Lecture 1 Introduction to Human Anatomy and Physiology























- Slides: 60

Lecture 1 Introduction to Human Anatomy and Physiology

Anatomy and Physiology n Anatomy deals with the structure (morphology) of the body and its parts, in other words, what are things called? n Physiology studies the functions of these parts or asks the question, “how do they work? n The two disciplines are closely interrelated because the functional role of a part depends on how it is constructed. Pathophysiology is the study of disorders of functioning, e. g Anemia n

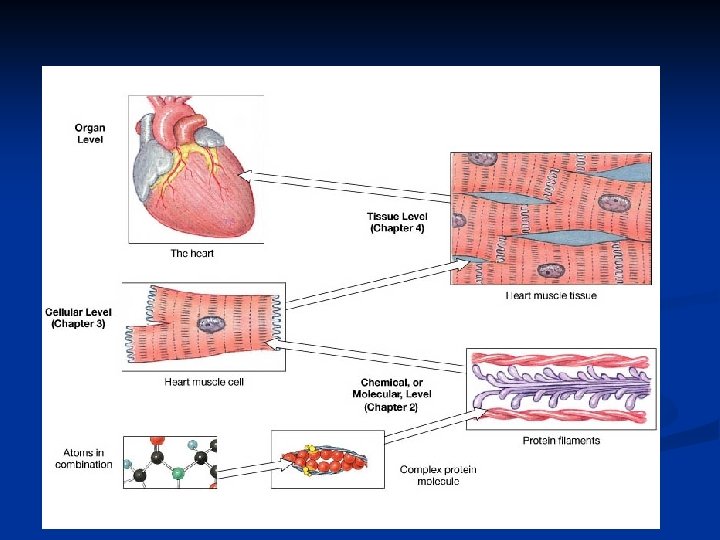
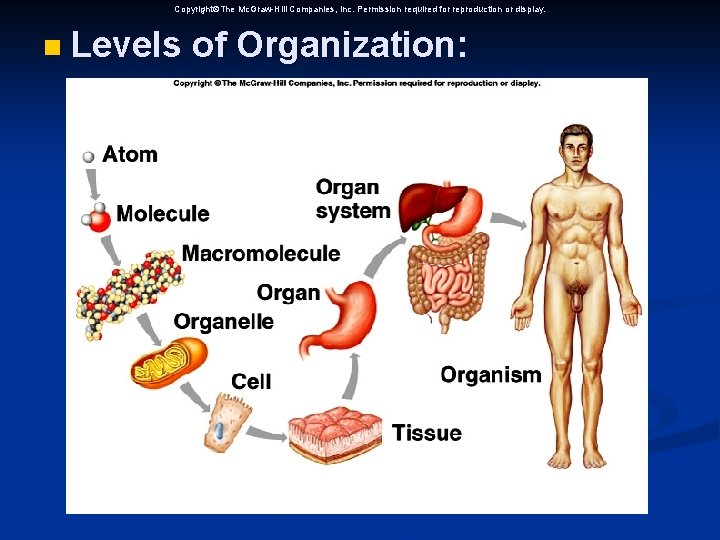
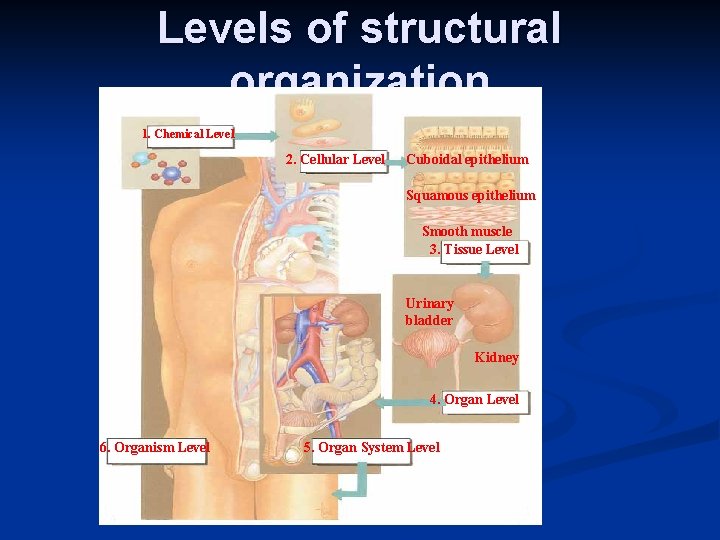
n Levels of organization n n Chemicals Cells Tissues (Epithelial, Connective, Muscle, Nerve tissues) n Organs n Replacing Tissues and organs (Blood), (Corneas, kidneys, liver, heart and lungs) Skin is organ in skin burns n


Copyright The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. n Levels of Organization:

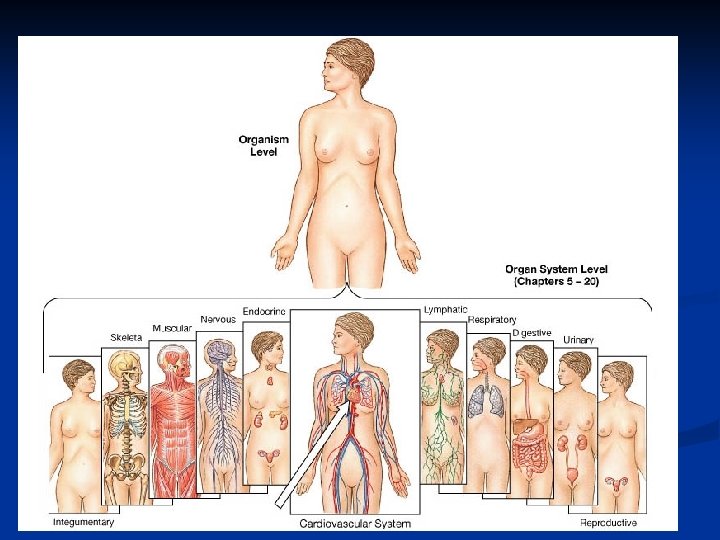
Levels of structural organization 1. Chemical Level 2. Cellular Level Cuboidal epithelium Squamous epithelium Smooth muscle 3. Tissue Level Urinary bladder Kidney 4. Organ Level 6. Organism Level 5. Organ System Level

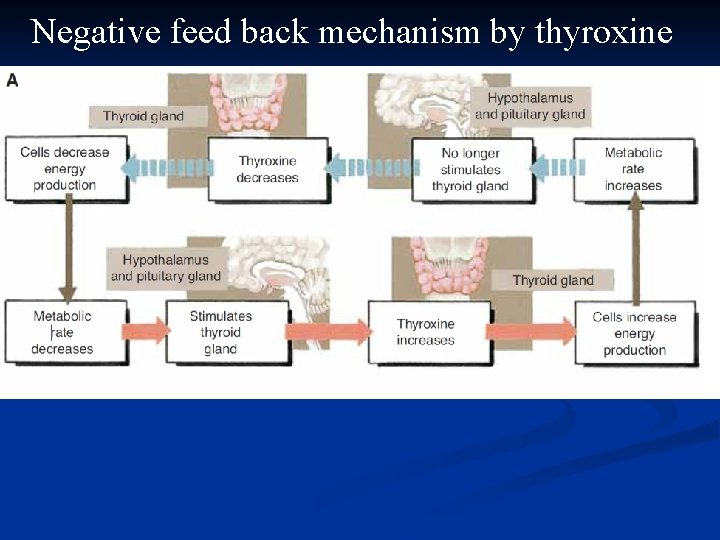
n n n Metabolism and Homeostasis: Metabolism includes growing, repairing, reacting and reproducing Pumping, digestion, diffusion and production of energy Greek word Metabolic rate Maintenance of a stable internal environment is called homeostasis. Homeostasis is regulated through control systems which have receptors, a set point and effectors in common. Examples include: a. Homeostatic mechanisms regulate body temperature in a manner similar to the functioning of a home heating thermostat. b. Another homeostatic mechanism employs pressuresensitive receptors to regulate blood pressure.

Homeostasis: Many of the body's homeostatic controls are negative feedback mechanisms. n Each individual uses homeostatic mechanisms to keep body levels within a normal range; normal ranges can vary from one individual to the next. n

Negative feed back mechanism by thyroxine

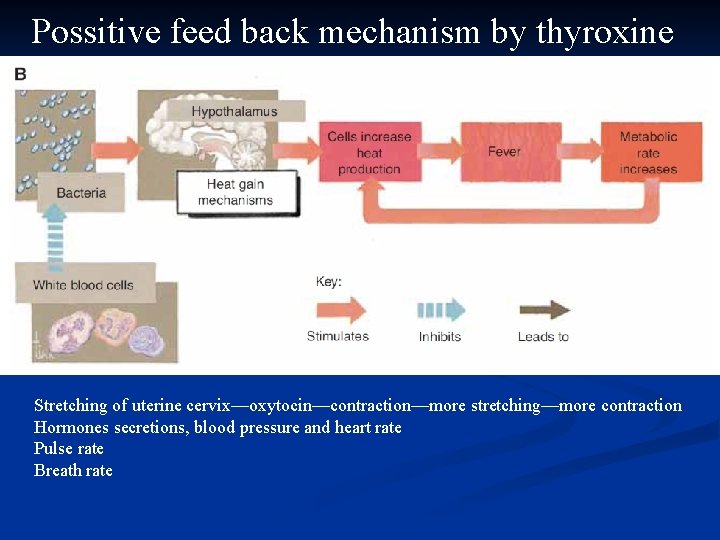
Possitive feed back mechanism by thyroxine Stretching of uterine cervix—oxytocin—contraction—more stretching—more contraction Hormones secretions, blood pressure and heart rate Pulse rate Breath rate

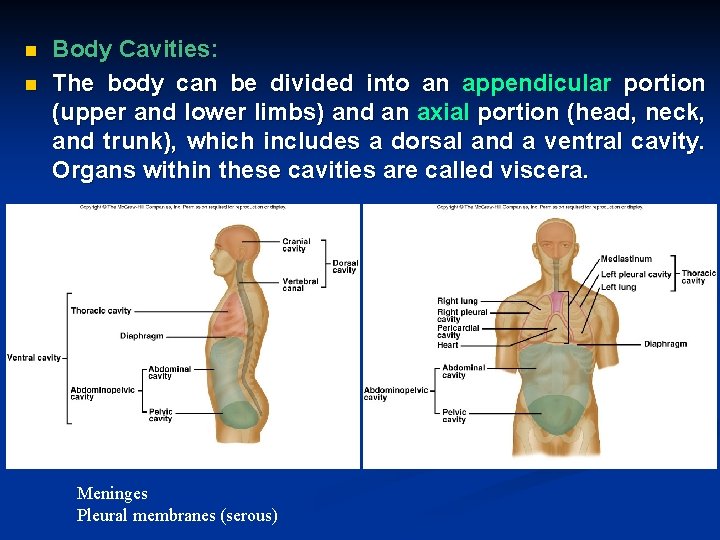
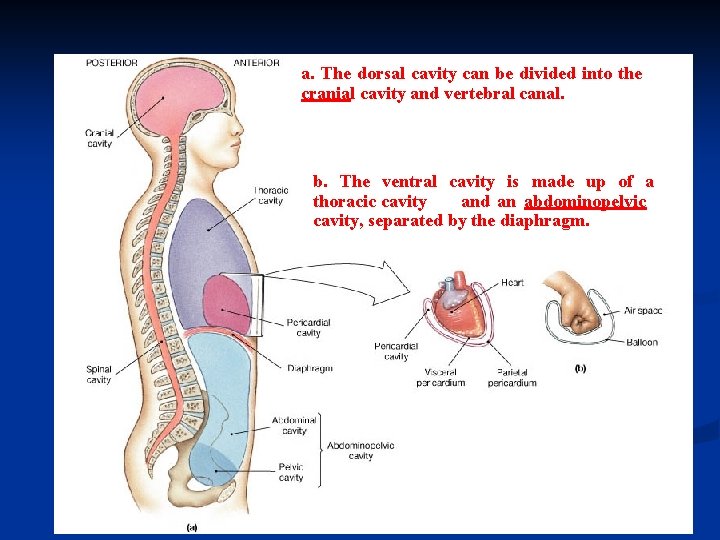
n n Body Cavities: The body can be divided into an appendicular portion (upper and lower limbs) and an axial portion (head, neck, and trunk), which includes a dorsal and a ventral cavity. Organs within these cavities are called viscera. Meninges Pleural membranes (serous)


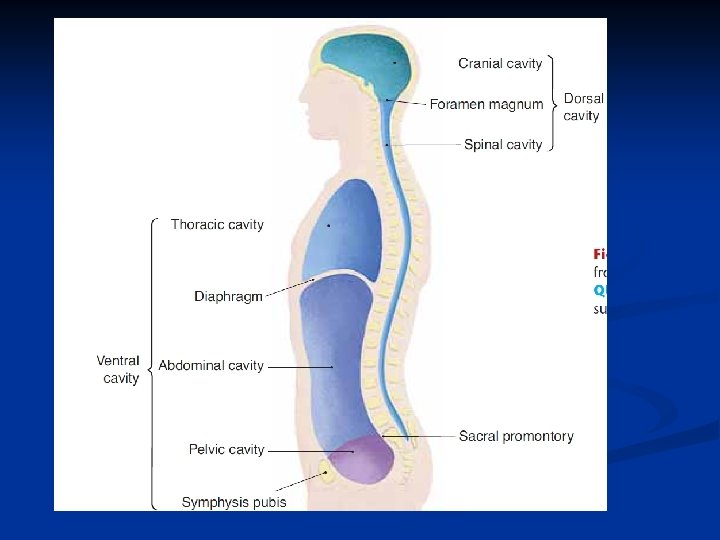
a. The dorsal cavity can be divided into the cranial cavity and vertebral canal. b. The ventral cavity is made up of a thoracic cavity and an abdominopelvic cavity, separated by the diaphragm.

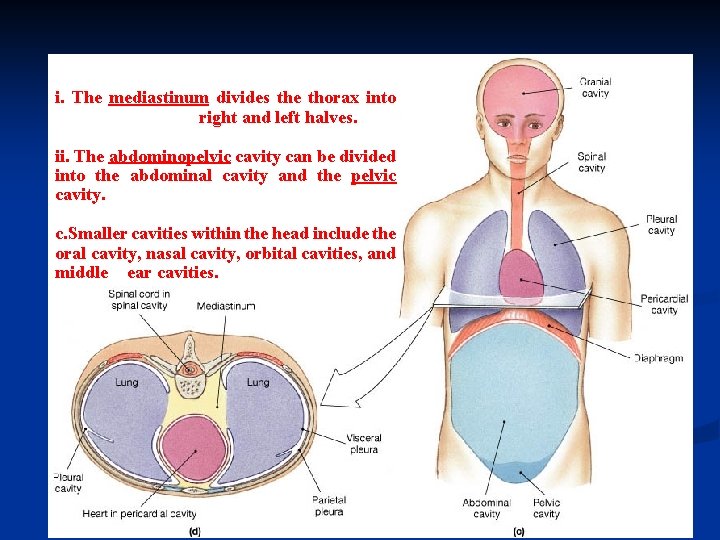
i. The mediastinum divides the thorax into right and left halves. ii. The abdominopelvic cavity can be divided into the abdominal cavity and the pelvic cavity. c. Smaller cavities within the head include the oral cavity, nasal cavity, orbital cavities, and middle ear cavities.



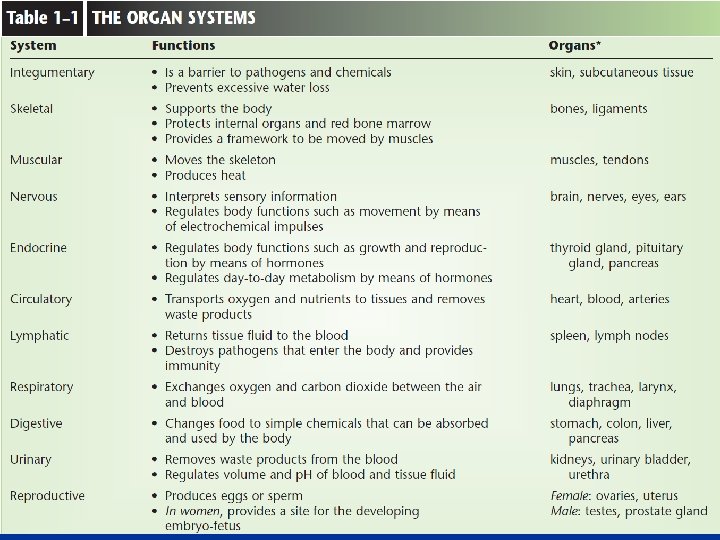
Organ Systems: n Body Covering a. The integumentary system, including skin, hair, nails, and various glands, covers the body, senses changes outside the body, and helps regulate body temperature. n

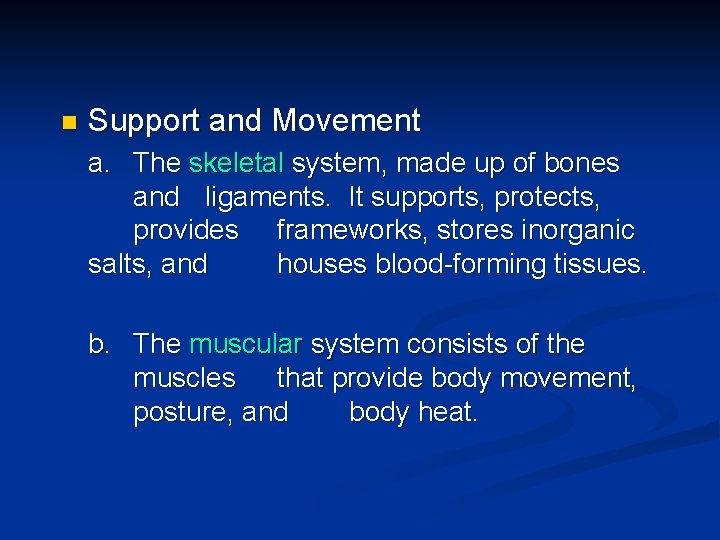
n Support and Movement a. The skeletal system, made up of bones and ligaments. It supports, protects, provides frameworks, stores inorganic salts, and houses blood-forming tissues. b. The muscular system consists of the muscles that provide body movement, posture, and body heat.

n Integration and Coordination a. The nervous system consists of the brain, spinal cord, nerves, and sense organs. It integrates information incoming information from receptors and sends impulses to muscles and glands. b. The endocrine system, including all of the glands that secrete hormones, helps to integrate metabolic functions

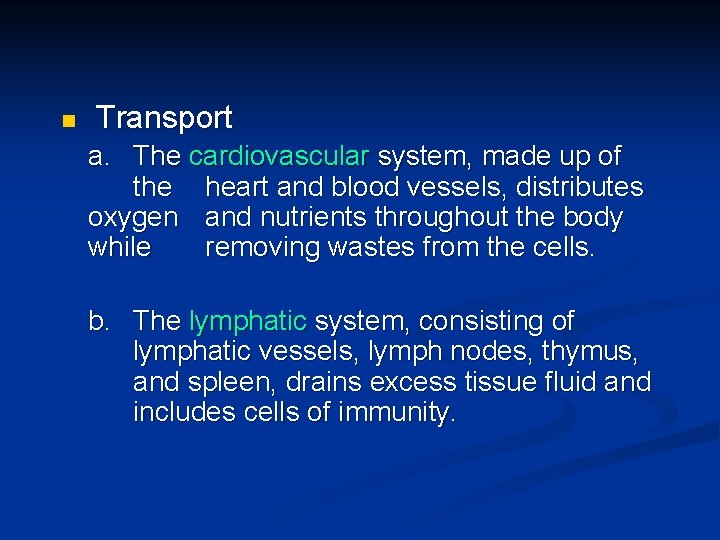
n Transport a. The cardiovascular system, made up of the heart and blood vessels, distributes oxygen and nutrients throughout the body while removing wastes from the cells. b. The lymphatic system, consisting of lymphatic vessels, lymph nodes, thymus, and spleen, drains excess tissue fluid and includes cells of immunity.

n n Absorption and Excretion a. The digestive system is made up of the mouth, esophagus, stomach, intestines and accessory organs. It receives, breaks down, and absorbs nutrients. b. The respiratory system exchanges gases between the blood and air and is made up of the lungs and passageways. c. The urinary system, consisting of the kidneys, ureters, bladder, and urethra, removes wastes from the blood and helps to maintain water and electrolyte balance.

n Reproduction a. The reproductive system produces new organisms. i. The male reproductive system consists of the testes, accessory organs, and vessels that conduct sperm to the penis. ii. The female reproductive system consists of ovaries, uterine tubes, uterus, vagina, and external genitalia. The female reproductive system also houses the developing offspring.

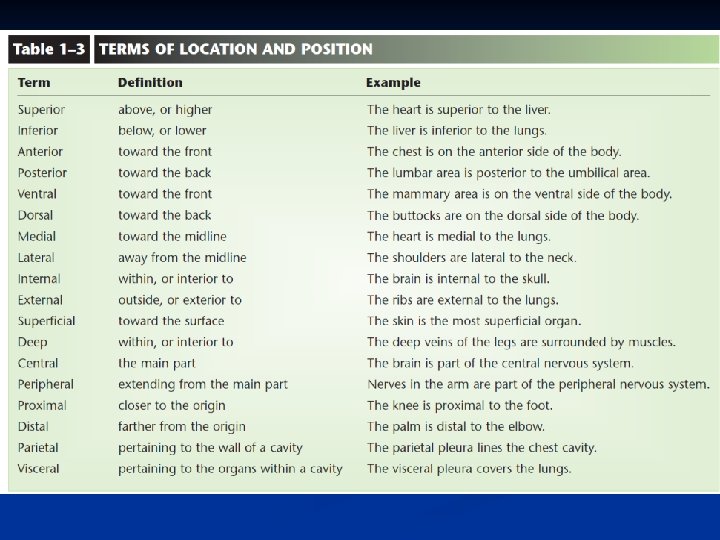
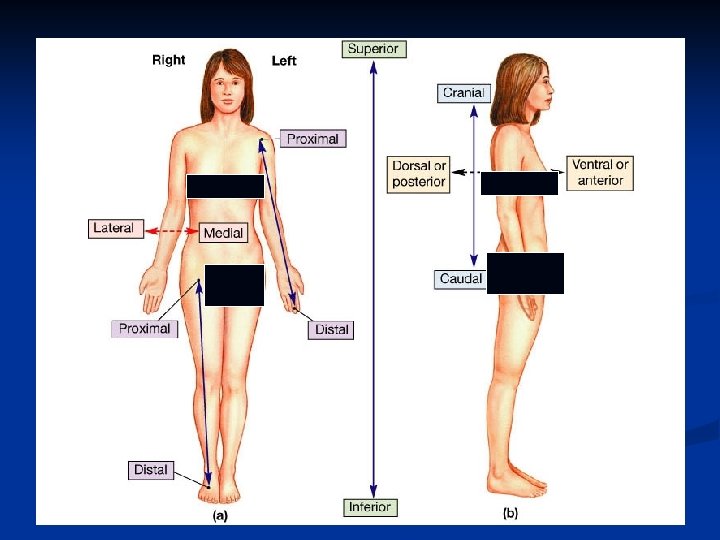
n n Anatomical Terminology Relative Positions: 1. Terms of relative position are used to describe the location of a part relative to another part. 2. Terms of relative position include: superior, inferior, anterior, posterior, medial, lateral, proximal, distal, superficial (peripheral), and deep.


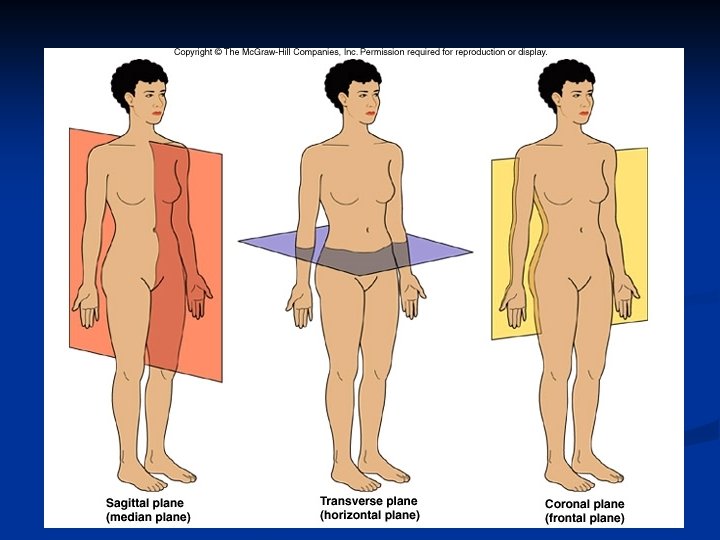
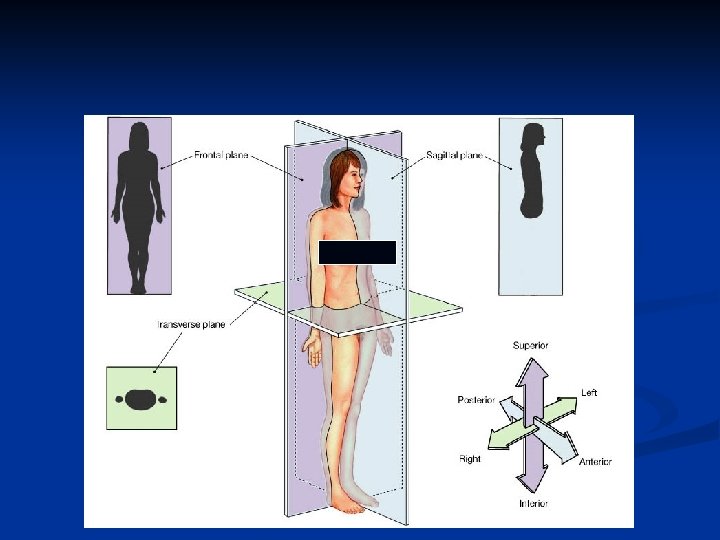
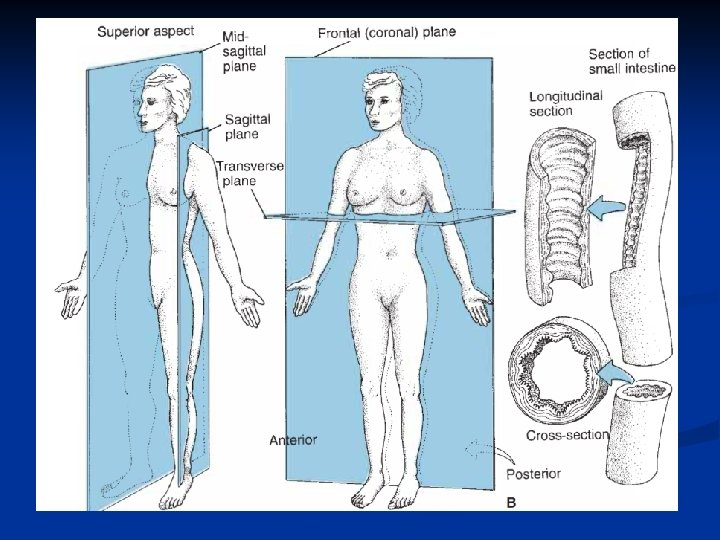
n Planes and Sections: 1. A sagittal section divides the body into right and left portions. 2. A transverse section divides the body into superior and inferior portions. It is often called a “cross section”. 3. A coronal section divides the body into anterior and posterior sections.





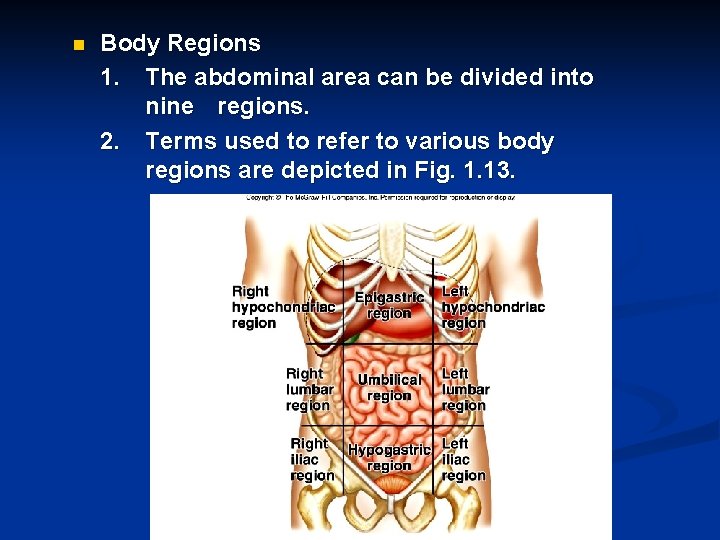
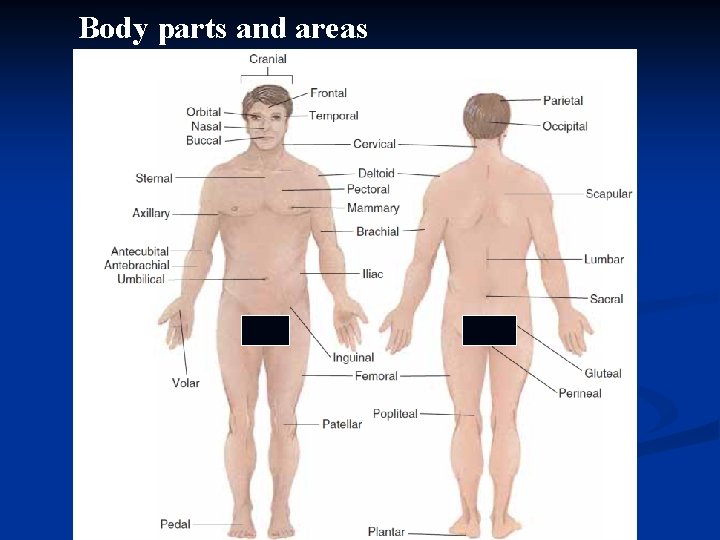
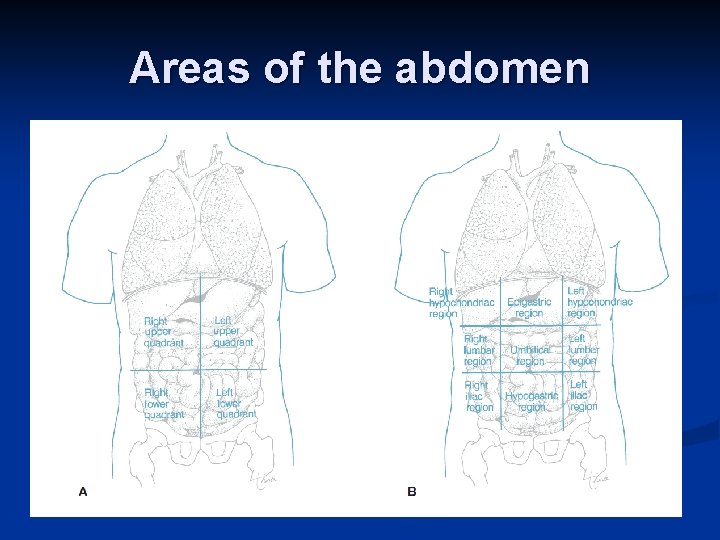
n Body Regions 1. The abdominal area can be divided into nine regions. 2. Terms used to refer to various body regions are depicted in Fig. 1. 13.

Body parts and areas

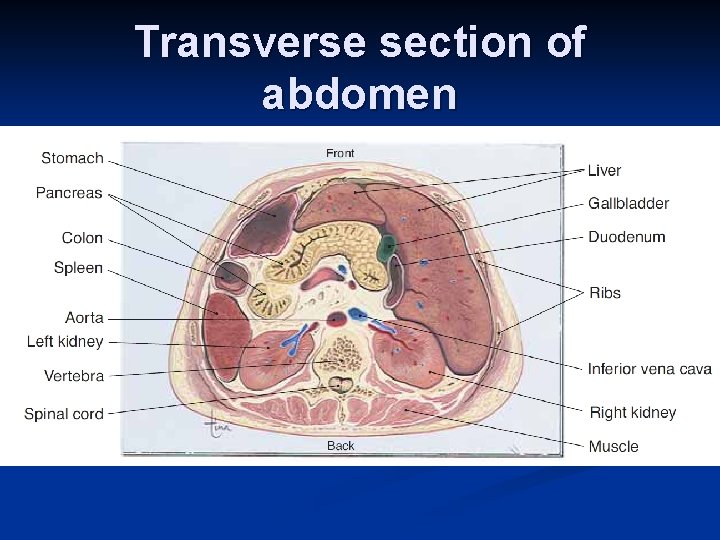
Transverse section of abdomen

Areas of the abdomen


Chapter 2 Chemical Basis of Life

X Introduction: A. Chemistry deals with the composition of substances and how they change. B. A knowledge of chemistry is necessary for the understanding of physiology because of the importance of chemicals in body processes.

Structure of Matter: A. Elements and Atoms: 1. Matter is anything that takes up space. 2. All matter is composed of elements, 92 of which occur naturally. 3. Living organisms require about 20 elements, of which oxygen, carbon, hydrogen, and nitrogen are most abundant. 4. Elements are composed of atoms; atoms of different elements vary in size and in how they interact.

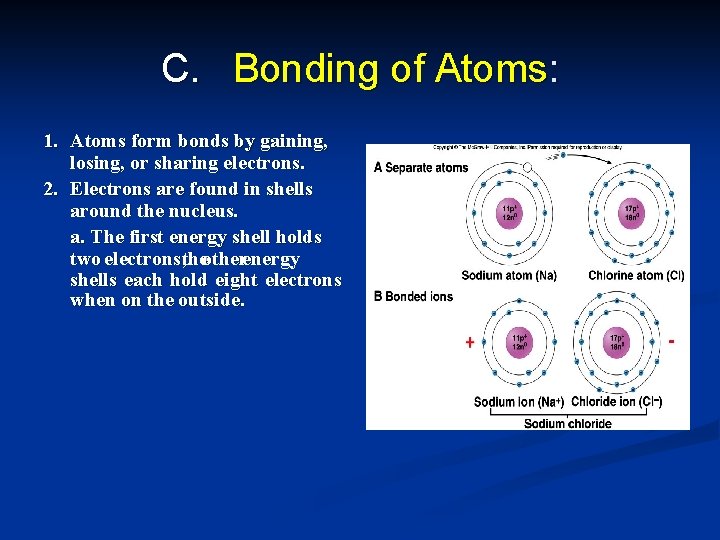
C. Bonding of Atoms: 1. Atoms form bonds by gaining, losing, or sharing electrons. 2. Electrons are found in shells around the nucleus. a. The first energy shell holds two electrons; theotherenergy shells each hold eight electrons when on the outside.

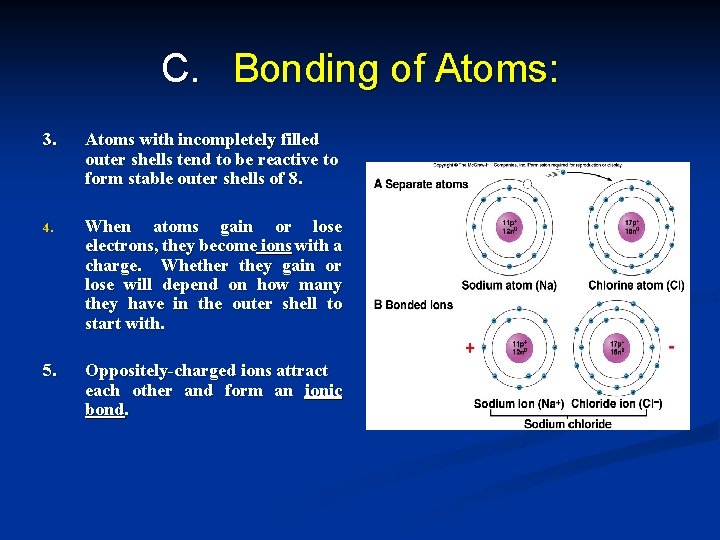
C. Bonding of Atoms: 3. Atoms with incompletely filled outer shells tend to be reactive to form stable outer shells of 8. 4. When atoms gain or lose electrons, they become ions with a charge. Whether they gain or lose will depend on how many they have in the outer shell to start with. 5. Oppositely-charged ions attract each other and form an ionic bond.

E. Formulas: 1. A molecular formula represents the numbers and types of atoms in a molecule. 2. Various representations, called structural formulas, can be used to illustrate molecules.

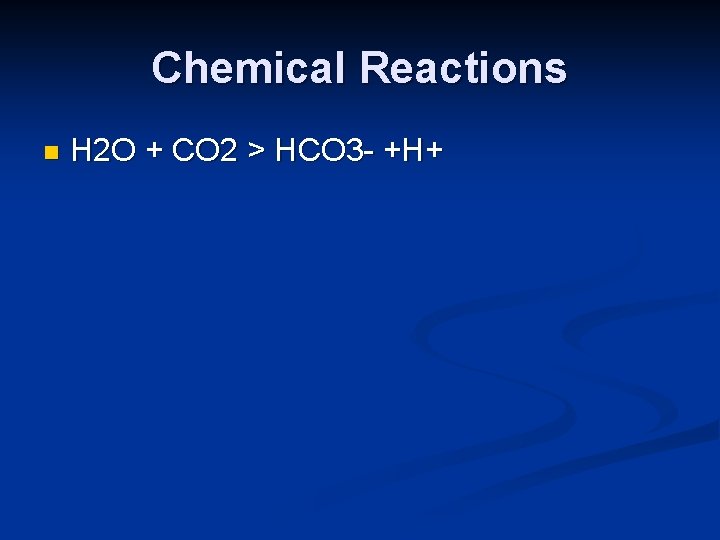
Chemical Reactions n H 2 O + CO 2 > HCO 3 - +H+

G. Acids and Bases: 1. Substances that release ions in water are called electrolytes. 2. Electrolytes that release hydrogen ions in water are called acids. 3. Electrolytes that release ions that combine with hydrogen ions in water are called bases.

G. Acids and Bases: 4. The concentrations of H+ & OHin the body is very important to physiology. 5. p. H represents the concentration of hydrogen ions [H+] in solution.

Chemical Constituents of Cells: A. Compounds that contain both hydrogen and carbon are called organic, the others are inorganic

1. Water B. Inorganic Substances a. Water is the most abundant compound in living things and makes up two-thirds of the weight of adults. b. Water is an important solvent so most metabolic reactions occur in water.

B. Inorganic Substances 2. Oxygen a. Oxygen is needed to release energy from nutrients and is used to drive the cell's metabolism. 3. Carbon Dioxide a. Carbon dioxide is released as a waste product during energy-releasing metabolic reactions.

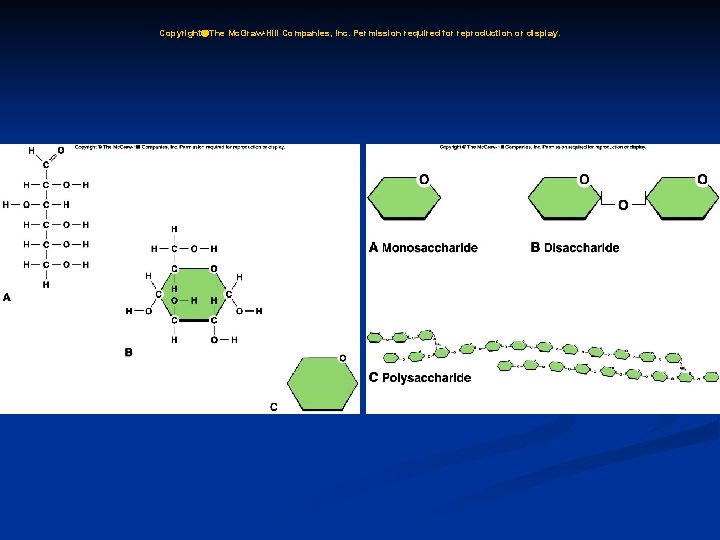
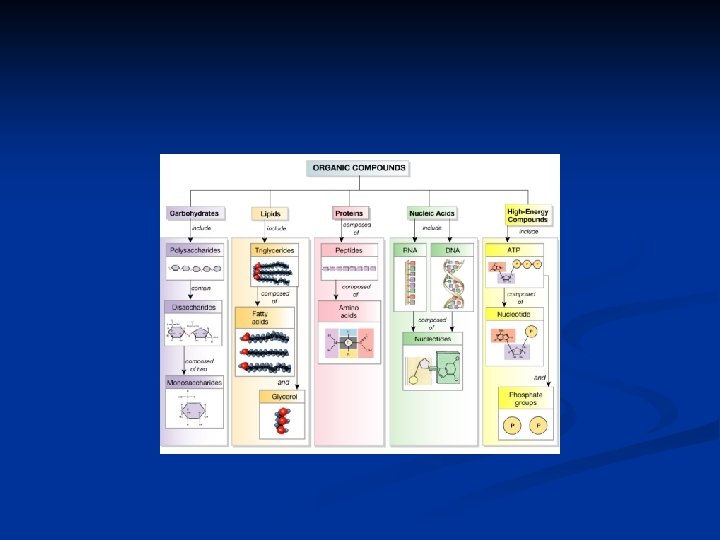
C. Organic Substances: 1. Carbohydrates a. Carbohydrates provide energy for cellular activities and are composed of carbon, hydrogen, and oxygen. b. Carbohydrates are made from monosaccharides (simple sugars); disaccharides are two monosaccharides joined together; complex carbohydrates (polysaccharides), such as starch, are built of many sugars.

Copyright The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display.

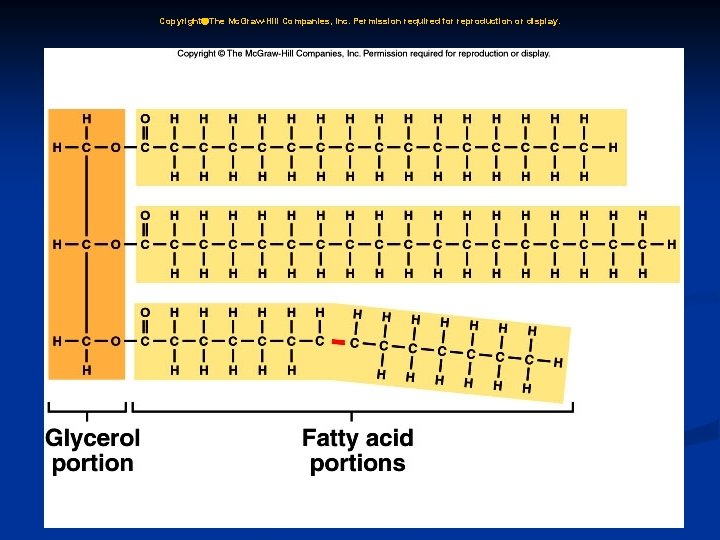
2. Lipids: a. Lipids are insoluble in water and include fats, phospholipids, and steroids. b. Fats supply energy, are composed of oxygen, carbon, and hydrogen, and are built from glycerol and three fatty acids.

2. Lipids: c. Phospholipids contain glycerol, two fatty acids, and a phosphate group, and are important in cell structures. d. Steroids are complex ring structures, and include cholesterol, which is used to synthesize the sex hormones.

Copyright The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display.

3. Proteins: a. Proteins have a great variety of functions in the body---as structural materials, as energy sources, as certain hormones, as receptors on cell membranes, as antibodies, and as enzymes to catalyze metabolic reactions.

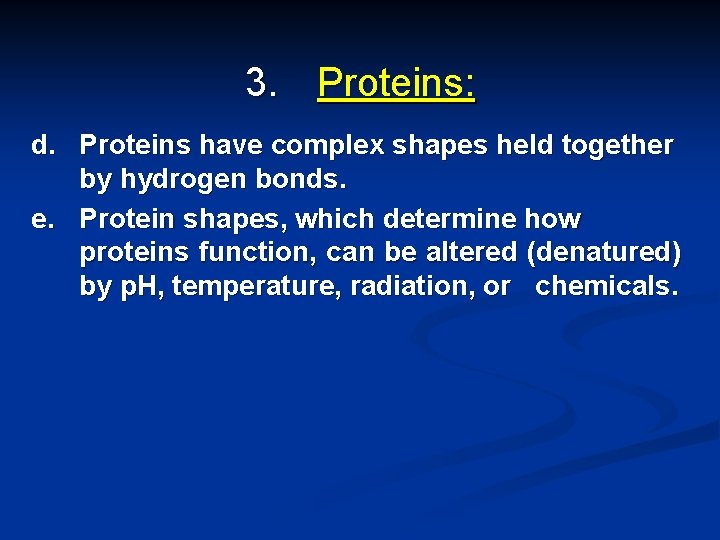
3. Proteins: b. Proteins contain C, O, H, and nitrogen atoms; some also contain sulfur. c. Building blocks of proteins are the amino acids, each of which has a carboxyl group, an amino group and a side chain called the R group.

3. Proteins: d. Proteins have complex shapes held together by hydrogen bonds. e. Protein shapes, which determine how proteins function, can be altered (denatured) by p. H, temperature, radiation, or chemicals.

Copyright The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display.

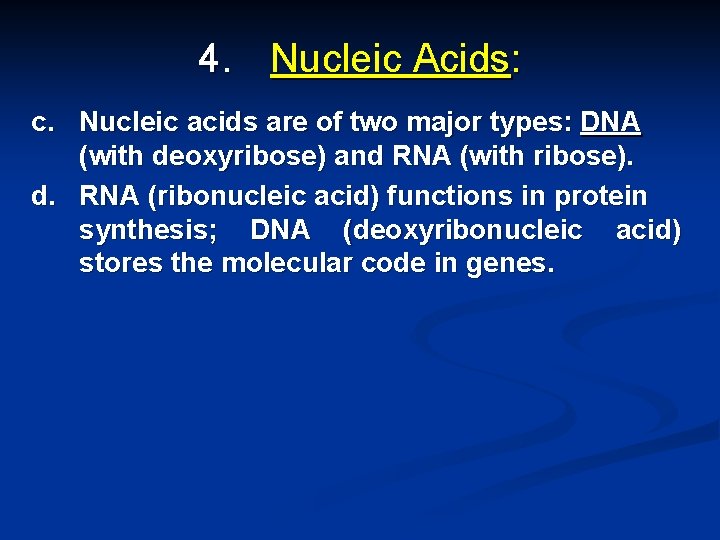
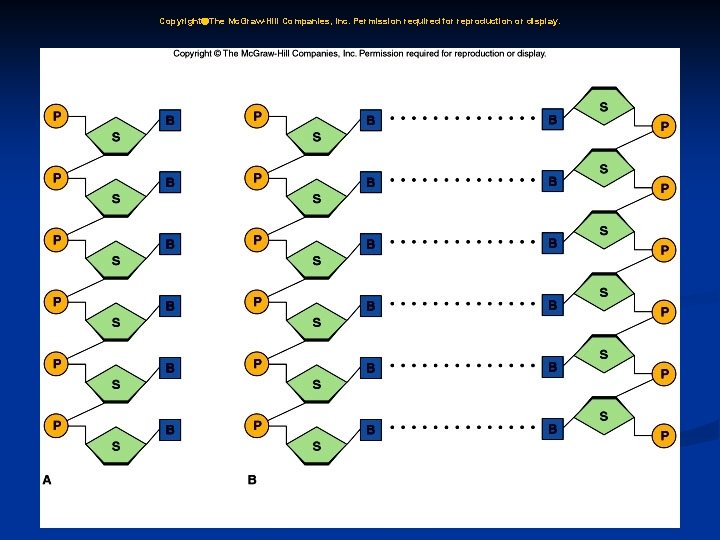
4. Nucleic Acids: a. Nucleic acids form genes and take part protein synthesis. b. They contain carbon, hydrogen, oxygen, nitrogen, and phosphorus, which are bound into building blocks called nucleotides.

4. Nucleic Acids: c. Nucleic acids are of two major types: DNA (with deoxyribose) and RNA (with ribose). d. RNA (ribonucleic acid) functions in protein synthesis; DNA (deoxyribonucleic acid) stores the molecular code in genes.

Copyright The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display.


Chapter 3 Cells