Lecture 11 Chemical Reaction Engineering CRE is the




















- Slides: 22

Lecture 11 Chemical Reaction Engineering (CRE) is the field that studies the rates and mechanisms of chemical reactions and the design of the reactors in which they take place.

Today’s lecture Determining the Rate Law from Experimental Data Integral Method Differential (Graphical) Method Nonlinear Least Regression Multiple Reactions The Four Types What’s New to Our Algorithm Reactions in Series 2

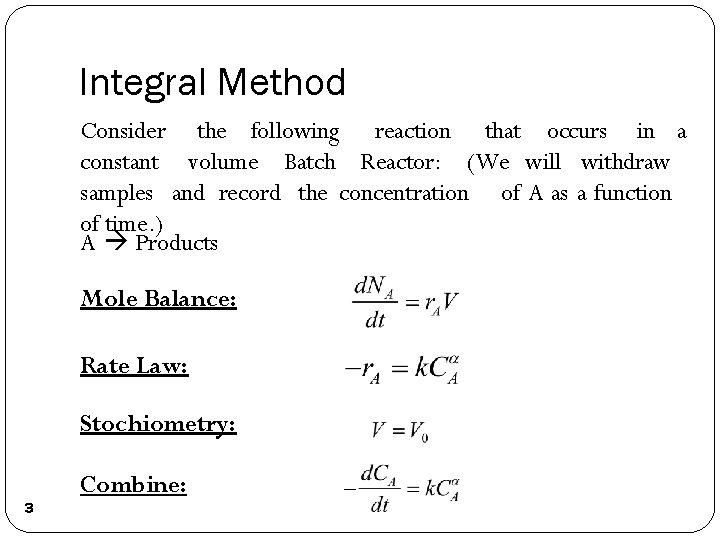
Integral Method Consider the following reaction that occurs in a constant volume Batch Reactor: (We will withdraw samples and record the concentration of A as a function of time. ) A Products Mole Balance: Rate Law: Stochiometry: 3 Combine:

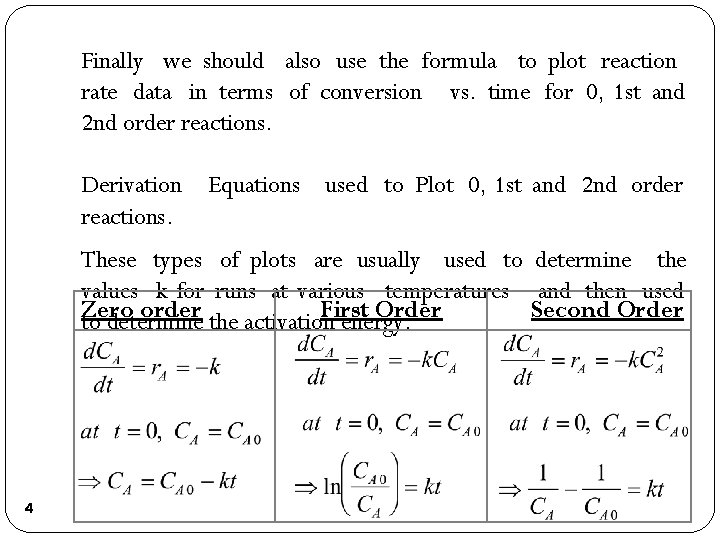
Finally we should also use the formula to plot reaction rate data in terms of conversion vs. time for 0, 1 st and 2 nd order reactions. Derivation reactions. Equations used to Plot 0, 1 st and 2 nd order These types of plots are usually used to determine the values k for runs at various temperatures and then used Zero order the activation First Order Second Order to determine energy. 4

Finding the Rate law (Integral Method) Guess and check for experimental plot CA α = 0, 1, 2 and check against 1/C A ln(C A 0/C A) t t t

Differential Method Taking the natural log of The reaction order can be found from a ln-ln plot of: ln Slope = α 6 ln

Methods for finding the slope of log-log and semi-log graph papers may be found at http: //www. physics. uoguelph. ca/tutorials/GLP/ . However, we are usually given concentration as a function of time from batch reactor experiments: time (s) concentration (moles/dm 3) 7 0 CA 0 t 1 CA 1 t 2 CA 2 t 3 CA 3

Three Ways to determine (-d. C A /dt) from Concentration. Time Data Graphical differentiation Numerical differentiation formulas Differentiation of a polynomial fit to the data 1. Graphical 8

9 The method accentuates measurement error!

Example - Finding Rate Law t(min) 0 CA(mol/L) 1 1 2 3 0. 7 0. 5 0. 3 0. 2 0. 15 . 3 Areas equal for both sides of the histogram . 2. 1 10 1 2 3 t

Example - Finding Rate Law Find the f(t) of differentiation CA 1 -d. C A /dt 0. 35 using equal area 0. 7 0. 25 0. 175 Plot –d. C A /dt as a function of CA ln d. C A /dt Slope = α ln 11 CA 0. 35 0. 12

Non-Linear Least-Square Analysis We want to find the parameter values ( α, k, E) for which the sum of the squares of the differences, the measured rate (r m ), and the calculated rate (r c ) is a minimum. That is we want 12 to be a minimum.

Non-Linear Least-Square Analysis For concentration-time data, we can integrate the mole balance equation for to obtain 13

Non-Linear Least-Square Analysis We find the values of alpha and k which minimize s 14 2

Regression Method Guess values for α and k and solve for measured data points then sum squared differences: Cmeas 1 0. 7 0. 5 0. 35 CCalc 1 0. 5 0. 33 0. 25 (C c -C m ) 0 -0. 2 -0. 17 -0. 10 (C c -C m ) 2 0 0. 04 0. 029 0. 01 for α= 2, k = 1 → S 2 = 0. 07 for α = 2, k = 2 → S 2 = 0. 27 15 etc. until S 2 is a minimum 0. 07

16

17

18

Residuals 19

20

21

End of Lecture 11 22