Learning Outcomes I can explain what reaction rate






- Slides: 16

Learning Outcomes • I can explain what ‘reaction rate’ means and how to measure the rate of reaction • I can explain how reactions happen due to collisions between particles. • I can explain how surface area, concentration and temperature affect the reaction rate using collision theory.


Rate of Reaction the speed at which a chemical reaction occurs. Some reactions are quick Some are not The rate of a reaction can be altered

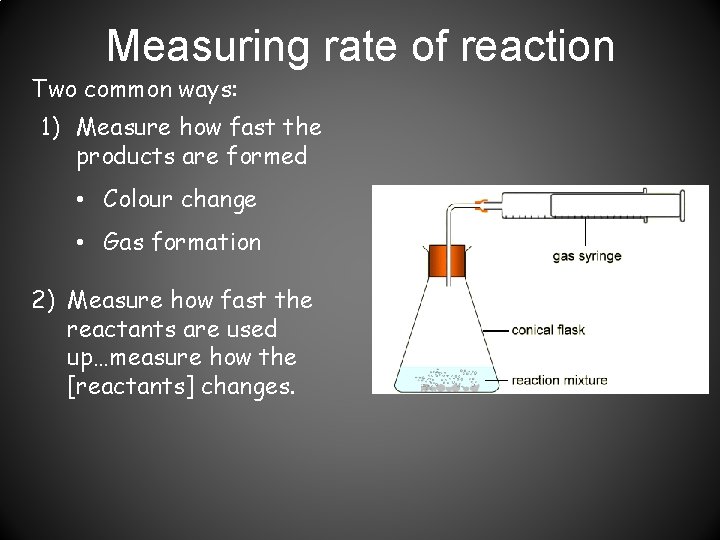
Measuring rate of reaction Two common ways: 1) Measure how fast the products are formed • Colour change • Gas formation 2) Measure how fast the reactants are used up…measure how the [reactants] changes.

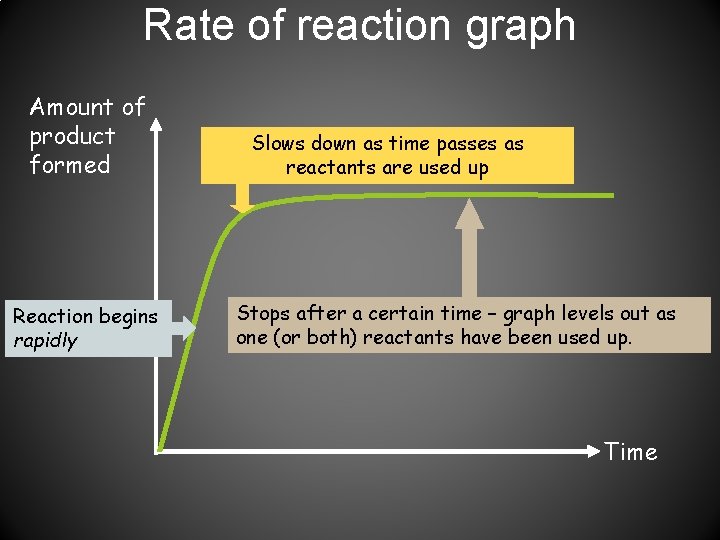
Rate of reaction graph Amount of product formed Reaction begins rapidly Slows down as time passes as reactants are used up Stops after a certain time – graph levels out as one (or both) reactants have been used up. Time

Collision Theory “For a reaction between two particles to occur, the particles must collide and the collision must be effective”

Effective Collisions • When the particles collide with enough kinetic energy and the correct orientation to break the bonds between particles so new bonds can form. Collisions can occur and yet result in no reaction if there is insufficient energy or incorrect orientation.

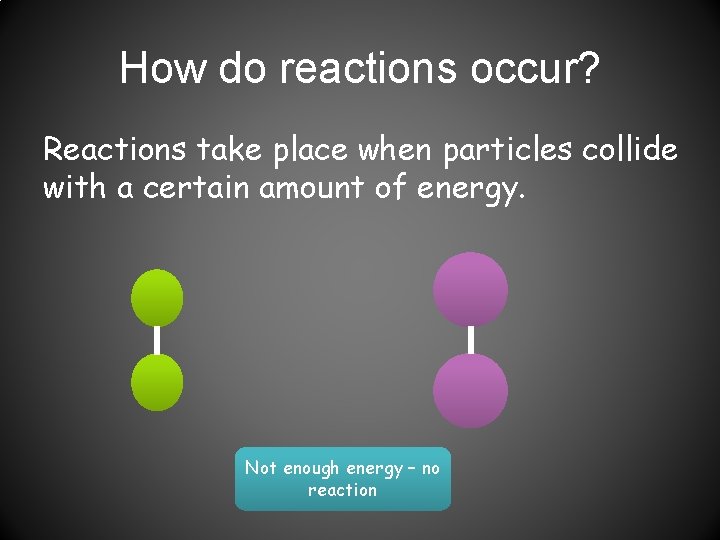
How do reactions occur? Reactions take place when particles collide with a certain amount of energy. Not enough energy – no reaction

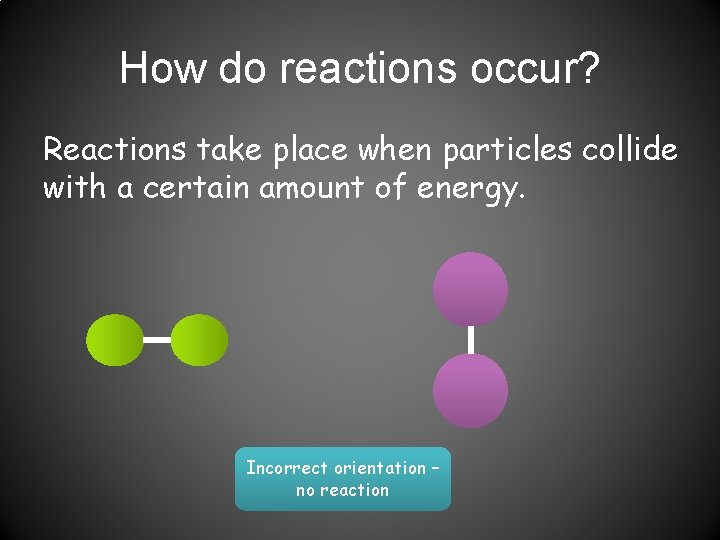
How do reactions occur? Reactions take place when particles collide with a certain amount of energy. Incorrect orientation – no reaction

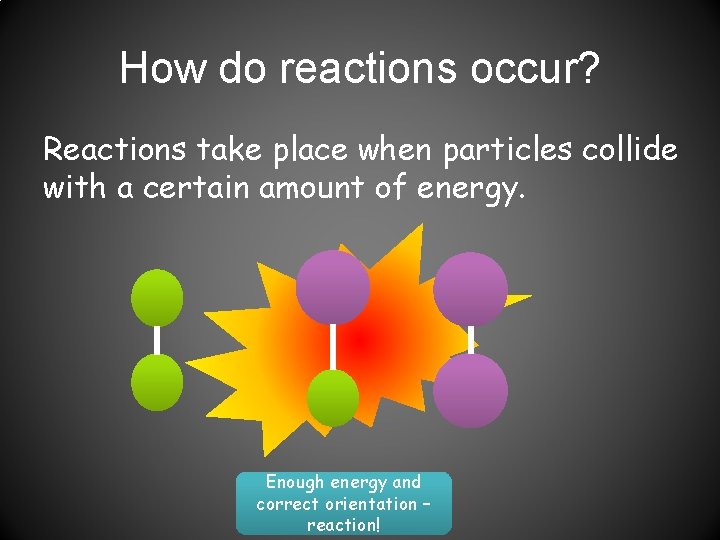
How do reactions occur? Reactions take place when particles collide with a certain amount of energy. Enough energy and correct orientation – reaction!

Activation Energy • The minimum amount of energy needed for the particles to react is called the activation energy, and is different for each reaction. • If particles collide with less energy than the activation energy, they will not react.

Collisions and Rate How to speed up chemical reactions (and get a date). • The more frequently effective collisions occur, the faster the rate of reaction. To change the rate of reaction we can: • • P (increase pressure, increase rate of reaction) T (increase temperature, increase rate of rxn. ) SA (increase surface area, increase rate or rxn) C (increase concentration of reactants, increase rate or rxn) • C (increase catalyst (by having one), increase rate or rxn)

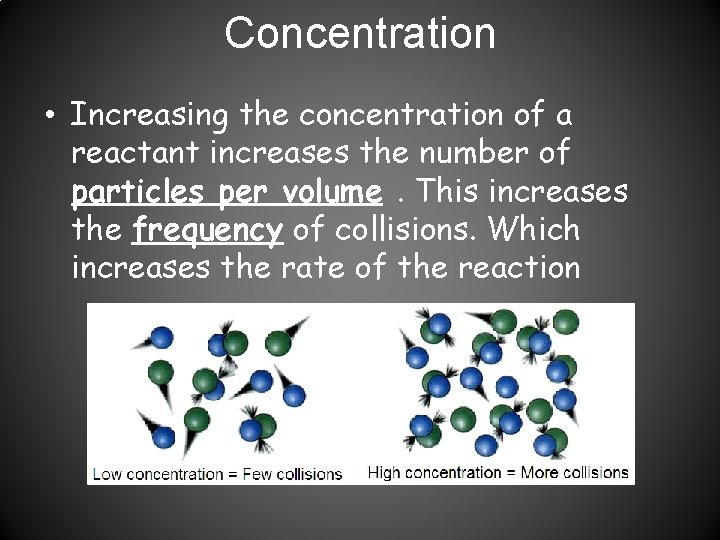
Concentration • Increasing the concentration of a reactant increases the number of particles per volume. This increases the frequency of collisions. Which increases the rate of the reaction

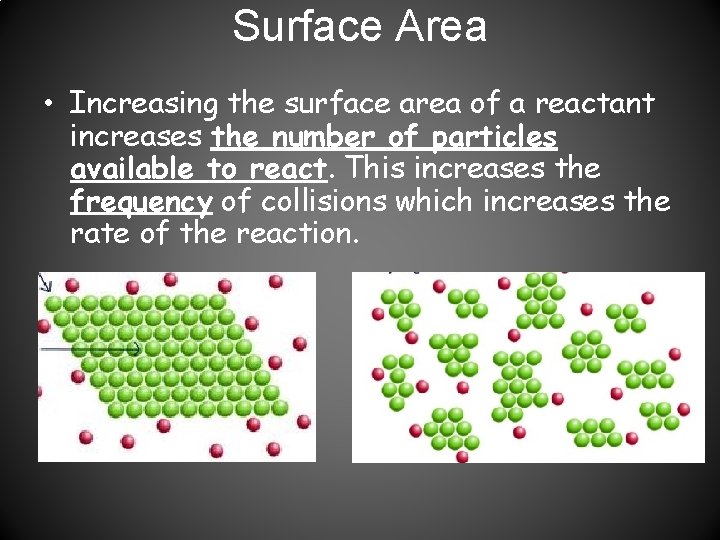
Surface Area • Increasing the surface area of a reactant increases the number of particles available to react. This increases the frequency of collisions which increases the rate of the reaction.

Temperature • Increasing the temperature of a reaction increases the average kinetic energy of the particles. • The particles collide more often and with more energy. • This increases the frequency of effective collisions which increases the rate of reaction.

Homework: Read chapters 17. 1 & 17. 2. THEN write the question and answer in notebook: § Page 535 # 4 -9 § Page 541 # 11 -15 (for 15, a short paragraph is fine. )