Lecture 11 Cofactors Vitamins Cofactors Introduction Many enzymes






- Slides: 21

Lecture 11 Cofactors & Vitamins

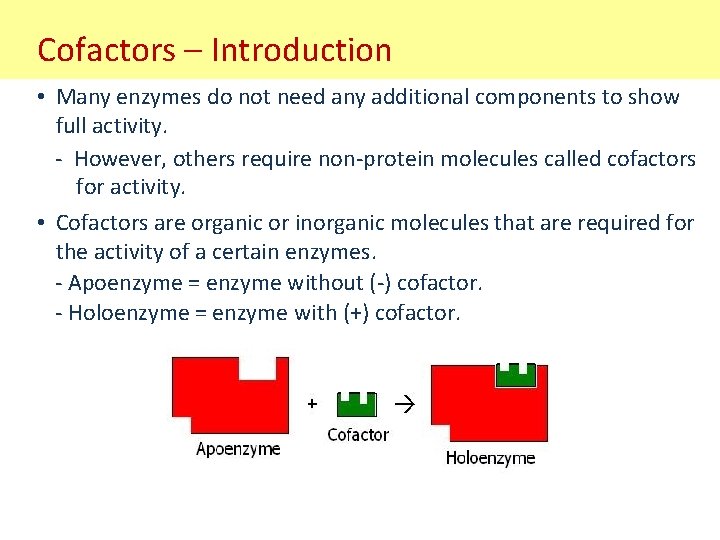
Cofactors – Introduction • Many enzymes do not need any additional components to show full activity. - However, others require non-protein molecules called cofactors for activity. • Cofactors are organic or inorganic molecules that are required for the activity of a certain enzymes. - Apoenzyme = enzyme without (-) cofactor. - Holoenzyme = enzyme with (+) cofactor.

Cofactors – Introduction • Two types of cofactors: 1. Inorganic cofactors – essential ions (e. g. metal ions and ironsulfur clusters). 2. Organic cofactors – coenzymes (e. g. flavin and heme).

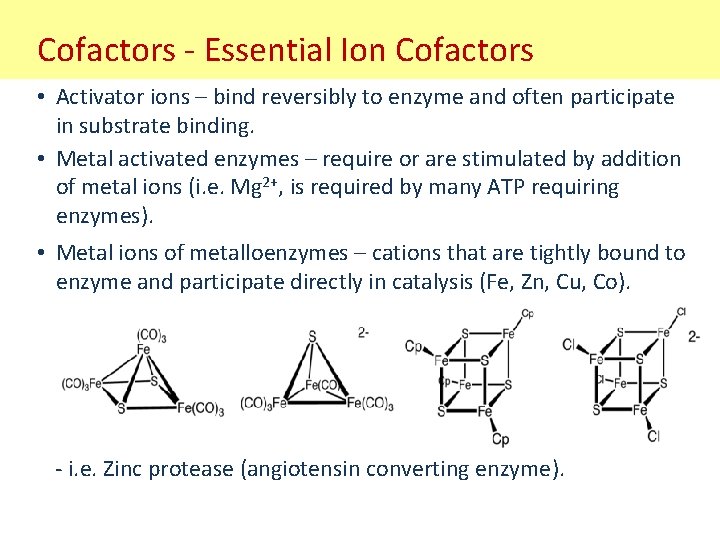
Cofactors - Essential Ion Cofactors • Activator ions – bind reversibly to enzyme and often participate in substrate binding. • Metal activated enzymes – require or are stimulated by addition of metal ions (i. e. Mg 2+, is required by many ATP requiring enzymes). • Metal ions of metalloenzymes – cations that are tightly bound to enzyme and participate directly in catalysis (Fe, Zn, Cu, Co). - i. e. Zinc protease (angiotensin converting enzyme).

Coenzymes • Coenzymes are small organic molecules (organic cofactors) that can be loosely or tightly bound to an enzyme. • Coenzymes include NADH, NADPH and adenosine triphosphate. These molecules transfer chemical groups between enzymes. • Coenzymes are usually continuously regenerated and their concentrations maintained at a steady level inside the cell. • Two classes of coenzymes: 1. Co-substrates. - altered in reactions and regenerated to original structure in subsequent reactions. - disassociated from active site. - shuttle chemical groups among different enzyme reactions. 2. Prosthetic groups. - remains bound to enzyme.

Coenzymes • Examples: 1. Metabolite coenzymes – synthesized from common metabolites. 2. Nucleoside triphosphates – (ATP) can donate phosphates, pyrophosphates, adenosyl groups. 3. S-adenosylmethionine (SAM) – donates methyl groups. 4. Nucleotide sugars (uridine diphosphate glucose = UDP-glucose) - transfer sugars in carbohydrate metabolism.

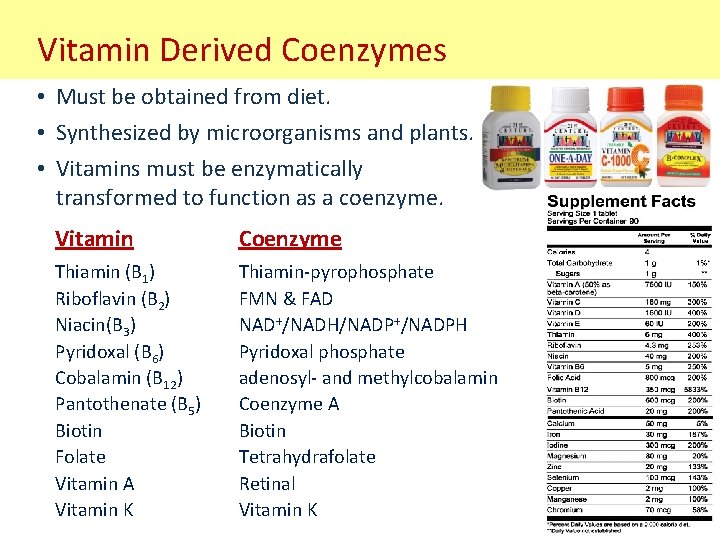
Vitamin Derived Coenzymes • Must be obtained from diet. • Synthesized by microorganisms and plants. • Vitamins must be enzymatically transformed to function as a coenzyme. Vitamin Coenzyme Thiamin (B 1) Riboflavin (B 2) Niacin(B 3) Pyridoxal (B 6) Cobalamin (B 12) Pantothenate (B 5) Biotin Folate Vitamin A Vitamin K Thiamin-pyrophosphate FMN & FAD NAD+/NADH/NADP+/NADPH Pyridoxal phosphate adenosyl- and methylcobalamin Coenzyme A Biotin Tetrahydrafolate Retinal Vitamin K

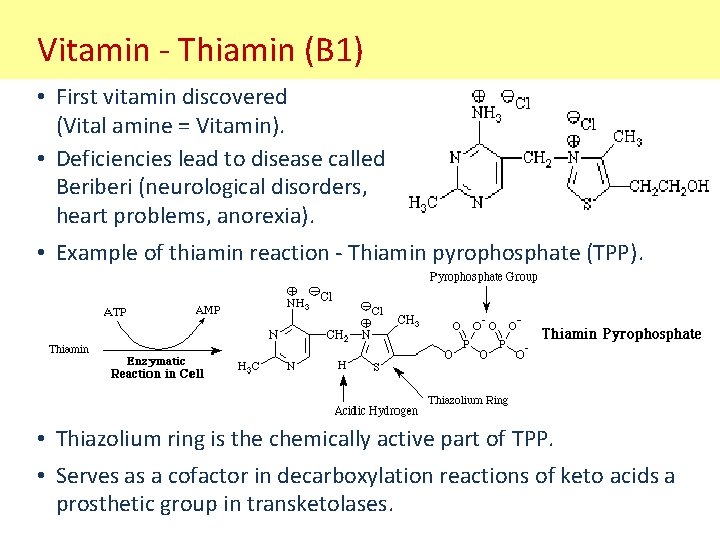
Vitamin - Thiamin (B 1) • First vitamin discovered (Vital amine = Vitamin). • Deficiencies lead to disease called Beriberi (neurological disorders, heart problems, anorexia). • Example of thiamin reaction - Thiamin pyrophosphate (TPP). • Thiazolium ring is the chemically active part of TPP. • Serves as a cofactor in decarboxylation reactions of keto acids a prosthetic group in transketolases.

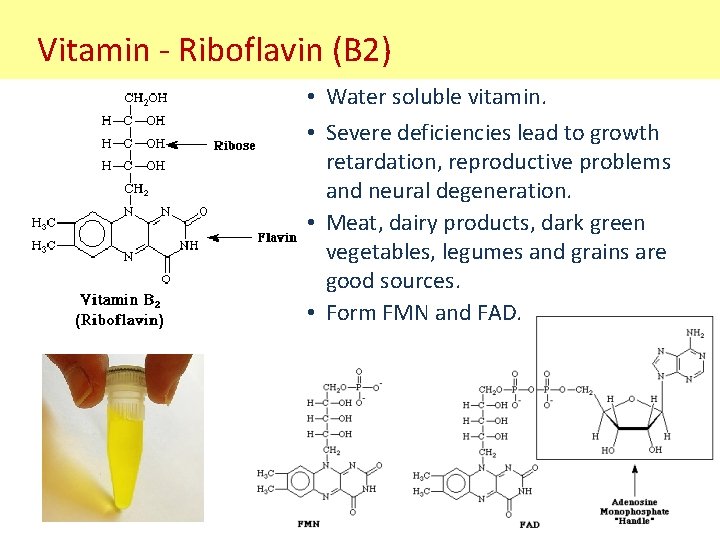
Vitamin - Riboflavin (B 2) • Water soluble vitamin. • Severe deficiencies lead to growth retardation, reproductive problems and neural degeneration. • Meat, dairy products, dark green vegetables, legumes and grains are good sources. • Form FMN and FAD.

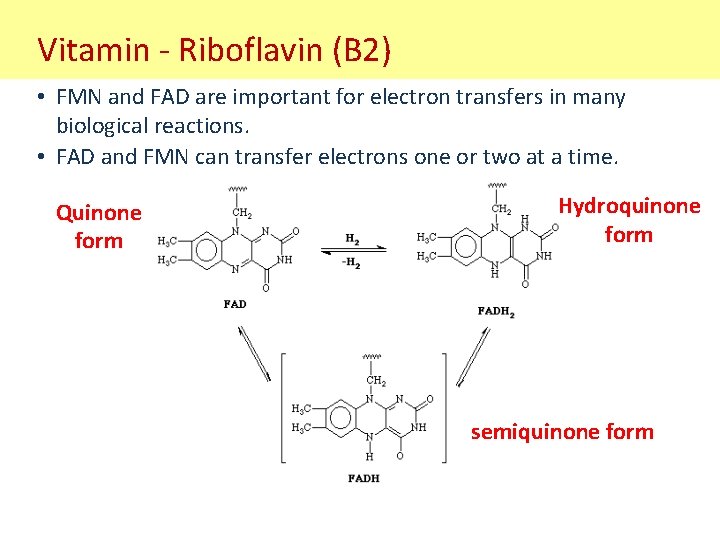
Vitamin - Riboflavin (B 2) • FMN and FAD are important for electron transfers in many biological reactions. • FAD and FMN can transfer electrons one or two at a time. Quinone form Hydroquinone form semiquinone form

Vitamin - Niacin (B 3) • Also known as nicotinic acid. • Deficiencies lead to pellagra (dermatitis, diarrhea, dementia). • Can be synthesized from tryptophan in the liver. • Required in relatively high amounts compared to other vitamins. - Niacin is a precursor to NAD+/NADH and NADP+/NADPH, which play essential metabolic roles in living cells. - Niacin is involved in both DNA repair, and the production of steroid hormones in the adrenal gland.

Vitamin - Niacin (B 3) • NAD+ / NADP+. - Serve as cofactors in oxidation/reduction reactions. - Act as co-substrates for dehydrogenases. - Reduction of NAD+/NADP+ and oxidation of NADH/NADPH occurs 2 e- at a time. - NADH is coupled with ATP production in mitochondria. - NADPH is an important reducing agent in biosynthetic reactions.

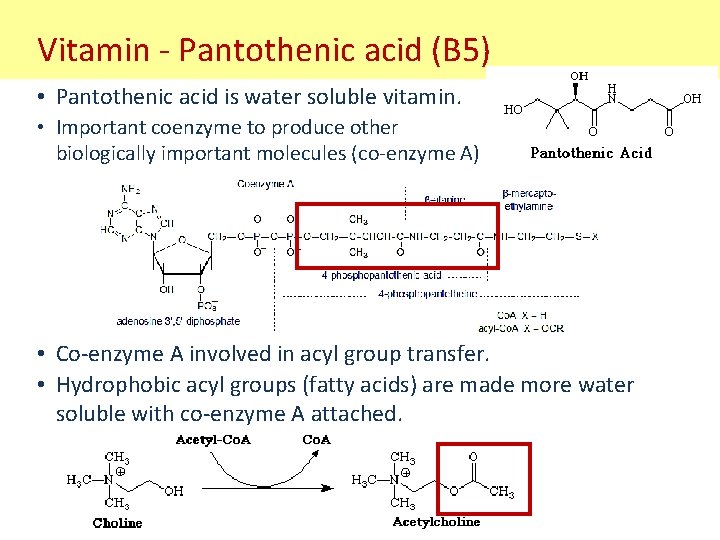
Vitamin - Pantothenic acid (B 5) • Pantothenic acid is water soluble vitamin. • Important coenzyme to produce other biologically important molecules (co-enzyme A). • Co-enzyme A involved in acyl group transfer. • Hydrophobic acyl groups (fatty acids) are made more water soluble with co-enzyme A attached.

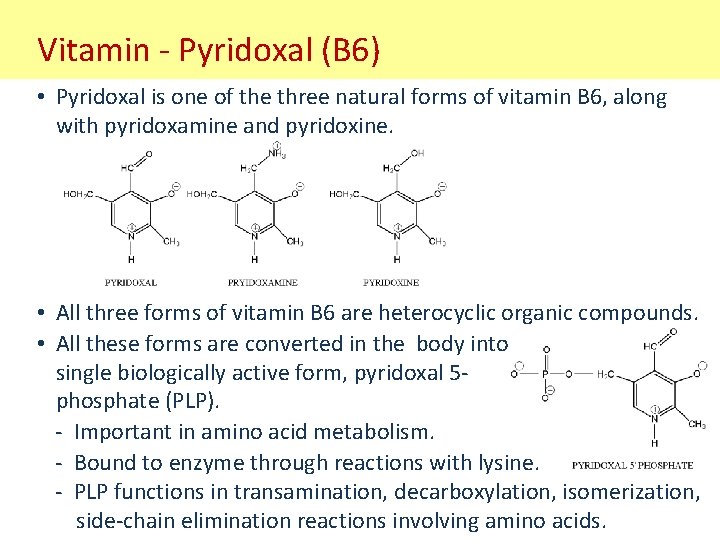
Vitamin - Pyridoxal (B 6) • Pyridoxal is one of the three natural forms of vitamin B 6, along with pyridoxamine and pyridoxine. • All three forms of vitamin B 6 are heterocyclic organic compounds. • All these forms are converted in the body into a single biologically active form, pyridoxal 5 phosphate (PLP). - Important in amino acid metabolism. - Bound to enzyme through reactions with lysine. - PLP functions in transamination, decarboxylation, isomerization, side-chain elimination reactions involving amino acids.

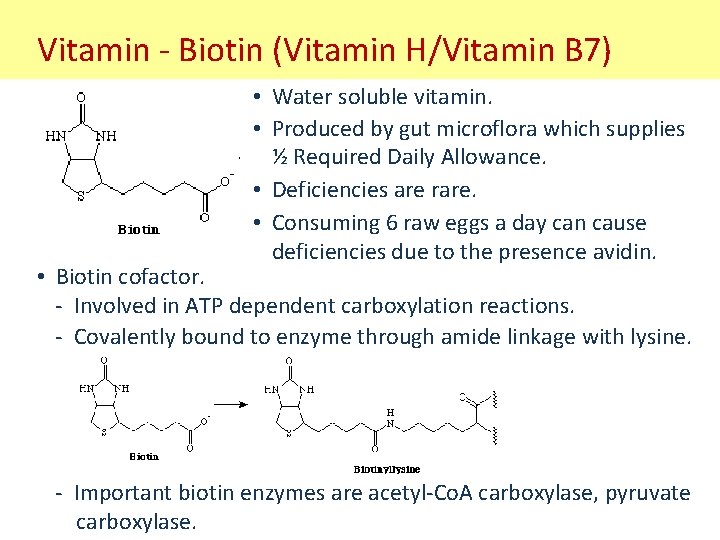
Vitamin - Biotin (Vitamin H/Vitamin B 7) • Water soluble vitamin. • Produced by gut microflora which supplies ½ Required Daily Allowance. • Deficiencies are rare. • Consuming 6 raw eggs a day can cause deficiencies due to the presence avidin. • Biotin cofactor. - Involved in ATP dependent carboxylation reactions. - Covalently bound to enzyme through amide linkage with lysine. - Important biotin enzymes are acetyl-Co. A carboxylase, pyruvate carboxylase.

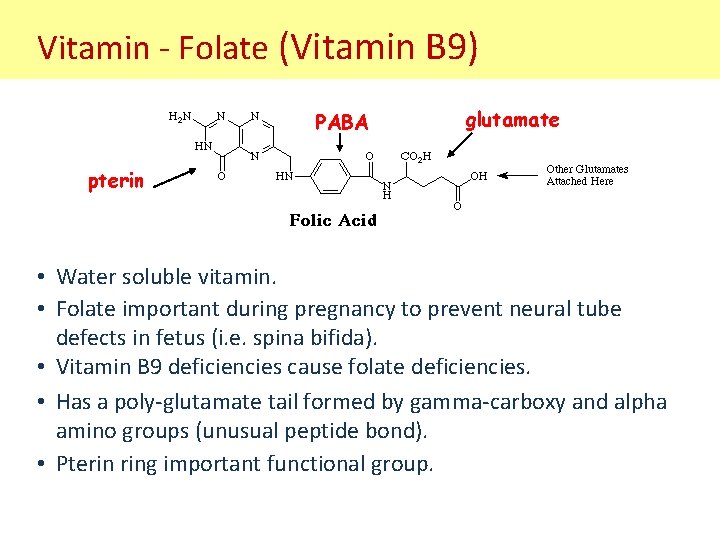
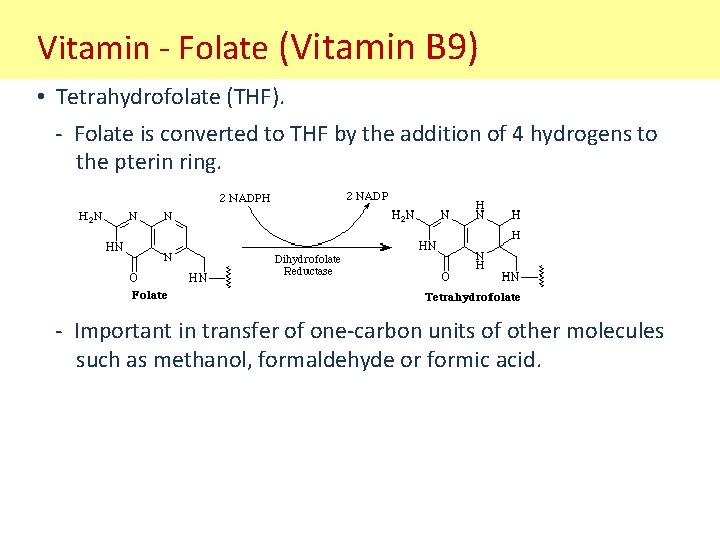
Vitamin - Folate (Vitamin B 9) PABA glutamate pterin • Water soluble vitamin. • Folate important during pregnancy to prevent neural tube defects in fetus (i. e. spina bifida). • Vitamin B 9 deficiencies cause folate deficiencies. • Has a poly-glutamate tail formed by gamma-carboxy and alpha amino groups (unusual peptide bond). • Pterin ring important functional group.

Vitamin - Folate (Vitamin B 9) • Tetrahydrofolate (THF). - Folate is converted to THF by the addition of 4 hydrogens to the pterin ring. - Important in transfer of one-carbon units of other molecules such as methanol, formaldehyde or formic acid.

Vitamin - Cobalamin (B 12) • Water soluble Vitamin. • Corrin ring with Cobalt cation. • Involved in intramolecular rearrangements, methyl group transfer, reduction of ribonucleotides to deoxyribonucleotides • Forms radical species.

Vitamin - Fat soluble Vitamins • Some vitamins are fat soluble (i. e. vitamins A, D, E, K). - Vitamin A (retinol) derived from β-carotene is important for vision, regulation of gene expression during cell differentiation and teratogenic. - Vitamin D – important in calcium absorption, regulates intestinal absorption and deposition in bones. - Vitamin E – antioxidant. - Vitamin K –needed for the posttranslational modification of certain proteins required for blood coagulation and in metabolic pathways in bone and other tissue.

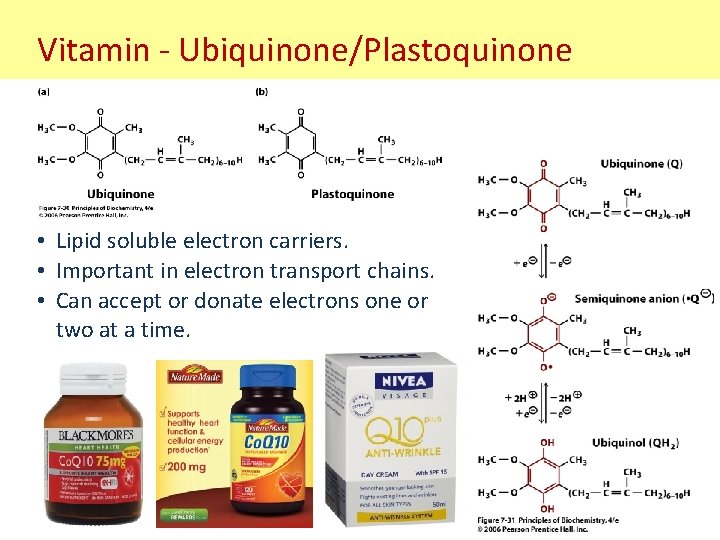
Vitamin - Ubiquinone/Plastoquinone • Lipid soluble electron carriers. • Important in electron transport chains. • Can accept or donate electrons one or two at a time.

Vitamin - Cytochromes • Protein coenzyme. • Heme containing proteins. • Fe 3+ can undergo reversible one electron reduction. • Important in redox reactions.
 Water soluble vitamins vs fat soluble vitamins
Water soluble vitamins vs fat soluble vitamins Organic and inorganic cofactors
Organic and inorganic cofactors Cofactor
Cofactor Organic and inorganic cofactors
Organic and inorganic cofactors Vitamin cofactors
Vitamin cofactors Cofactor expansion theorem
Cofactor expansion theorem Dao cofactors
Dao cofactors 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Introduction to biochemistry lecture notes
Introduction to biochemistry lecture notes Introduction to psychology lecture
Introduction to psychology lecture Introduction to algorithms lecture notes
Introduction to algorithms lecture notes Water soluble vitamins characteristics
Water soluble vitamins characteristics Are vitamins tasteless
Are vitamins tasteless Minerals and their functions sources and deficiency chart
Minerals and their functions sources and deficiency chart Vitamins name
Vitamins name Vitamin flowchart
Vitamin flowchart Vital amine
Vital amine Water soluble vitamins characteristics
Water soluble vitamins characteristics Kellogg's pep vitamins advertisement analysis
Kellogg's pep vitamins advertisement analysis Categories of vitamins
Categories of vitamins Orange vegetables list
Orange vegetables list Vitamins functions
Vitamins functions






































