Lec 17 Carnot principles entropy 1 For next







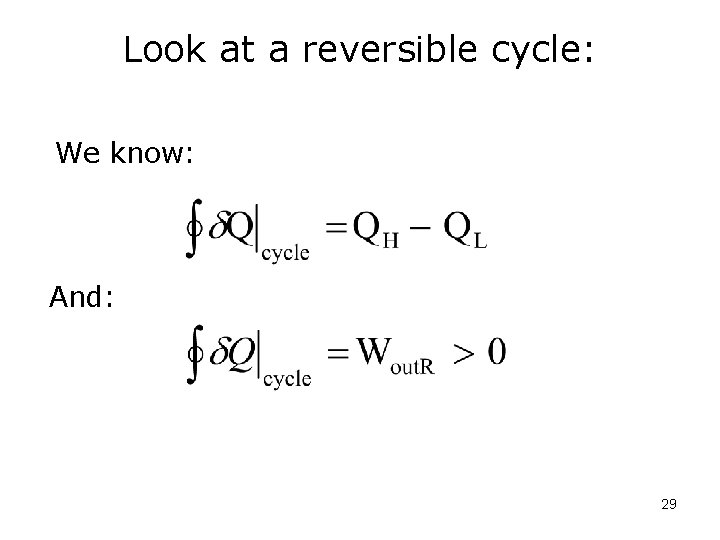
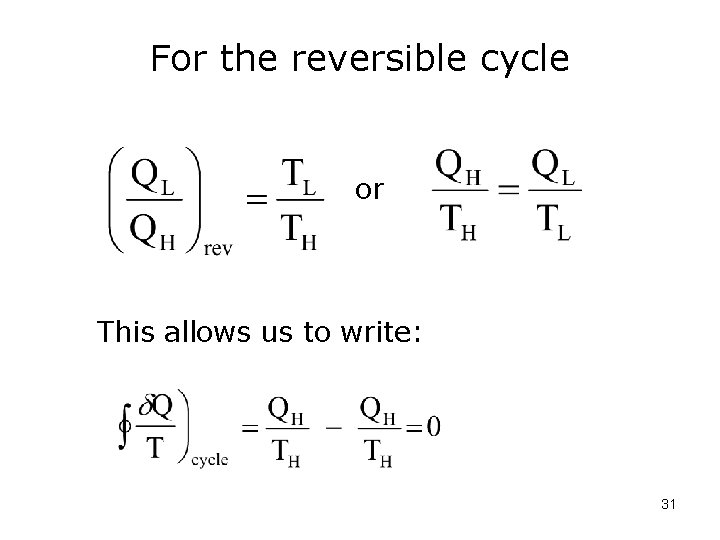

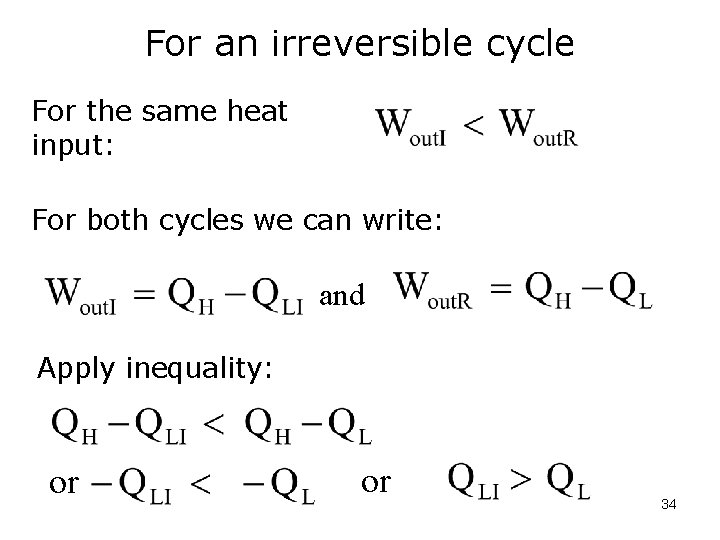
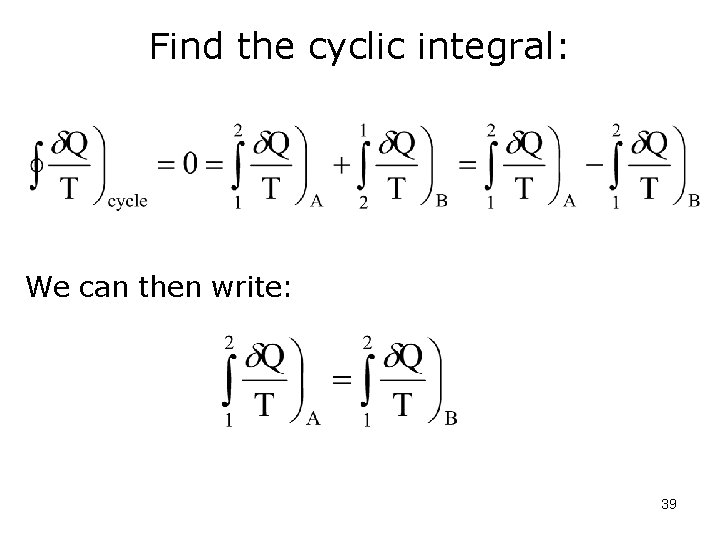
- Slides: 41

Lec 17: Carnot principles, entropy 1

• For next time: – Read: § 6 -9 to 6 -14 and 7 -1 – HW 9 due October 29, 2003 • Outline: – Carnot’s corollaries – Kelvin temperature scale – Clausius inequality and definition of entropy • Important points: – Do not forget the first law of thermodynamics and the conservation of mass – we still need these to solve problems – Kelvin temperature scale helps us find maximum efficiencies for power cycles – Understand how entropy is defined as a system property 2

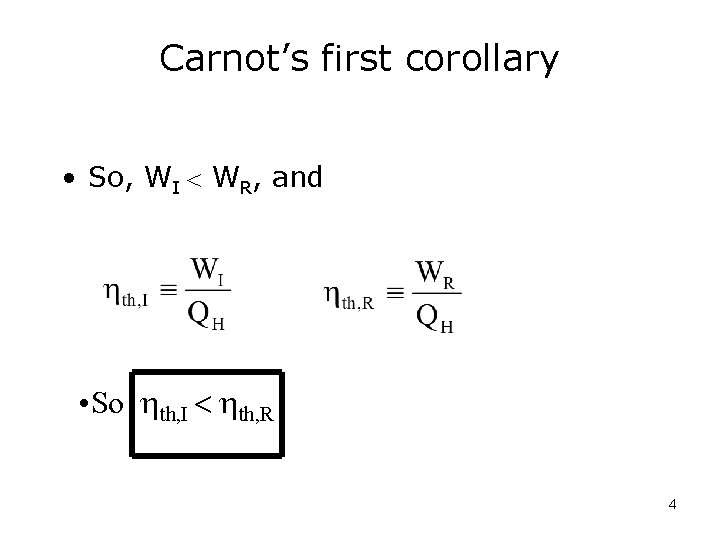
Carnot’s first corollary The thermal efficiency of an irreversible power cycle is always less than thermal efficiency of a reversible power cycle when each operates between the same two reservoirs. 3

Carnot’s first corollary • So, WI WR, and • So th, I th, R 4

Carnot’s second corollary • All reversible power cycles operating between the same two thermal reservoirs have the same thermal efficiencies. 5

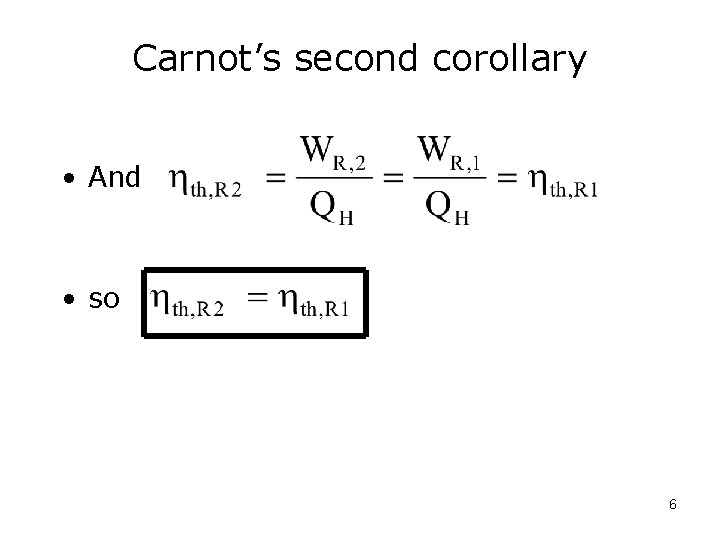
Carnot’s second corollary • And • so 6

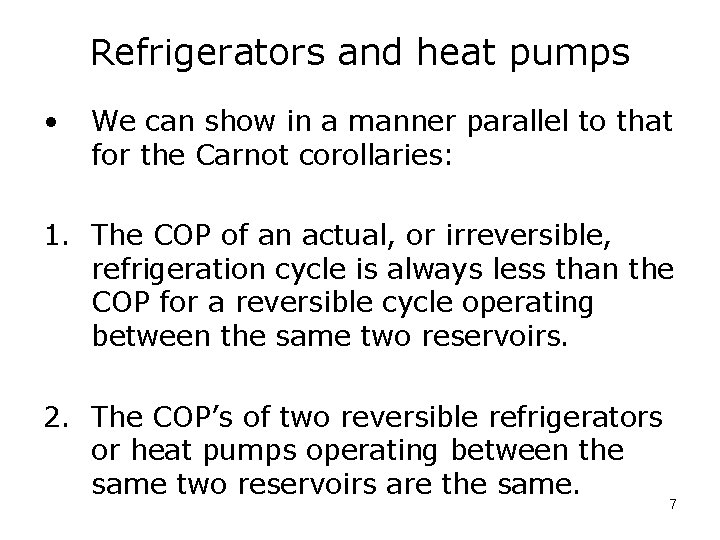
Refrigerators and heat pumps • We can show in a manner parallel to that for the Carnot corollaries: 1. The COP of an actual, or irreversible, refrigeration cycle is always less than the COP for a reversible cycle operating between the same two reservoirs. 2. The COP’s of two reversible refrigerators or heat pumps operating between the same two reservoirs are the same. 7

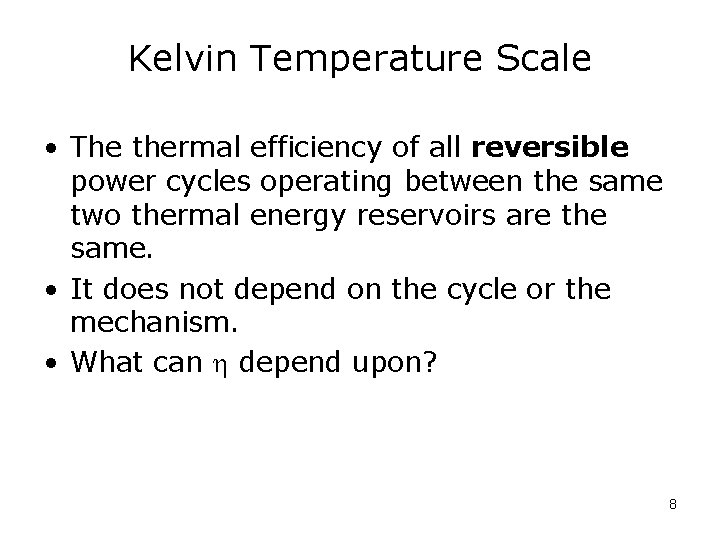
Kelvin Temperature Scale • The thermal efficiency of all reversible power cycles operating between the same two thermal energy reservoirs are the same. • It does not depend on the cycle or the mechanism. • What can depend upon? 8

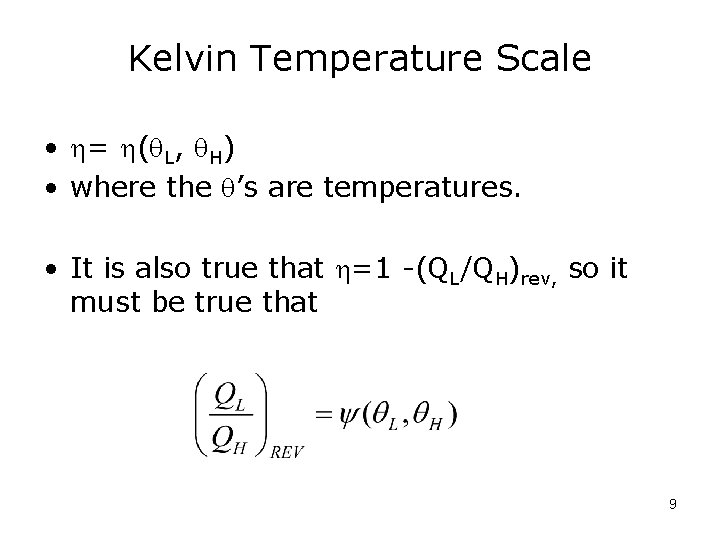
Kelvin Temperature Scale • = ( L, H) • where the ’s are temperatures. • It is also true that =1 -(QL/QH)rev, so it must be true that 9

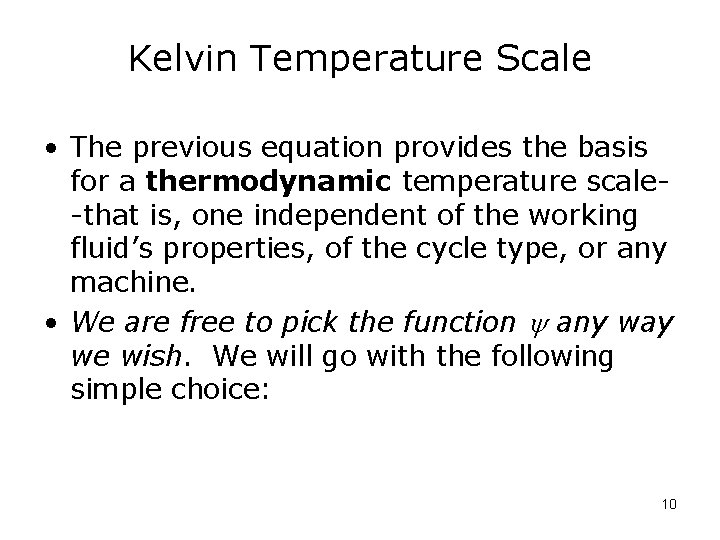
Kelvin Temperature Scale • The previous equation provides the basis for a thermodynamic temperature scale-that is, one independent of the working fluid’s properties, of the cycle type, or any machine. • We are free to pick the function any way we wish. We will go with the following simple choice: 10

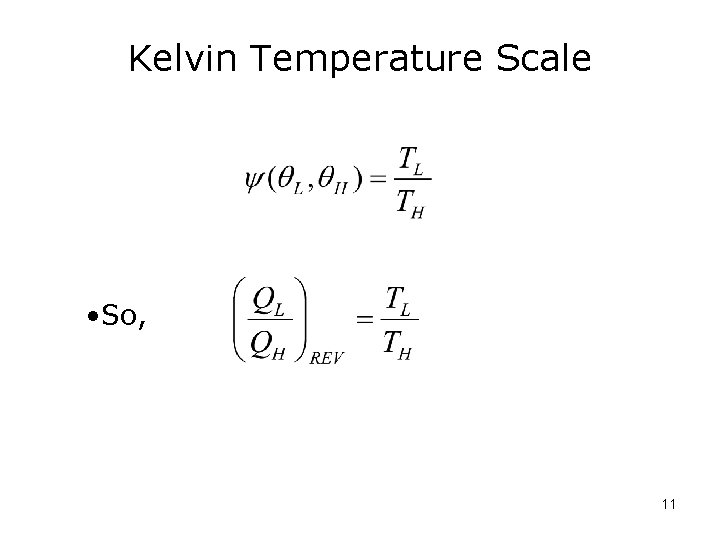
Kelvin Temperature Scale • So, 11

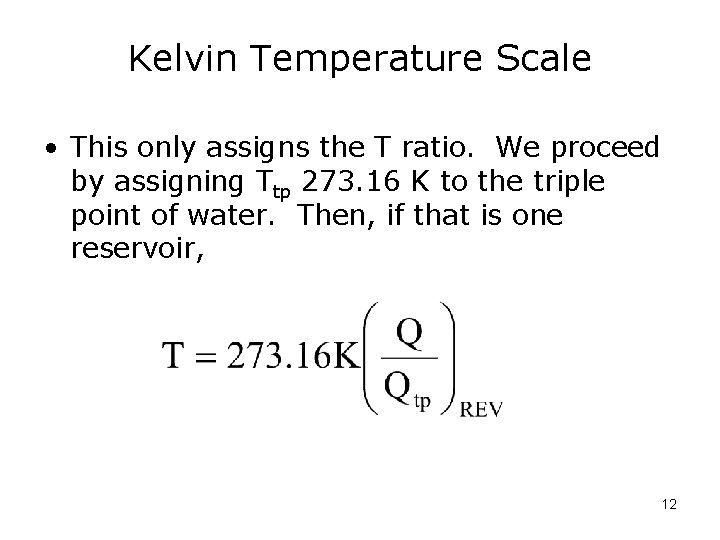
Kelvin Temperature Scale • This only assigns the T ratio. We proceed by assigning Ttp 273. 16 K to the triple point of water. Then, if that is one reservoir, 12

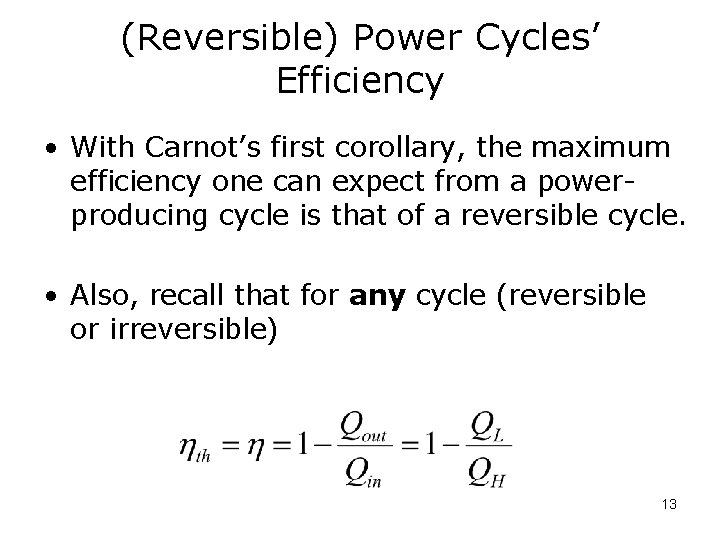
(Reversible) Power Cycles’ Efficiency • With Carnot’s first corollary, the maximum efficiency one can expect from a powerproducing cycle is that of a reversible cycle. • Also, recall that for any cycle (reversible or irreversible) 13

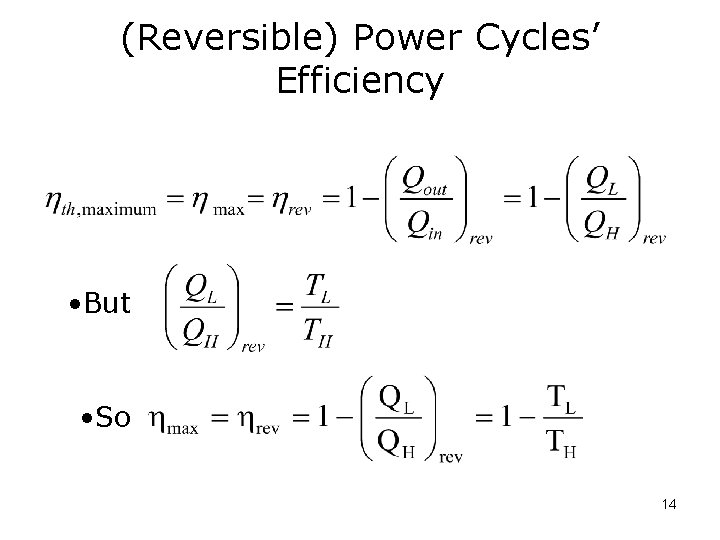
(Reversible) Power Cycles’ Efficiency • But • So 14

TEAMPLAY • Many power cycles for electricity supply operate between a steam supply reservoir of about 1, 000 °F and a heat rejection reservoir of about 70 °F. • What is the maximum thermal efficiency you can expect from such a system? 15

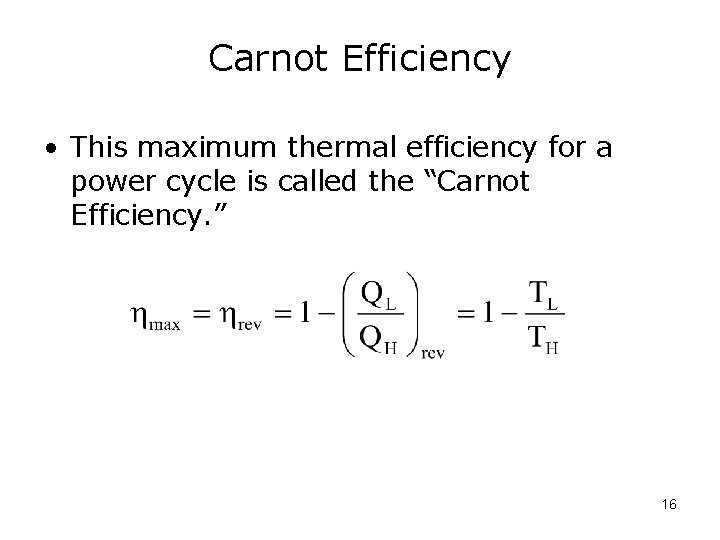
Carnot Efficiency • This maximum thermal efficiency for a power cycle is called the “Carnot Efficiency. ” 16

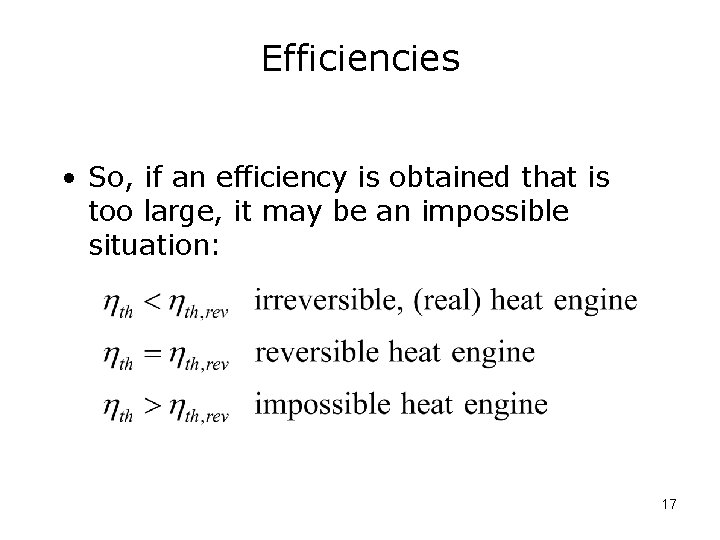
Efficiencies • So, if an efficiency is obtained that is too large, it may be an impossible situation: 17

TEAMPLAY Problem 6 -90 E 18

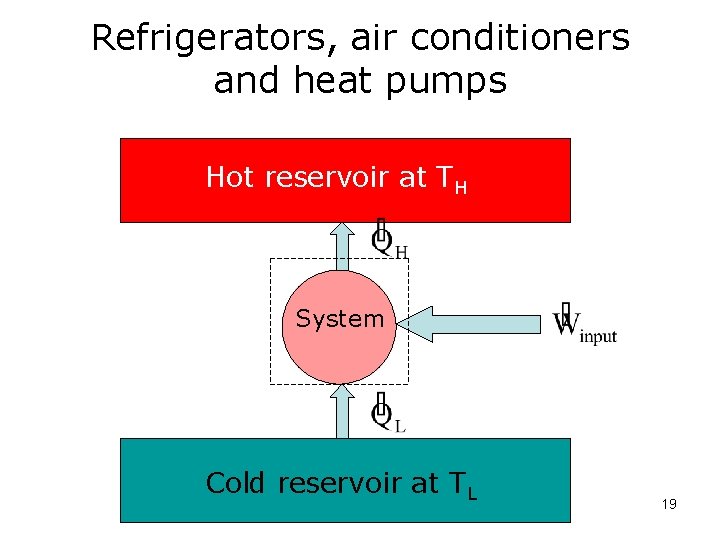
Refrigerators, air conditioners and heat pumps Hot reservoir at TH System Cold reservoir at TL 19

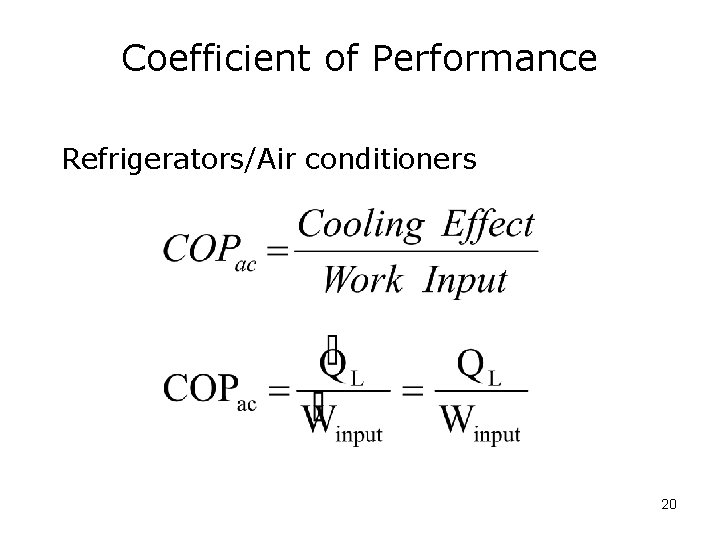
Coefficient of Performance Refrigerators/Air conditioners 20

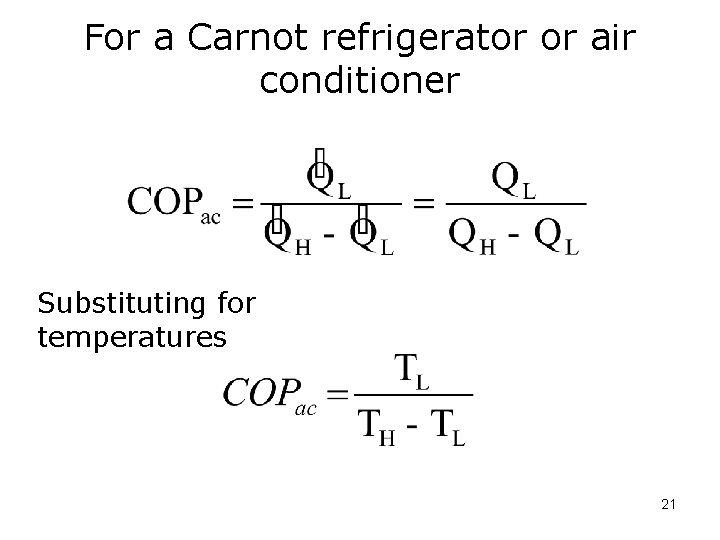
For a Carnot refrigerator or air conditioner Substituting for temperatures 21

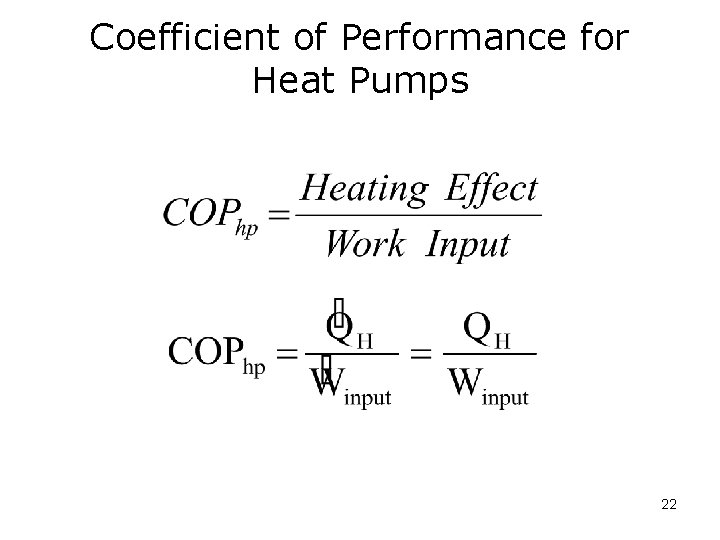
Coefficient of Performance for Heat Pumps 22

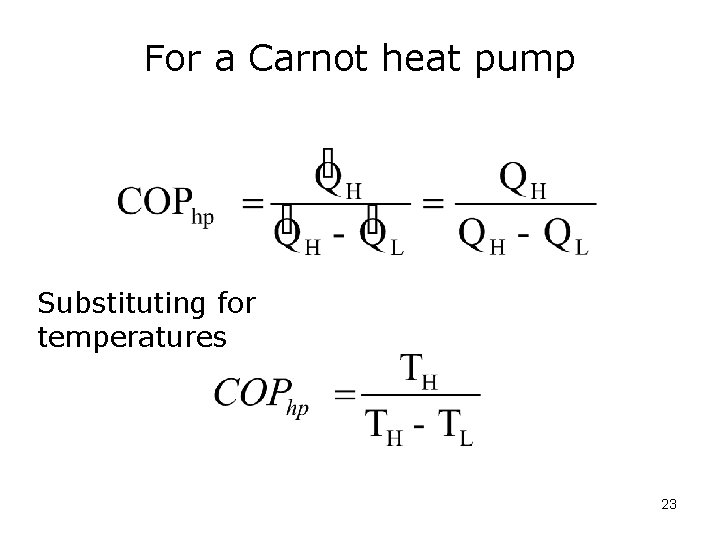
For a Carnot heat pump Substituting for temperatures 23

TEAMPLAY Problem 6 -104 24

This is going to seem pretty abstract. . so hang on for the ride! 25

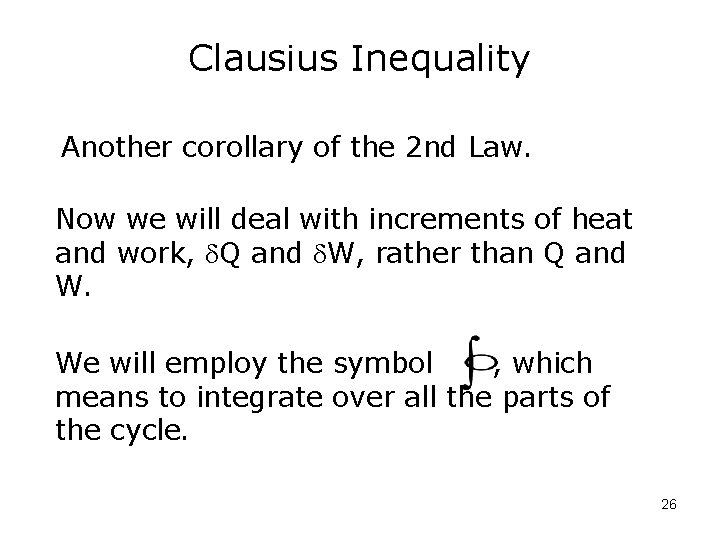
Clausius Inequality Another corollary of the 2 nd Law. Now we will deal with increments of heat and work, Q and W, rather than Q and W. We will employ the symbol , which means to integrate over all the parts of the cycle. 26

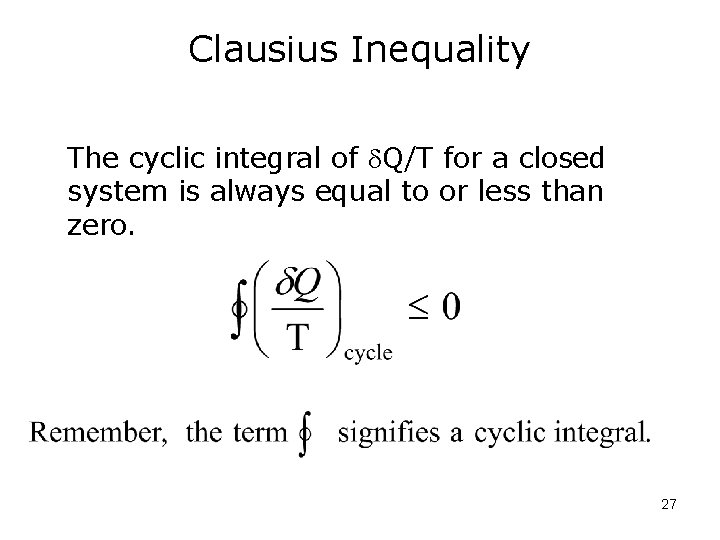
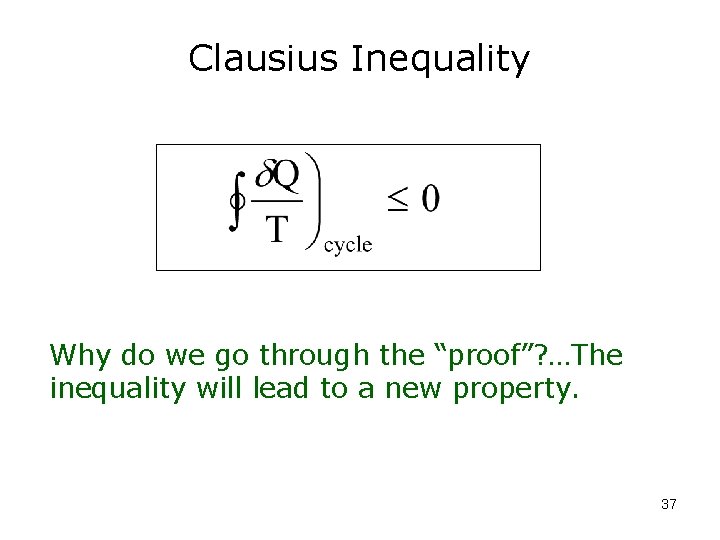
Clausius Inequality The cyclic integral of Q/T for a closed system is always equal to or less than zero. 27

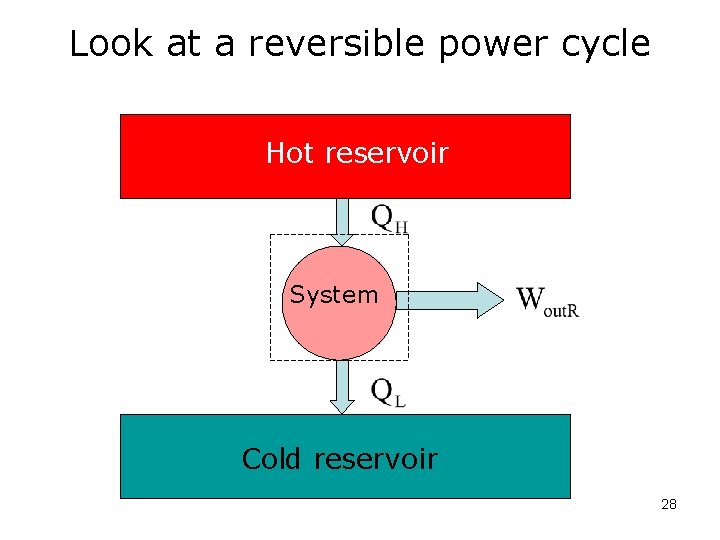
Look at a reversible power cycle Hot reservoir System Cold reservoir 28
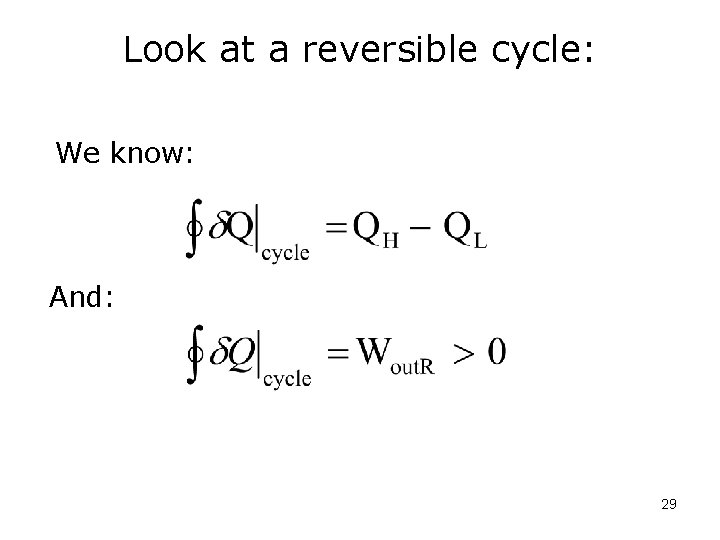
Look at a reversible cycle: We know: And: 29

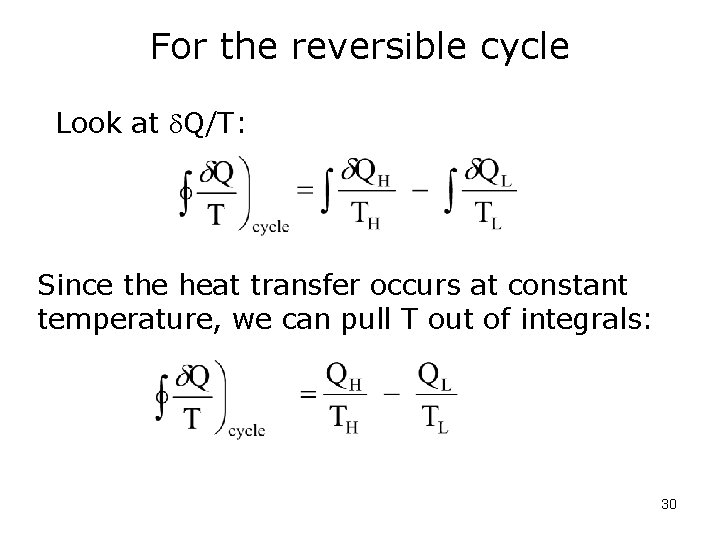
For the reversible cycle Look at Q/T: Since the heat transfer occurs at constant temperature, we can pull T out of integrals: 30
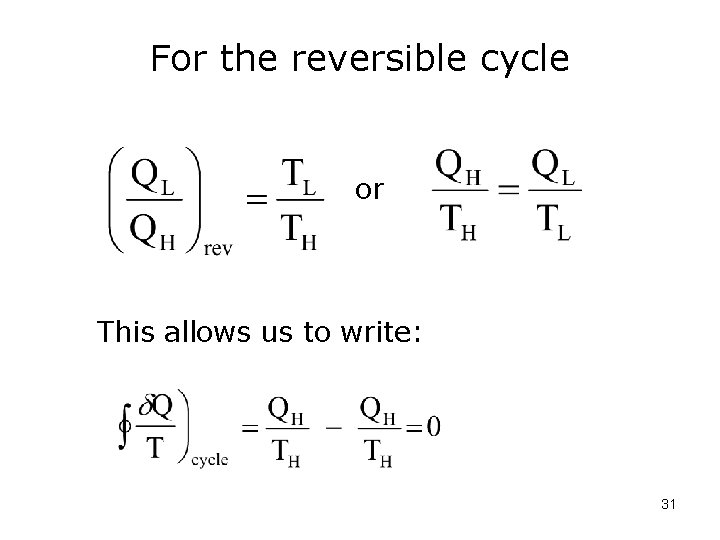
For the reversible cycle or This allows us to write: 31

What were signs of irreversibilities? • • Friction unrestrained expansion mixing heat transfer across a temperature difference • inelastic deformation 32

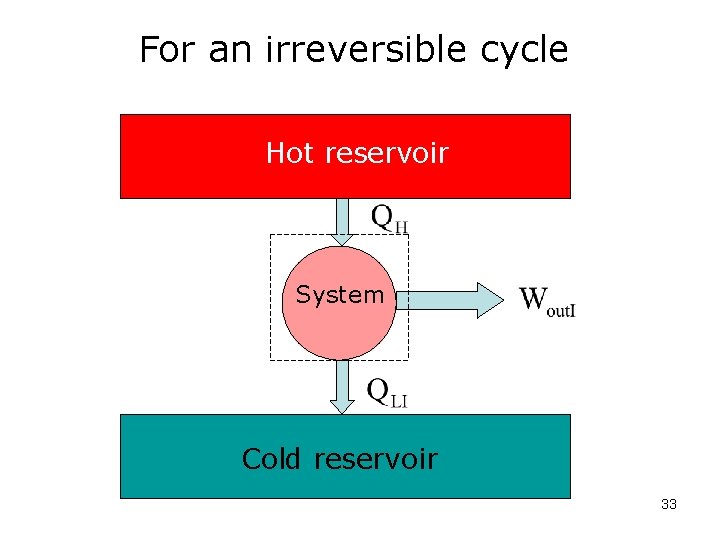
For an irreversible cycle Hot reservoir System Cold reservoir 33
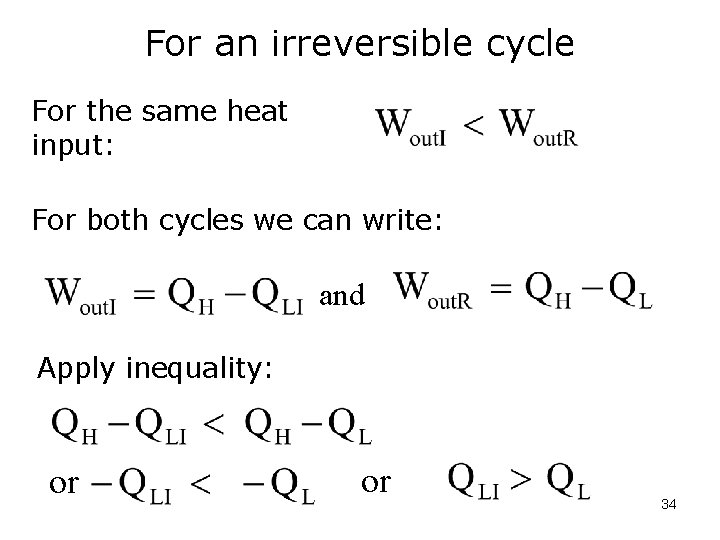
For an irreversible cycle For the same heat input: For both cycles we can write: and Apply inequality: or or 34

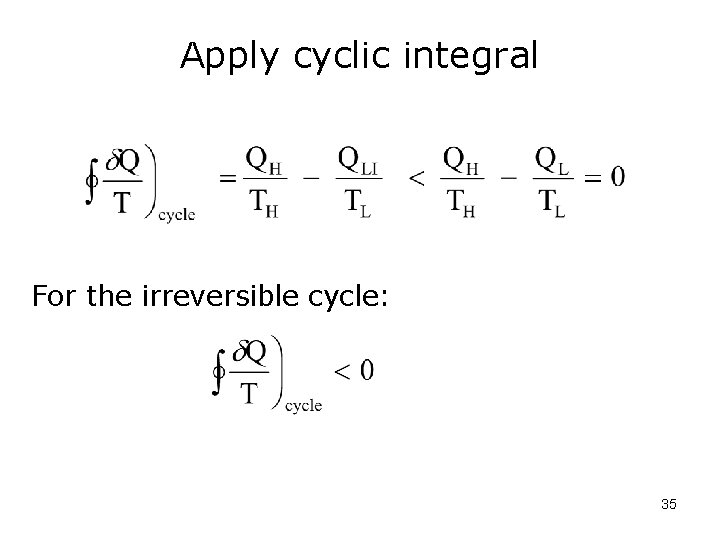
Apply cyclic integral For the irreversible cycle: 35

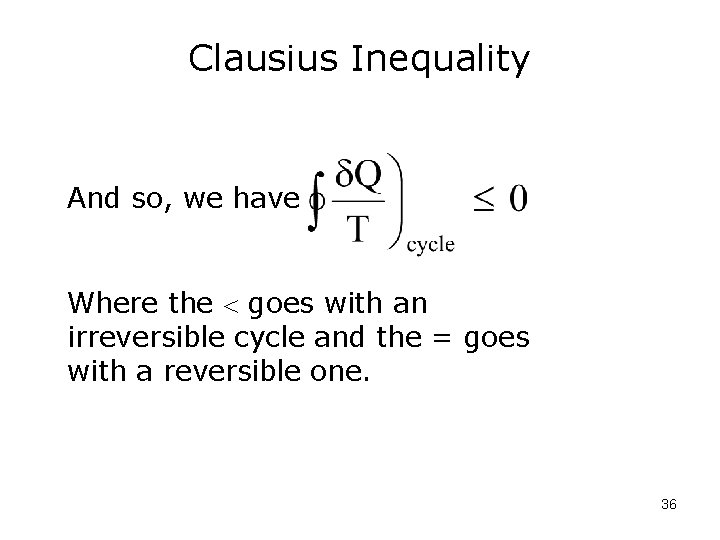
Clausius Inequality And so, we have Where the goes with an irreversible cycle and the = goes with a reversible one. 36

Clausius Inequality Why do we go through the “proof”? …The inequality will lead to a new property. 37

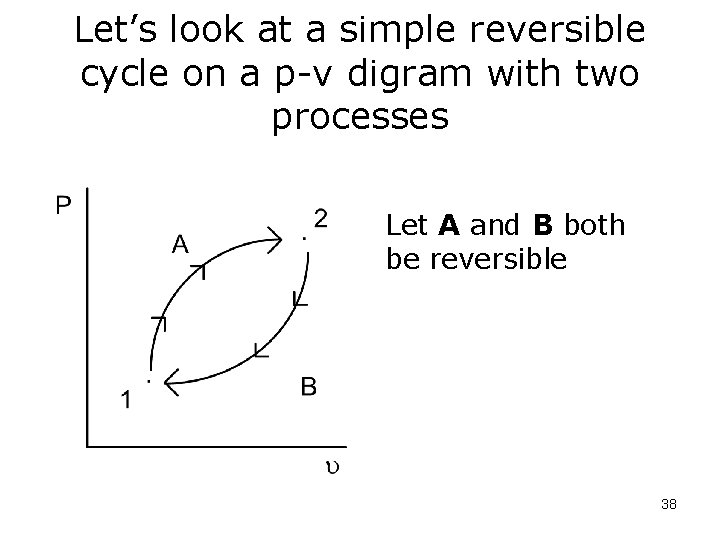
Let’s look at a simple reversible cycle on a p-v digram with two processes Let A and B both be reversible 38
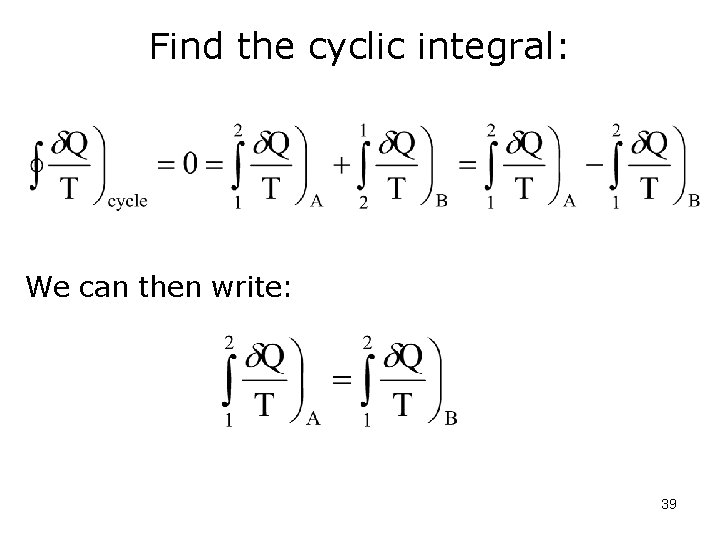
Find the cyclic integral: We can then write: 39

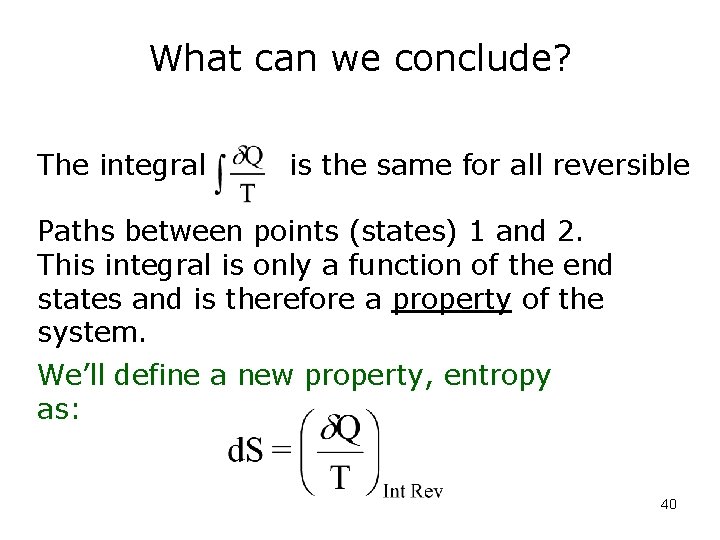
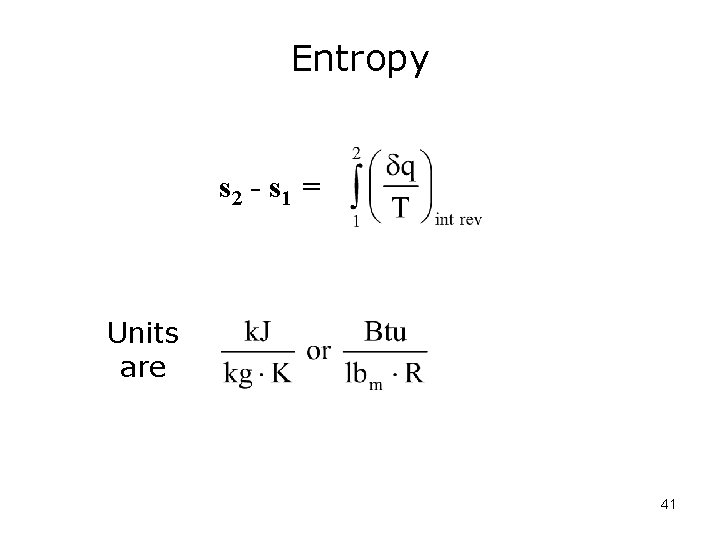
What can we conclude? The integral is the same for all reversible Paths between points (states) 1 and 2. This integral is only a function of the end states and is therefore a property of the system. We’ll define a new property, entropy as: 40

Entropy s 2 - s 1 = Units are 41