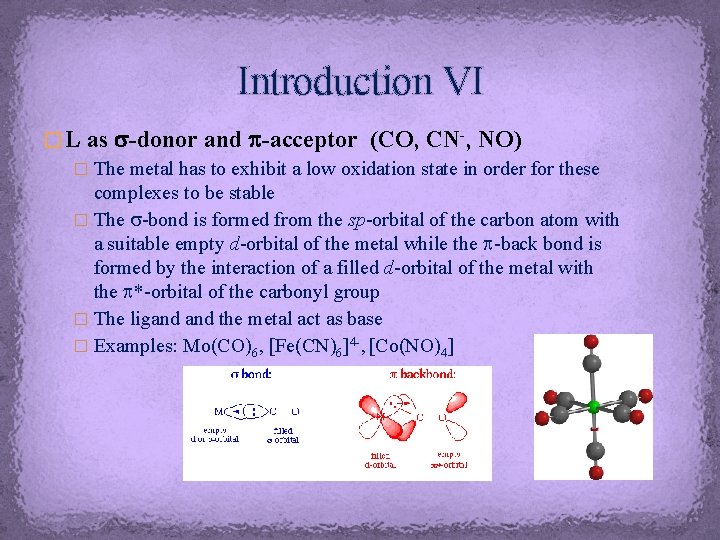
Introduction VI � L as s-donor and p-acceptor (CO, CN-, NO) � The metal has to exhibit a low oxidation state in order for these complexes to be stable � The s-bond is formed from the sp-orbital of the carbon atom with a suitable empty d-orbital of the metal while the p-back bond is formed by the interaction of a filled d-orbital of the metal with the p*-orbital of the carbonyl group � The ligand the metal act as base � Examples: Mo(CO)6, [Fe(CN)6]4 -, [Co(NO)4]

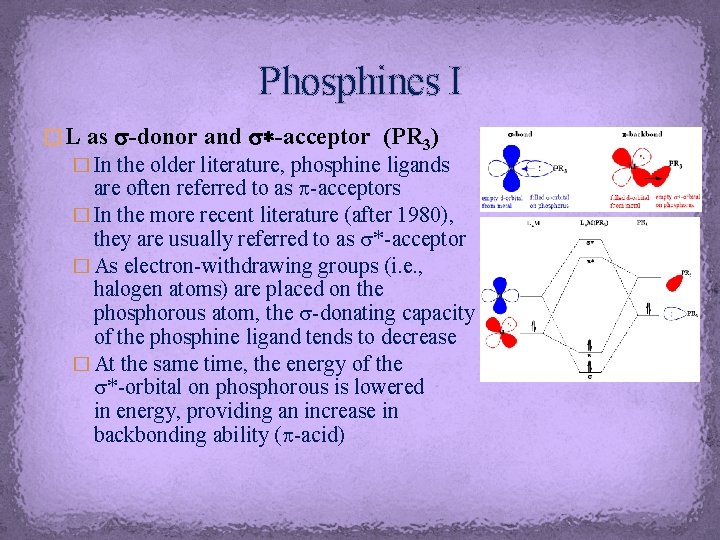
Phosphines I � L as s-donor and s*-acceptor (PR 3) � In the older literature, phosphine ligands are often referred to as p-acceptors � In the more recent literature (after 1980), they are usually referred to as s*-acceptor � As electron-withdrawing groups (i. e. , halogen atoms) are placed on the phosphorous atom, the s-donating capacity of the phosphine ligand tends to decrease � At the same time, the energy of the s*-orbital on phosphorous is lowered in energy, providing an increase in backbonding ability (p-acid)

Phosphines II � The degree of p-acidity largely depends on the substituents on the phosphorus atom � While alkyl phosphines are weak p-acids, the acidity increases for aryl, dialkylamino and alkoxy phosphines � The extreme cases are PCl 3 and PF 3, which is equivalent to CO in its p-acidity because more electronegative elements on the phosphorous atom stabilize the s-bond and lower the energy of the s*-orbital (see diagram) � The contribution of the phosphorus atom to the s*-orbital increases and the size of the orbital pointing towards the metal as well allowing for a better overlap � Based on this argument, the order of p-acidity of phosphines is � PMe 3 < PAr 3 < P(OMe)3 < P(OAr)3 < PCl 3 < PF 3 ≈ CO

Phosphines III � Aside of the p-acidity, the steric impact of the phosphine ligand has to be considered as well � C. A. Tolman (Chem. Rev. 1977, 313) summarizes the electronic parameters and cone angles of phosphine ligands: � The electronic parameter can be adjusted by changing the R-group (see above). Stronger donor groups increase the electron density on the metal atom, which is capable of more backbonding to ligands like CO, CN-, etc. � Tolman observed for Ni(CO)3 L that the carbonyl stretching frequency decreases as the donor ability of the R-group increases (i. e. , PCy 3 (2056 cm-1) vs. P(OMe)3 (2070 cm-1) vs. PF 3 (2111 cm-1)).

Phosphines IV � The second important parameter is the steric demand, which can also be controlled by changing the R-group. � Very bulky phosphines often favor low-coordinate compounds, which can coordinate additional small ligand as observed in catalytic cycles � Metals like Mo and W can coordinate up to six PMe 3 ligands (i. e. , M(PMe 3)6)), while a maximum of four PPh 3 ligands (i. e. , M(PPh 3)4, M=Pd, Cu+, Ag+, Au+) or two PCy 3 ligands (i. e. , Cu+, Ag+, Au+, Ni 2+, Pd 2+, Pt 2+) can be coordinated to a metal center � Thus, the bulkiness of the phosphine ligand can be quantified by its cone angle (Q) � The observed cone angles for phosphines range from Q=87 o (PH 3) to Q=212 o (P(mes)3) (neither one is shown in the diagram below). � The cone angles for PMe 3, PPh 3 and PCy 3 are Q=118 o, Q=145 o and Q=170 o, respectively, consistent with the observations above. � Generally, phosphines with aryl groups or highly branched alkyl chains exhibit large cone angles while phosphite have much smaller cone angles

Phosphines V

Phosphines VI �The ability of a metal to perform backbonding can easily be tuned by manipulating the electronic effect of the phosphine ligand. � For instance, a change of the ligand from PBu 3 to P(Oi. Pr)3, which possess virtually identical cone angles, decreases the ability of the metal for backbonding as can be seen from the higher carbonyl stretching frequency in Ni(CO)3 L. � If the same electronic effect is desired but a larger cone angle to lower the number of coordinated ligands, one could move from PBu 3 to P(i. Pr)3, which exhibits a 30 o larger cone angle, but is electronically speaking identical.

Mo(CO)5 L complexes I �These complexes can easily be prepared from Mo(CO)6 by the reaction with one equivalent of L �The resulting compounds exhibit colors ranging from white to red depending in the ligand L � 95 Mo-NMR and infrared spectroscopy can be used to assess the effect of the ligand L on the metal and the remaining CO ligands

Mo(CO)5 L complexes II Mo-NMR studies have shown that the chemical shift varies significantly with the ligand Ligand d(ppm) � 95 � Ligands that are good s-donors, but poor or Piperidine -1433 no p-acceptor causing a significant decrease in the HOMO-LUMO gap, which results in a deshielding of the Mo-nucleus � Ligands that are s-donors and good p-acceptor i. e. , PF 3 and P(OR)3 are comparable to the CO ligand itself CH 3 CN -1440 PCl 3 -1523 PCl 2 Ph -1615 PCl. Ph 2 -1702 PPh 3 -1743 PBr 3 -1396 PF 3 -1860 P(OPh)3 -1819 Mo(CO)6 -1857

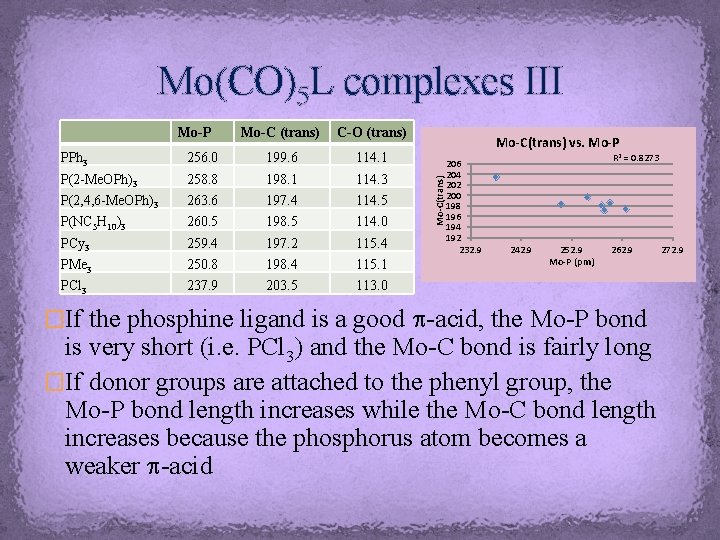
Mo(CO)5 L complexes III Mo-C (trans) C-O (trans) PPh 3 256. 0 199. 6 114. 1 P(2 -Me. OPh)3 258. 8 198. 1 114. 3 P(2, 4, 6 -Me. OPh)3 263. 6 197. 4 114. 5 P(NC 5 H 10)3 260. 5 198. 5 114. 0 PCy 3 259. 4 197. 2 115. 4 PMe 3 250. 8 198. 4 115. 1 PCl 3 237. 9 203. 5 113. 0 Mo-C(trans) vs. Mo-P Mo-C(trans) Mo-P 206 204 202 200 198 196 194 192 232. 9 R 2 = 0. 8273 242. 9 252. 9 Mo-P (pm) 262. 9 �If the phosphine ligand is a good p-acid, the Mo-P bond is very short (i. e. PCl 3) and the Mo-C bond is fairly long �If donor groups are attached to the phenyl group, the Mo-P bond length increases while the Mo-C bond length increases because the phosphorus atom becomes a weaker p-acid 272. 9

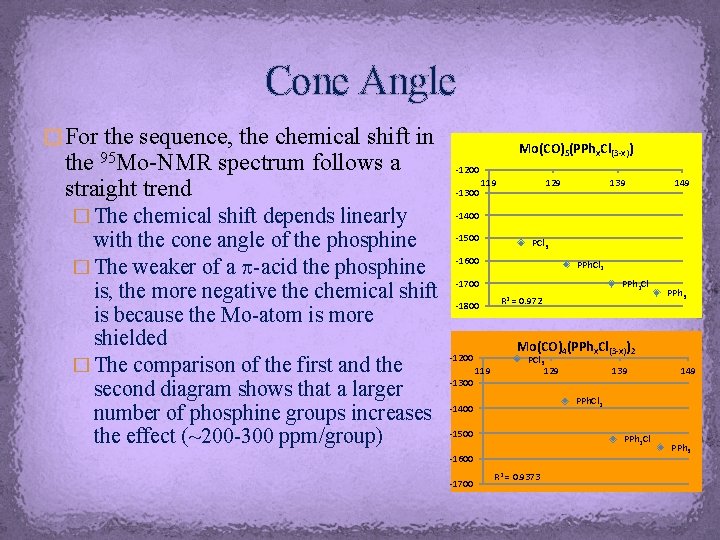
Cone Angle � For the sequence, the chemical shift in 95 Mo-NMR the straight trend spectrum follows a � The chemical shift depends linearly with the cone angle of the phosphine � The weaker of a p-acid the phosphine is, the more negative the chemical shift is because the Mo-atom is more shielded � The comparison of the first and the second diagram shows that a larger number of phosphine groups increases the effect (~200 -300 ppm/group) Mo(CO)5(PPhx. Cl(3 -x)) -1200 -1300 119 129 139 149 -1400 -1500 PCl 3 -1600 PPh. Cl 2 -1700 -1800 PPh 2 Cl R 2 = 0. 972 PPh 3 Mo(CO)4(PPhx. Cl(3 -x))2 -1200 119 PCl 3 129 139 149 -1300 PPh. Cl 2 -1400 -1500 PPh 2 Cl -1600 -1700 R 2 = 0. 9373 PPh 3

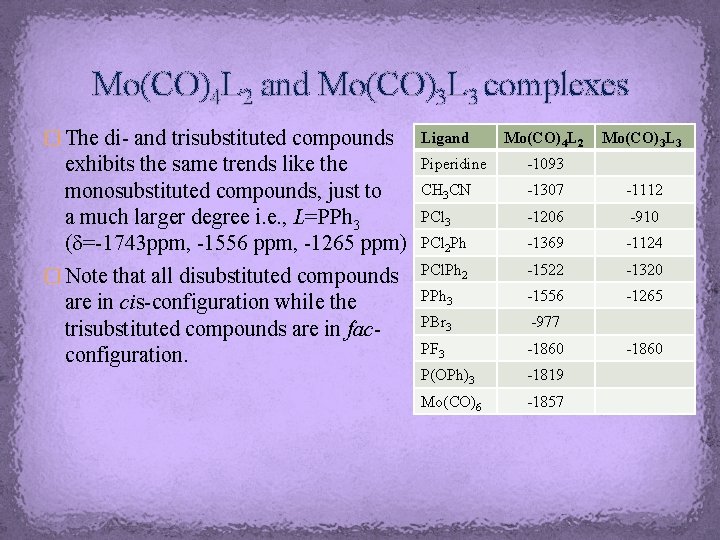
Mo(CO)4 L 2 and Mo(CO)3 L 3 complexes � The di- and trisubstituted compounds Ligand exhibits the same trends like the monosubstituted compounds, just to a much larger degree i. e. , L=PPh 3 (d=-1743 ppm, -1556 ppm, -1265 ppm) � Note that all disubstituted compounds are in cis-configuration while the trisubstituted compounds are in facconfiguration. Piperidine -1093 CH 3 CN -1307 -1112 PCl 3 -1206 -910 PCl 2 Ph -1369 -1124 PCl. Ph 2 -1522 -1320 PPh 3 -1556 -1265 PBr 3 -977 PF 3 -1860 P(OPh)3 -1819 Mo(CO)6 -1857 Mo(CO)4 L 2 Mo(CO)3 L 3 -1860

Catalysis I �Wilkinson’s catalyst (Rh. Cl(PPh 3)3) � It is obtained by the reaction of Rh. Cl 3 with four equivalents of triphenylphosphine as a red-violet solid (note that the phosphine acts as ligand as reducing reagent here) � It exhibits a square-planar coordination of around the Rh(I)-ion (d 8) � It catalyzes the hydrogenation of alkenes � The complex itself is the 16 VE system

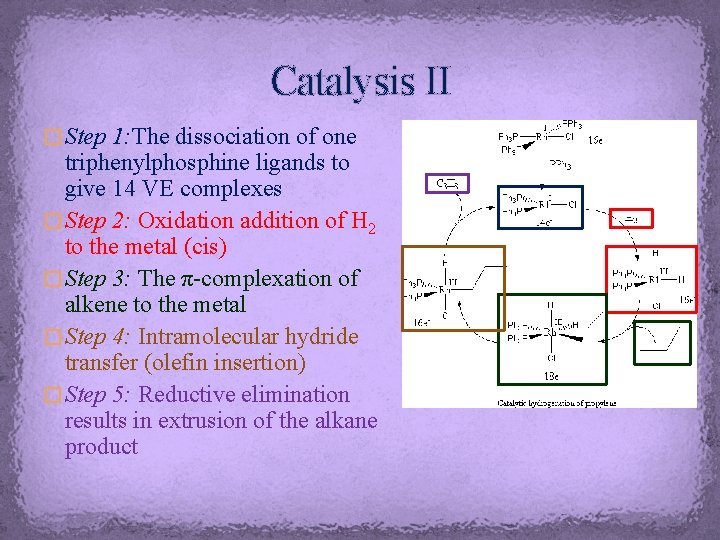
Catalysis II � Step 1: The dissociation of one triphenylphosphine ligands to give 14 VE complexes � Step 2: Oxidation addition of H 2 to the metal (cis) � Step 3: The π-complexation of alkene to the metal � Step 4: Intramolecular hydride transfer (olefin insertion) � Step 5: Reductive elimination results in extrusion of the alkane product

Catalysis III �When the triphenylphosphine ligands are replaced by chiral phosphines (i. e. , DIPAMP), the catalyst becomes chiral and converts prochiral alkenes into enantiomerically enriched alkanes via the process called asymmetric hydrogenation (i. e. , L-DOPA process, Monsanto)