LECTURE 10 Wednesday 2817 Chapter 13 Alcohols Phenols


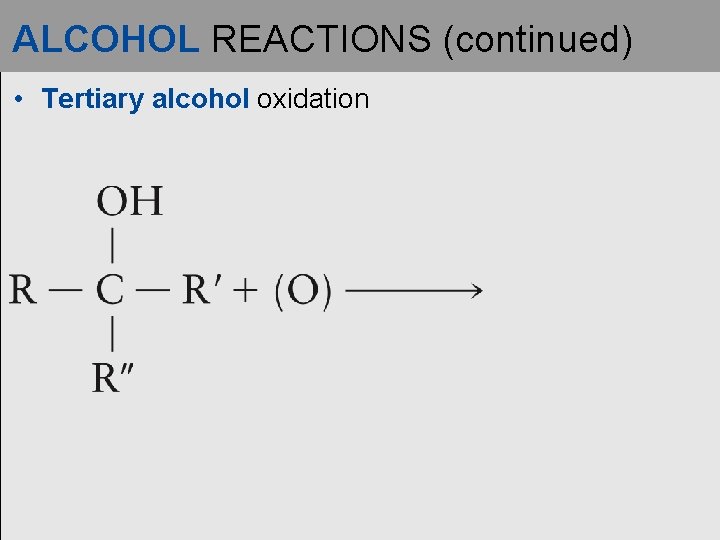






- Slides: 27

LECTURE 10 Wednesday 2/8/17

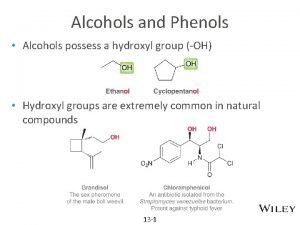
Chapter 13 Alcohols, Phenols, and Ethers

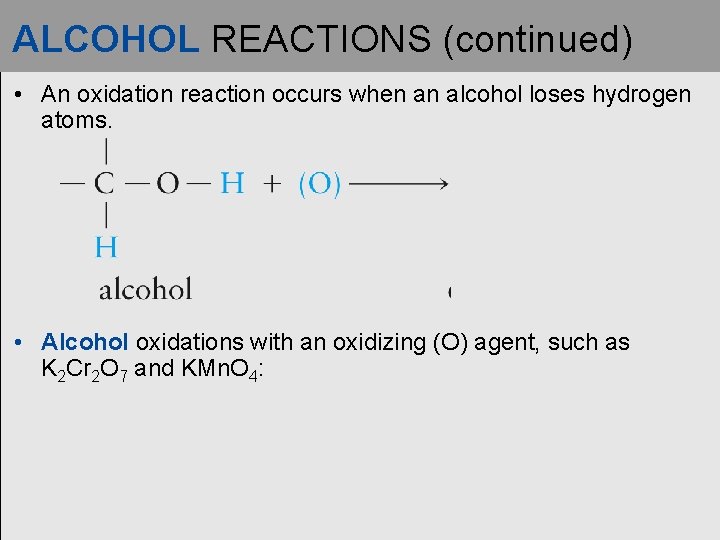
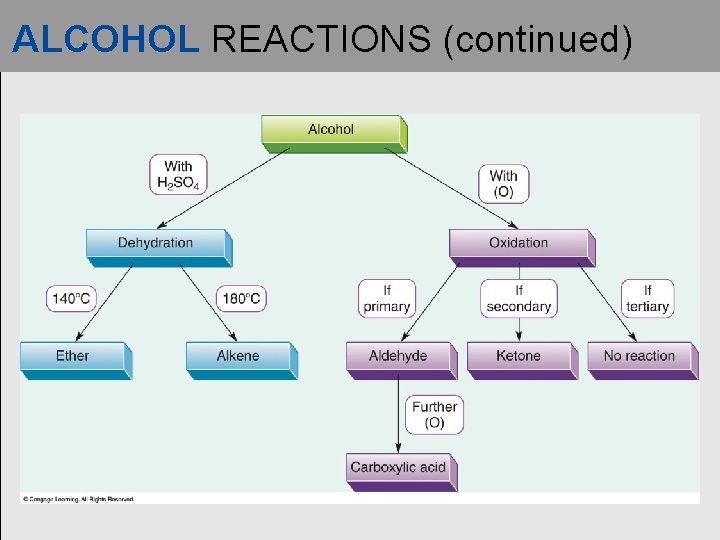
ALCOHOL REACTIONS (continued) • An oxidation reaction occurs when an alcohol loses hydrogen atoms. • Alcohol oxidations with an oxidizing (O) agent, such as K 2 Cr 2 O 7 and KMn. O 4:

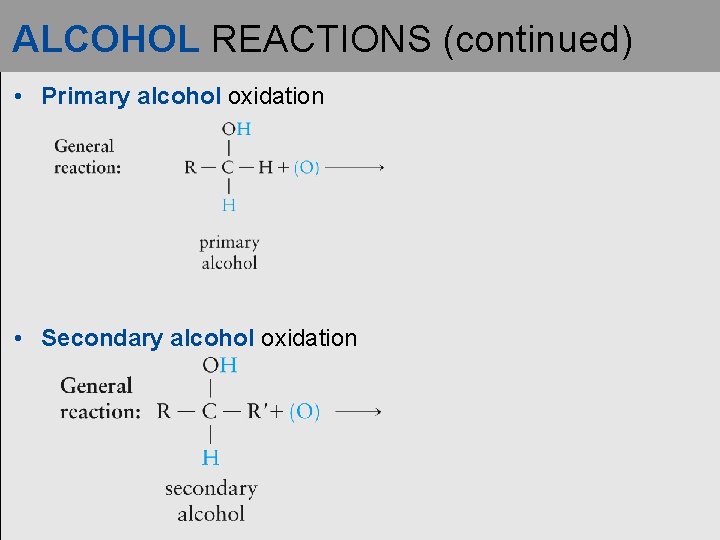
ALCOHOL REACTIONS (continued) • Primary alcohol oxidation • Secondary alcohol oxidation
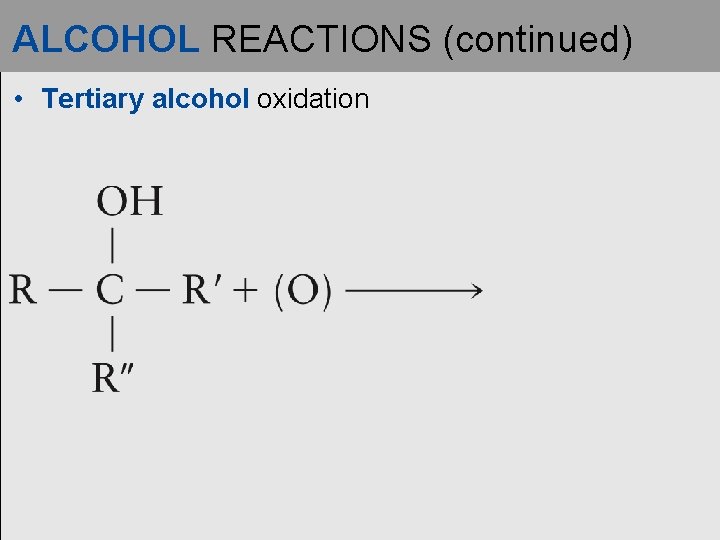
ALCOHOL REACTIONS (continued) • Tertiary alcohol oxidation

ALCOHOL OXIDATION EXAMPLES

ALCOHOL REACTIONS (continued)

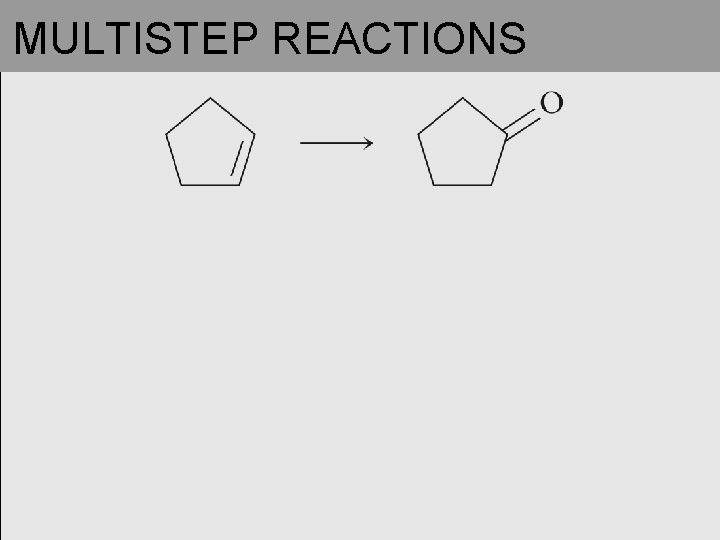
MULTISTEP REACTIONS

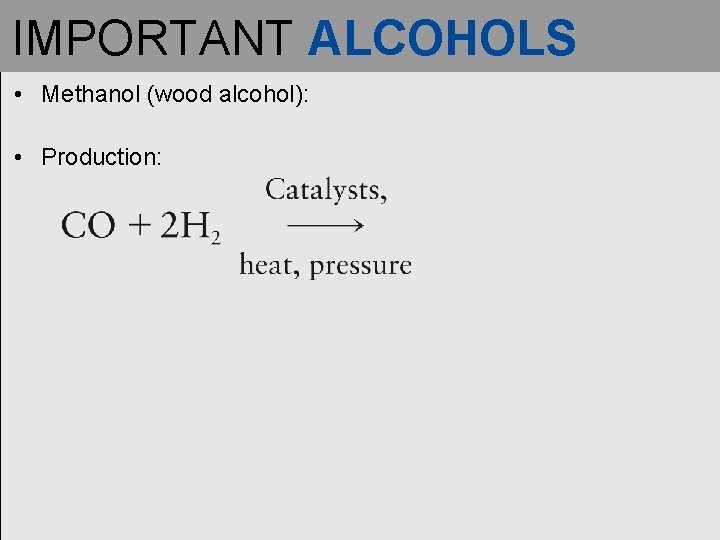
IMPORTANT ALCOHOLS • Methanol (wood alcohol): • Production:

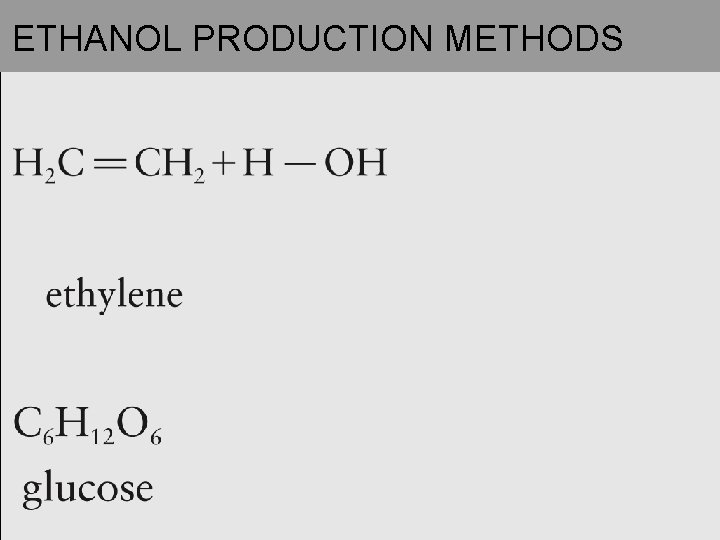
IMPORTANT ALCOHOLS (continued) • Ethanol (ethyl alcohol, grain alcohol): • Produced commercially from ethylene and through biological (yeast) ____________ of carbohydrates

ETHANOL PRODUCTION METHODS

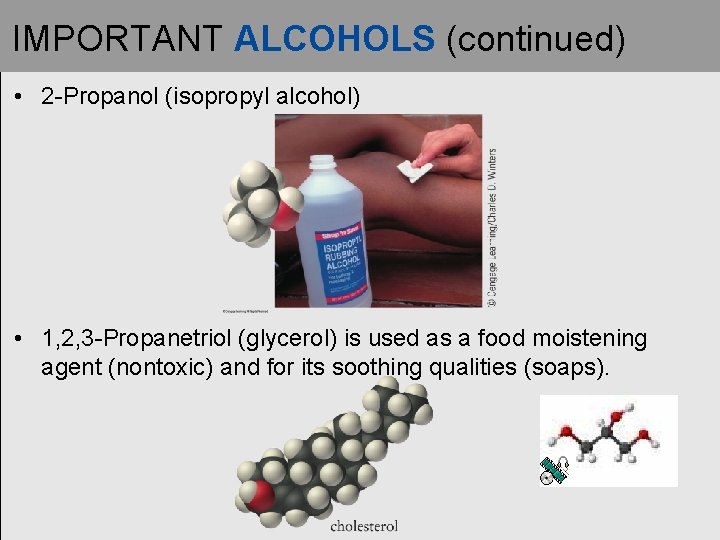
IMPORTANT ALCOHOLS (continued) • 2 -Propanol (isopropyl alcohol) • 1, 2, 3 -Propanetriol (glycerol) is used as a food moistening agent (nontoxic) and for its soothing qualities (soaps).

IMPORTANT ALCOHOLS (continued) • Antifreezes 1, 2 -ethanediol (ethylene glycol) • 1, 2 -propanediol (propylene glycol)

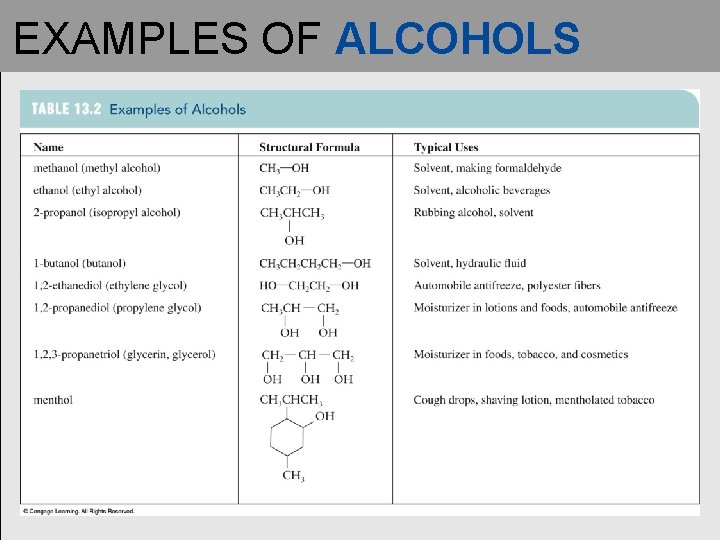
EXAMPLES OF ALCOHOLS

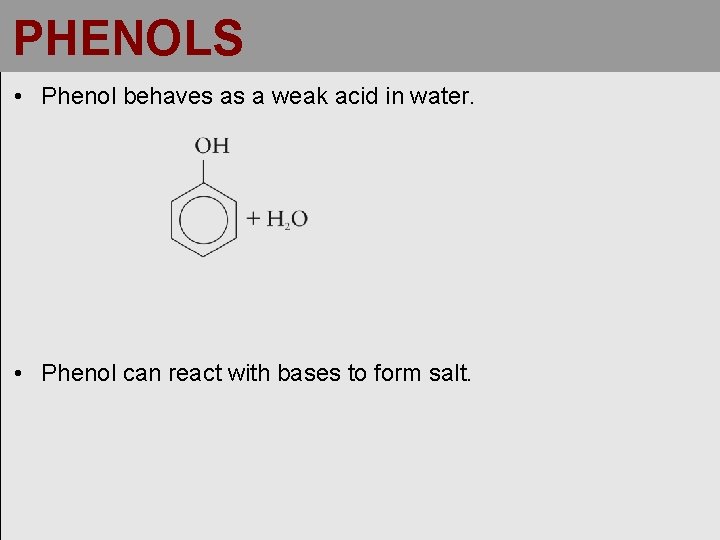
PHENOLS • Phenol behaves as a weak acid in water. • Phenol can react with bases to form salt.

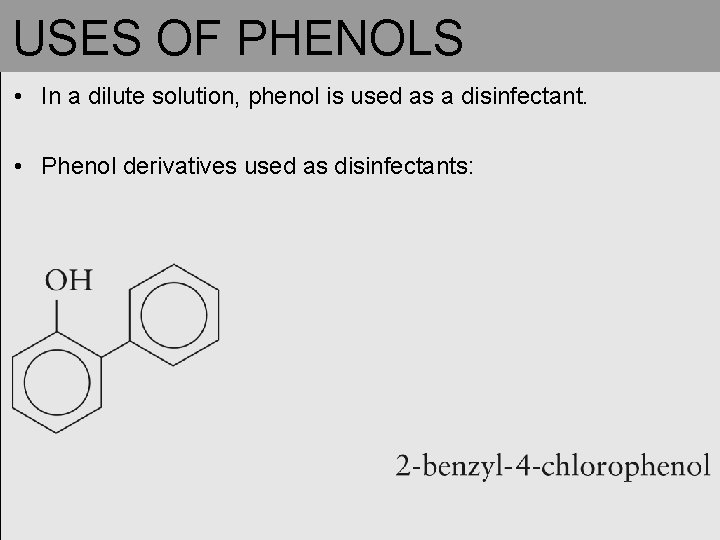
USES OF PHENOLS • In a dilute solution, phenol is used as a disinfectant. • Phenol derivatives used as disinfectants:

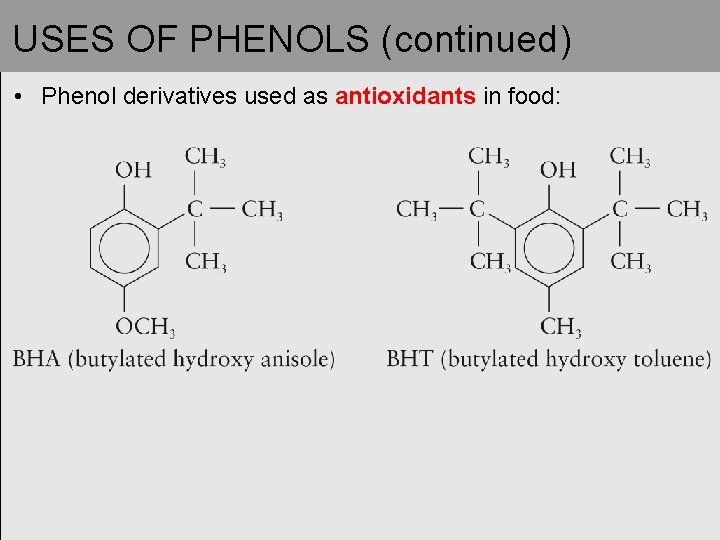
USES OF PHENOLS (continued) • Phenol derivatives used as antioxidants in food:

NAMING ETHERS • IUPAC name: Name the smaller of the two R groups as an alkoxy group attached to the parent chain by replacing the –yl ending of the R group with –oxy. • Common name: Name the groups attached to the oxygen alphabetically and add the word ether.

CYCLIC ETHERS • _________________ contain atoms other than carbon in the ring.

PROPERTIES OF ETHERS • Much less polar than • More soluble in water than _____ but less soluble than ______

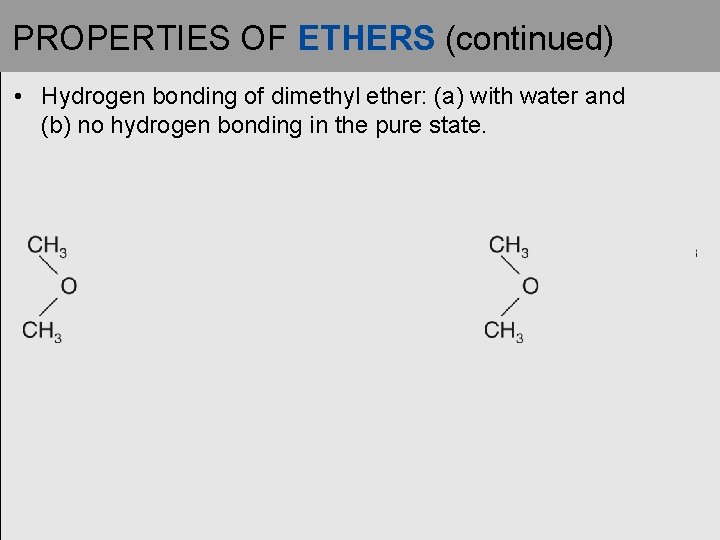
PROPERTIES OF ETHERS (continued) • Hydrogen bonding of dimethyl ether: (a) with water and (b) no hydrogen bonding in the pure state.

THIOLS: THE –SH (SULFHYDRYL) GROUP • Most distinguishing characteristic • ethanethiol – • propanethiol – • 1 -propene-3 -thiol and 3, 3 -di-(1 -propenyl)disulfide – • trans-2 -butene-1 -thiol, 3 -methyl-1 -butanethiol, and methyl 1 -(trans-2 -butenyl)disulfide –

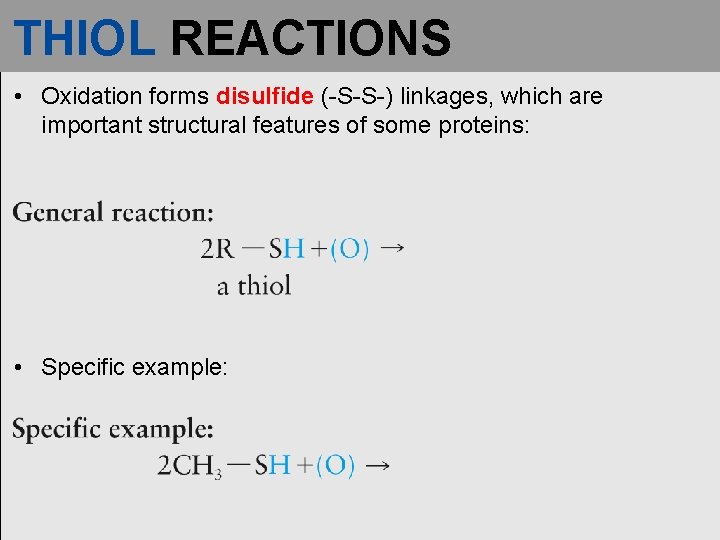
THIOL REACTIONS • Oxidation forms disulfide (-S-S-) linkages, which are important structural features of some proteins: • Specific example:

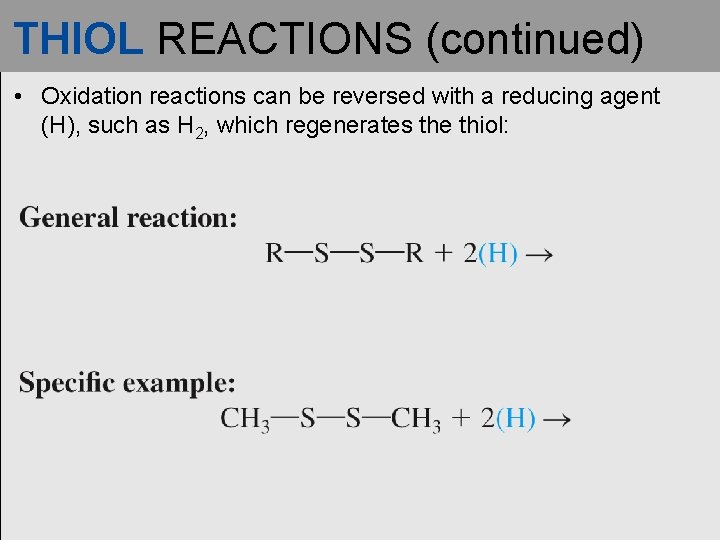
THIOL REACTIONS (continued) • Oxidation reactions can be reversed with a reducing agent (H), such as H 2, which regenerates the thiol:

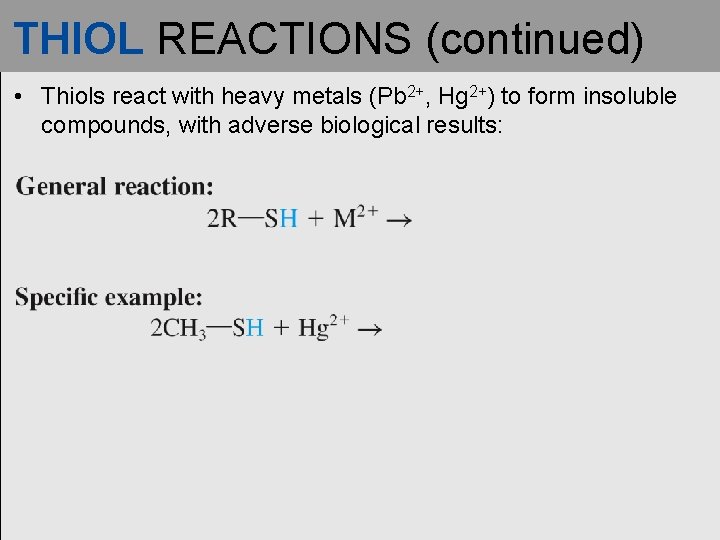
THIOL REACTIONS (continued) • Thiols react with heavy metals (Pb 2+, Hg 2+) to form insoluble compounds, with adverse biological results:

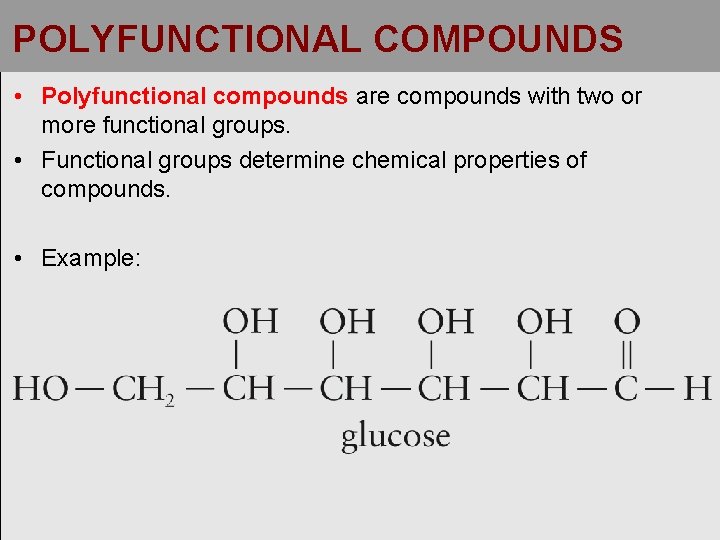
POLYFUNCTIONAL COMPOUNDS • Polyfunctional compounds are compounds with two or more functional groups. • Functional groups determine chemical properties of compounds. • Example:

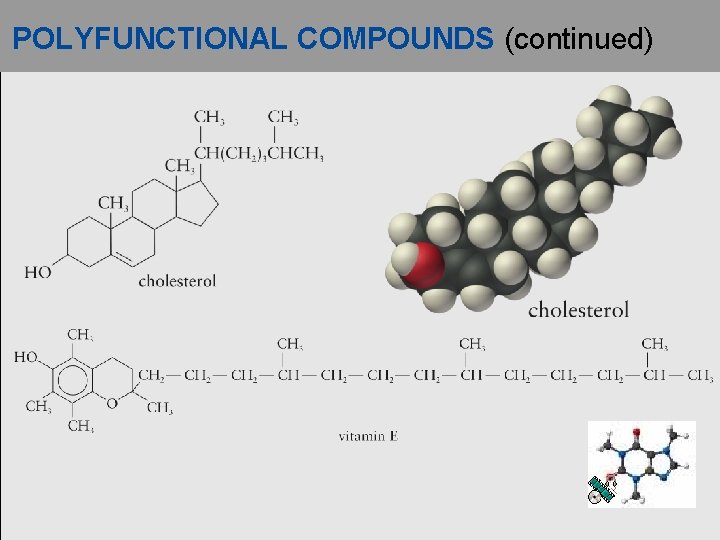
POLYFUNCTIONAL COMPOUNDS (continued)
 Alcohols phenols thiols and ethers
Alcohols phenols thiols and ethers Rfc 2817
Rfc 2817 How to name esters
How to name esters Simple phenols
Simple phenols 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Preparation of tertiary alcohol
Preparation of tertiary alcohol These are alcohols containing cppp nucleus?
These are alcohols containing cppp nucleus? Epoxide reaction with grignard reagent
Epoxide reaction with grignard reagent Oxidation of a primary alcohol
Oxidation of a primary alcohol Alcohol and hbr
Alcohol and hbr Lucas test
Lucas test Preparing haloalkanes from alcohols
Preparing haloalkanes from alcohols Reactions of alcohols 1 chemsheets answers
Reactions of alcohols 1 chemsheets answers Na2cr2o7 mechanism
Na2cr2o7 mechanism Acidity of alcohols
Acidity of alcohols Relative sweetness chart
Relative sweetness chart Alcohols nomenclature
Alcohols nomenclature Naming alkyl halides
Naming alkyl halides Alcohols nomenclature
Alcohols nomenclature Isopropyl methyl ether
Isopropyl methyl ether Oh group in phenol is
Oh group in phenol is Lucas reagent test
Lucas reagent test Monday tuesday wednesday thursday friday calendar
Monday tuesday wednesday thursday friday calendar Wednesday evening prayer
Wednesday evening prayer Wednesday seminar
Wednesday seminar History ia grade boundaries
History ia grade boundaries Michelle hansberry
Michelle hansberry Again
Again