Learning Objectives Clinical Implications of Acid Base Balance


Learning Objectives �Clinical Implications of Acid Base Balance
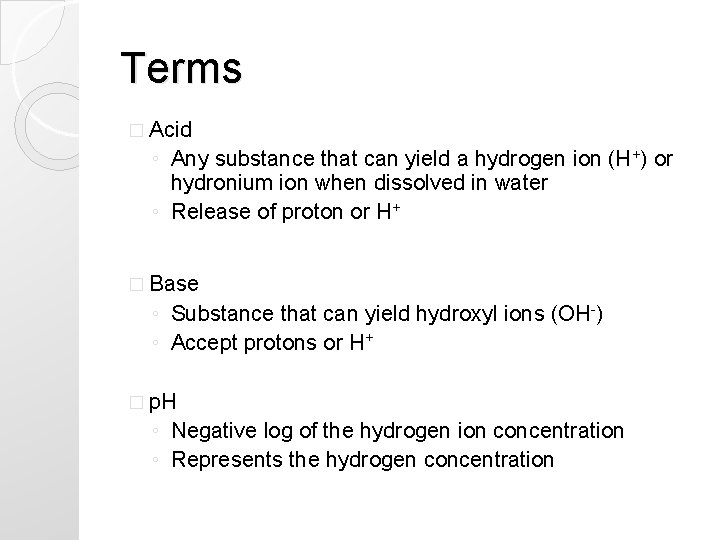
Terms � Acid ◦ Any substance that can yield a hydrogen ion (H+) or hydronium ion when dissolved in water ◦ Release of proton or H+ � Base ◦ Substance that can yield hydroxyl ions (OH-) ◦ Accept protons or H+ � p. H ◦ Negative log of the hydrogen ion concentration ◦ Represents the hydrogen concentration

Terms �Normal p. H is 7. 35 -7. 45 �Acidosis ◦ p. H less than 7. 35 �Alkalosis ◦ p. H greater than 7. 45
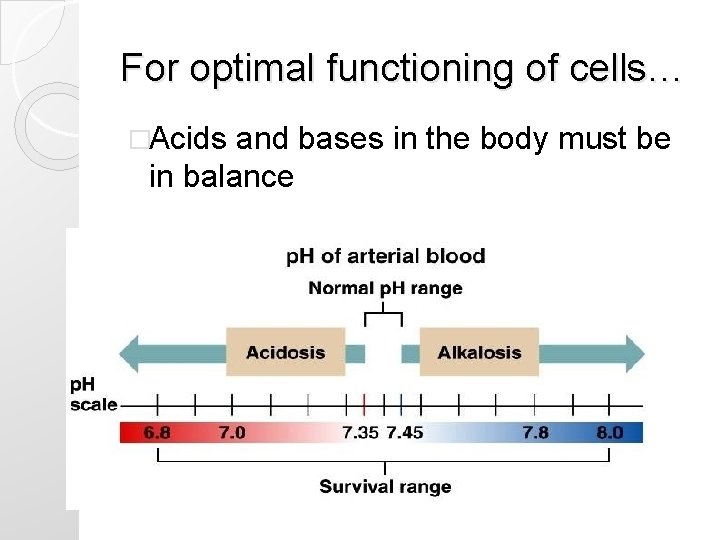
For optimal functioning of cells… �Acids and bases in the body must be in balance
The Body and p. H �Homeostasis of p. H is tightly controlled as p. H has an effects on: ◦ ◦ Protein function Enzyme function Hormones Electrolyte Balance (Na+, K+, Cl-) 6

2 Important Questions �Why does body p. H change? �How does body resist change in p. H?
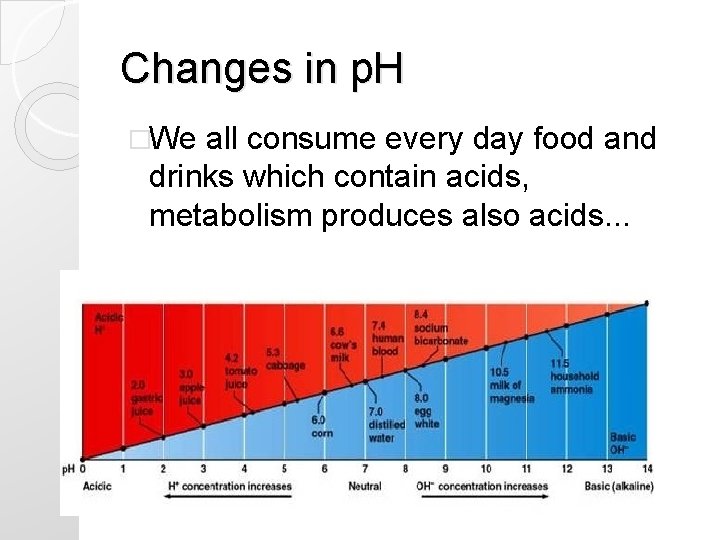
Changes in p. H �We all consume every day food and drinks which contain acids, metabolism produces also acids. . .
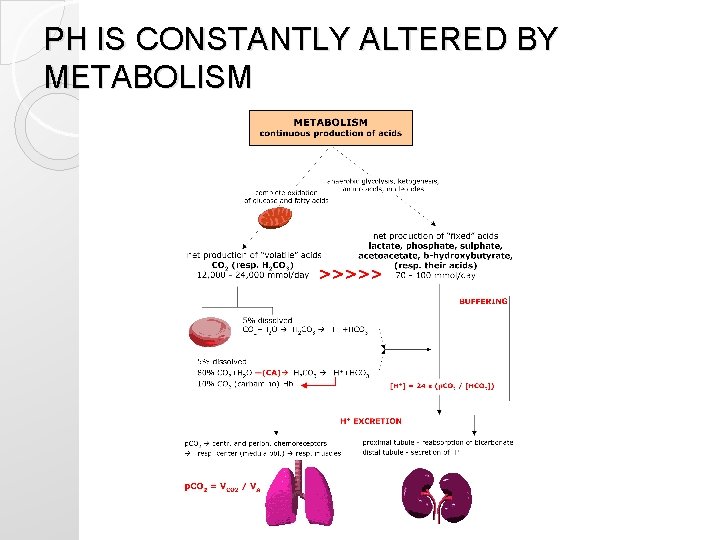
PH IS CONSTANTLY ALTERED BY METABOLISM

You get acidotic every day ! �While living, eating and drinking. . . there is. . �Production of 1 mmol of fixed acid/kg body weight per day (60 kg=60 mmol/day)
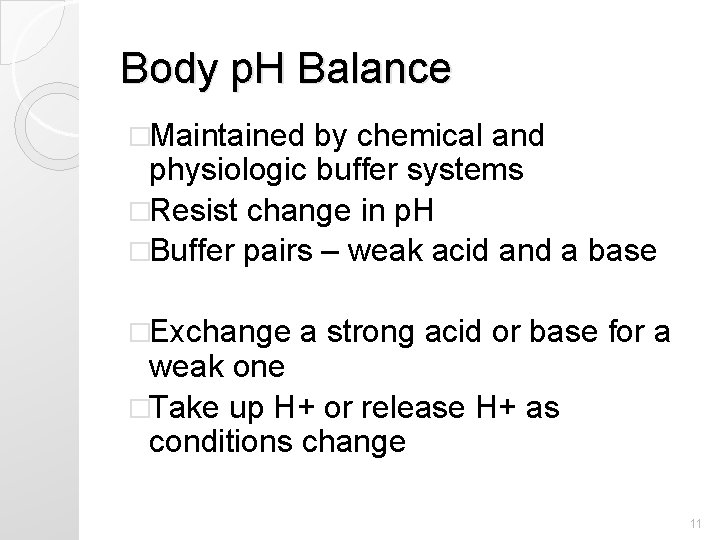
Body p. H Balance �Maintained by chemical and physiologic buffer systems �Resist change in p. H �Buffer pairs – weak acid and a base �Exchange a strong acid or base for a weak one �Take up H+ or release H+ as conditions change 11
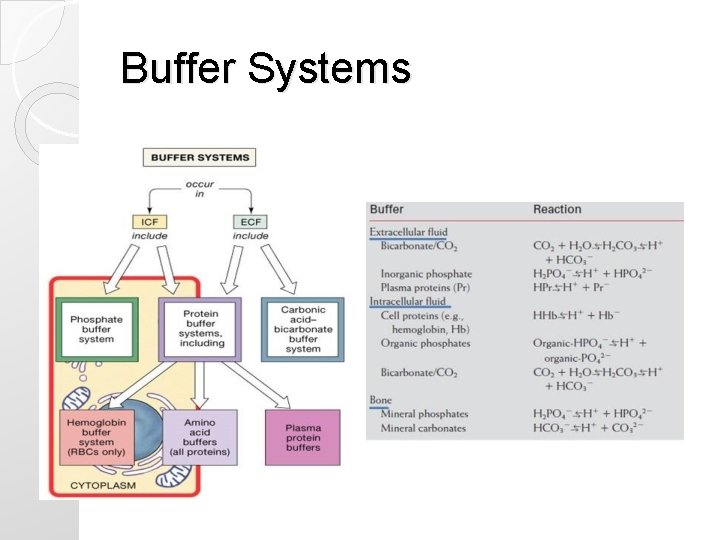
Buffer Systems
Organs involved in the regulation of Acid-Base Balance ◦ CO 2 production from complete oxidation of substrates � 20% of the body’s daily production ◦ metabolism of organic acid anions �such as lactate, ketones and amino acids ◦ metabolism of ammonium �conversion of NH 4+ to urea in the liver results in an equivalent production of H+ ◦ Production of plasma proteins �esp. albumin contributing to the anion gap ◦ Bone inorganic matrix consists of hydroxyapatite crystals (Ca 10(PO 4)6(OH)2] �bone can take up H+ in exchange for Ca 2+, Na+ and K+ (ionic exchange) or release of HCO 3 -, CO 3 - or HPO 42 -
Organs involved in the regulation of Acid-Base Balance ◦ Equilibrium with plasma ◦ High buffer capacity �Haemoglobin – main buffer for CO 2 ◦ Excretion of CO 2 by alveolar ventilation: minimally 12, 000 mmol/day ◦ Reabsorption of filtered bicarbonate: 4, 000 to 5, 000 mmol/day ◦ Excretion of the fixed acids (acid anion and associated H+): about 100 mmol/day
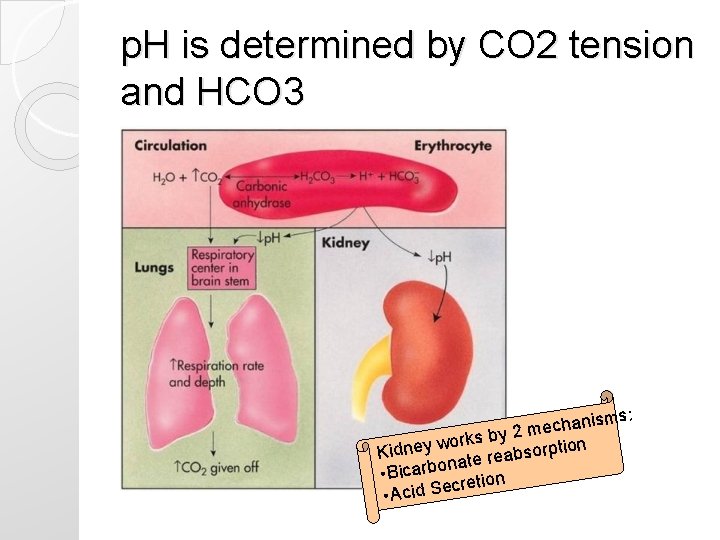
p. H is determined by CO 2 tension and HCO 3 isms: n a h c e s by 2 m ion k r o w y sorpt Kidne b a e r e t na • Bicarbo etion cr • Acid Se
Rates of correction �Buffers function: almost instantaneously �Respiratory mechanisms: take several minutes to hours �Renal mechanisms: may take several hours to days 16
Interpretation of ABGs
Interpretation of ABGs Respiratory failure • Type 1 • Type 2 Acid base disorder • Acidosis • Alkalosis
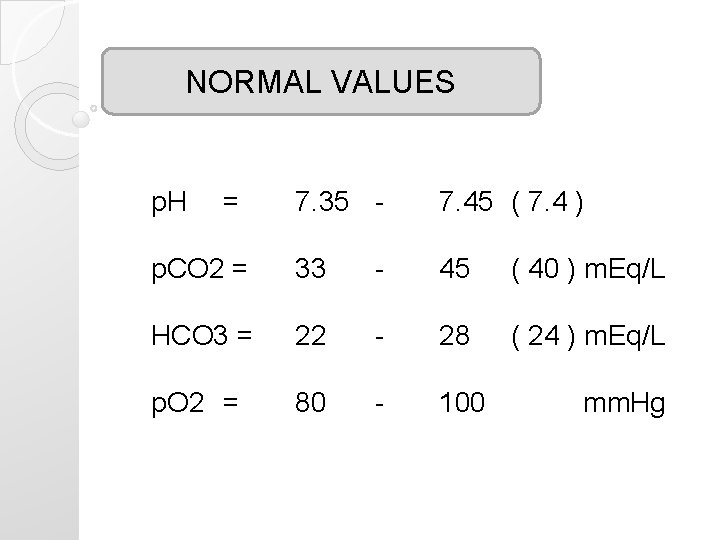
NORMAL VALUES p. H = 7. 35 - 7. 45 ( 7. 4 ) p. CO 2 = 33 - 45 ( 40 ) m. Eq/L HCO 3 = 22 - 28 ( 24 ) m. Eq/L p. O 2 = 80 - 100 mm. Hg
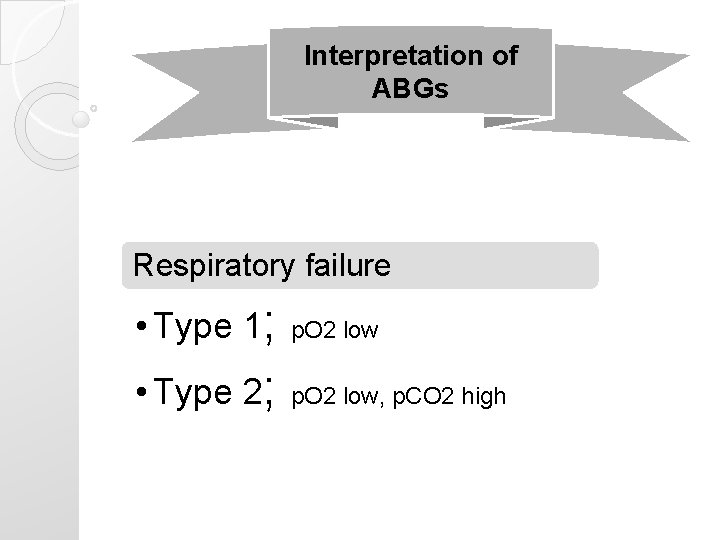
Interpretation of ABGs Respiratory failure • Type 1; p. O 2 low • Type 2; p. O 2 low, p. CO 2 high
Respiratory Acidosis Respiratory Alkalosis Acid Base disorder Metabolic Acidosis Metabolic Alkalosis
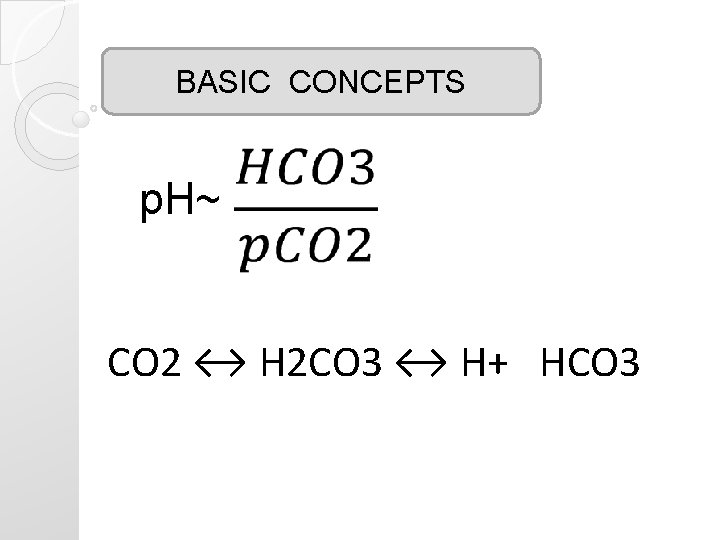
BASIC CONCEPTS p. H~ CO 2 ↔ H 2 CO 3 ↔ H+ HCO 3
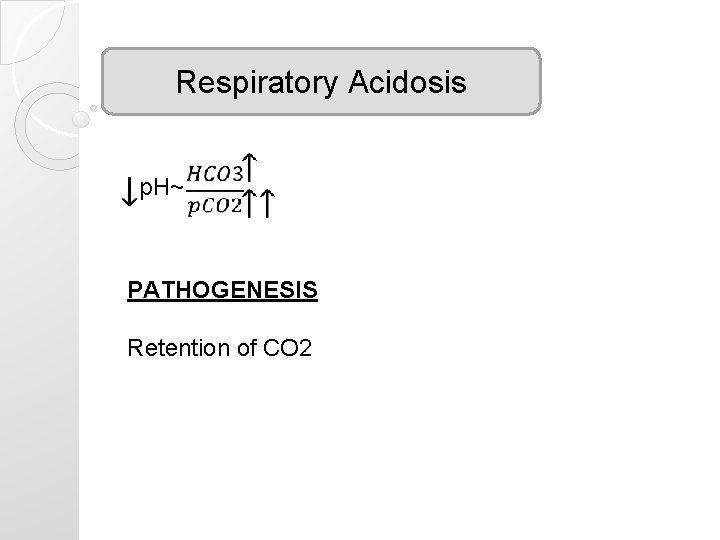
Respiratory Acidosis p. H~ PATHOGENESIS Retention of CO 2
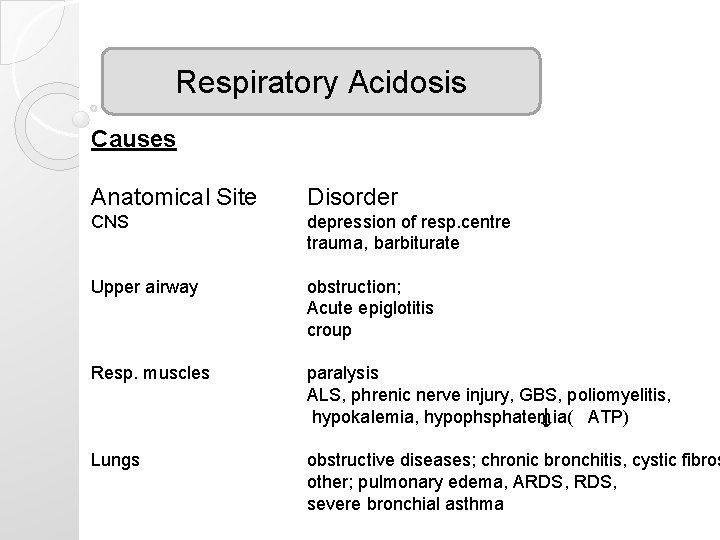
Respiratory Acidosis Causes Anatomical Site Disorder CNS depression of resp. centre trauma, barbiturate Upper airway obstruction; Acute epiglotitis croup Resp. muscles paralysis ALS, phrenic nerve injury, GBS, poliomyelitis, hypokalemia, hypophsphatemia( ATP) Lungs obstructive diseases; chronic bronchitis, cystic fibros other; pulmonary edema, ARDS, severe bronchial asthma
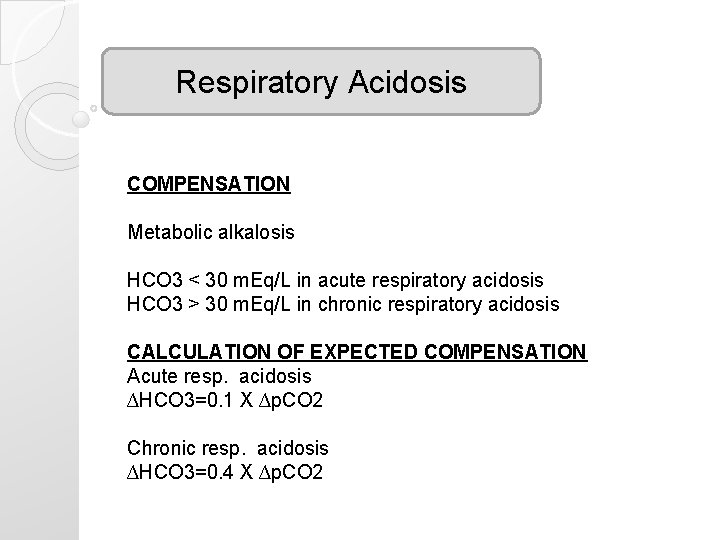
Respiratory Acidosis COMPENSATION Metabolic alkalosis HCO 3 < 30 m. Eq/L in acute respiratory acidosis HCO 3 > 30 m. Eq/L in chronic respiratory acidosis CALCULATION OF EXPECTED COMPENSATION Acute resp. acidosis ∆HCO 3=0. 1 X ∆p. CO 2 Chronic resp. acidosis ∆HCO 3=0. 4 X ∆p. CO 2
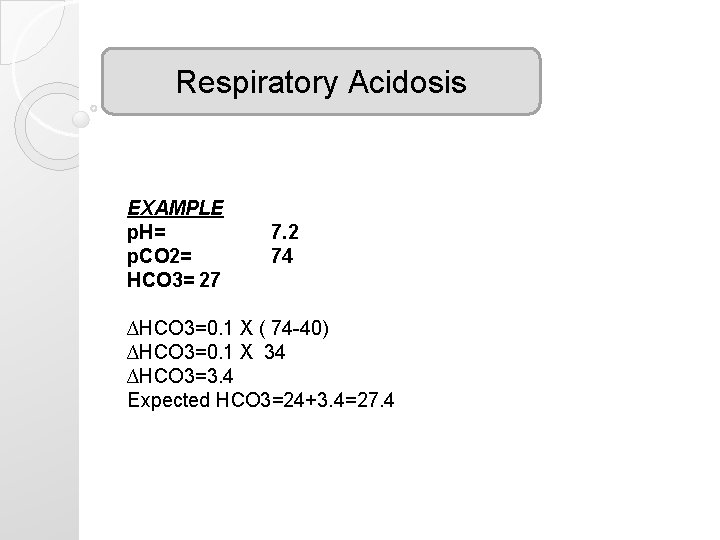
Respiratory Acidosis EXAMPLE p. H= p. CO 2= HCO 3= 27 7. 2 74 ∆HCO 3=0. 1 X ( 74 -40) ∆HCO 3=0. 1 X 34 ∆HCO 3=3. 4 Expected HCO 3=24+3. 4=27. 4
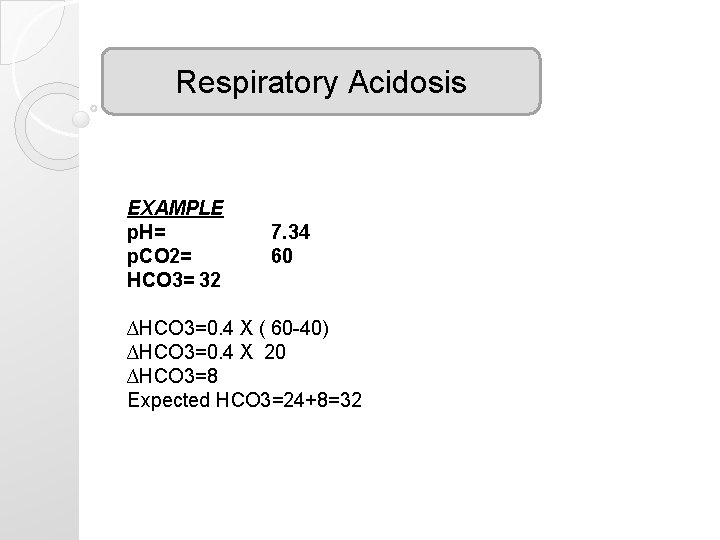
Respiratory Acidosis EXAMPLE p. H= p. CO 2= HCO 3= 32 7. 34 60 ∆HCO 3=0. 4 X ( 60 -40) ∆HCO 3=0. 4 X 20 ∆HCO 3=8 Expected HCO 3=24+8=32
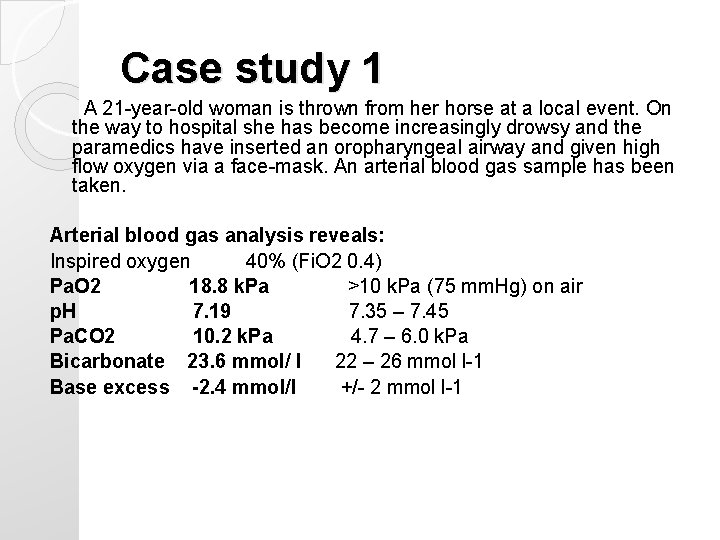
Case study 1 A 21 -year-old woman is thrown from her horse at a local event. On the way to hospital she has become increasingly drowsy and the paramedics have inserted an oropharyngeal airway and given high flow oxygen via a face-mask. An arterial blood gas sample has been taken. Arterial blood gas analysis reveals: Inspired oxygen 40% (Fi. O 2 0. 4) Pa. O 2 18. 8 k. Pa >10 k. Pa (75 mm. Hg) on air p. H 7. 19 7. 35 – 7. 45 Pa. CO 2 10. 2 k. Pa 4. 7 – 6. 0 k. Pa Bicarbonate 23. 6 mmol/ l 22 – 26 mmol l-1 Base excess -2. 4 mmol/l +/- 2 mmol l-1
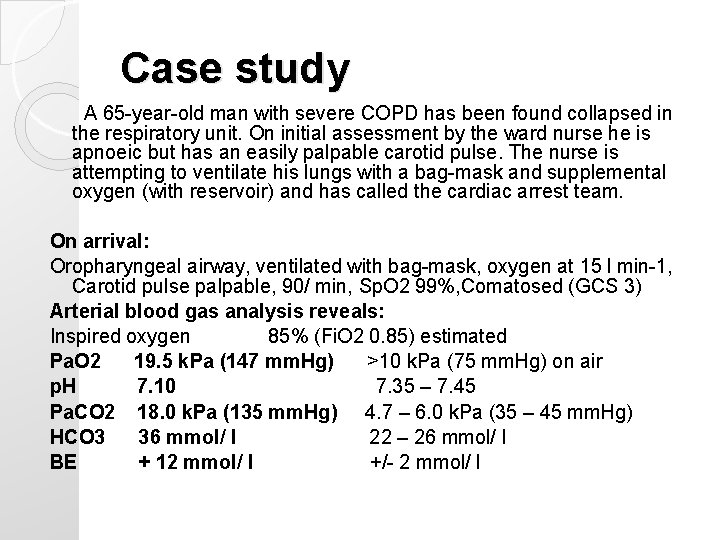
Case study A 65 -year-old man with severe COPD has been found collapsed in the respiratory unit. On initial assessment by the ward nurse he is apnoeic but has an easily palpable carotid pulse. The nurse is attempting to ventilate his lungs with a bag-mask and supplemental oxygen (with reservoir) and has called the cardiac arrest team. On arrival: Oropharyngeal airway, ventilated with bag-mask, oxygen at 15 l min-1, Carotid pulse palpable, 90/ min, Sp. O 2 99%, Comatosed (GCS 3) Arterial blood gas analysis reveals: Inspired oxygen 85% (Fi. O 2 0. 85) estimated Pa. O 2 19. 5 k. Pa (147 mm. Hg) >10 k. Pa (75 mm. Hg) on air p. H 7. 10 7. 35 – 7. 45 Pa. CO 2 18. 0 k. Pa (135 mm. Hg) 4. 7 – 6. 0 k. Pa (35 – 45 mm. Hg) HCO 3 36 mmol/ l 22 – 26 mmol/ l BE + 12 mmol/ l +/- 2 mmol/ l
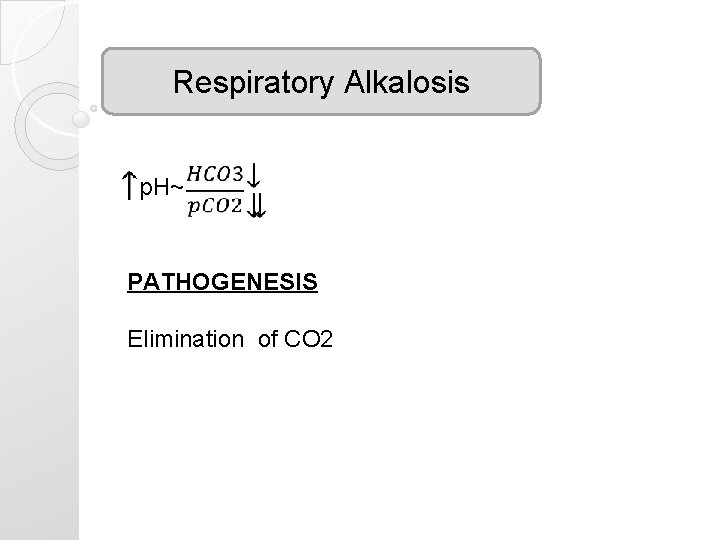
Respiratory Alkalosis p. H~ PATHOGENESIS Elimination of CO 2
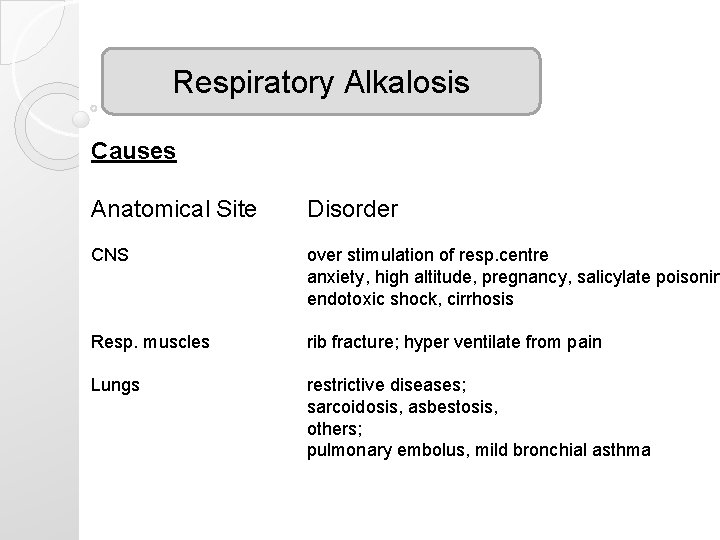
Respiratory Alkalosis Causes Anatomical Site Disorder CNS over stimulation of resp. centre anxiety, high altitude, pregnancy, salicylate poisonin endotoxic shock, cirrhosis Resp. muscles rib fracture; hyper ventilate from pain Lungs restrictive diseases; sarcoidosis, asbestosis, others; pulmonary embolus, mild bronchial asthma
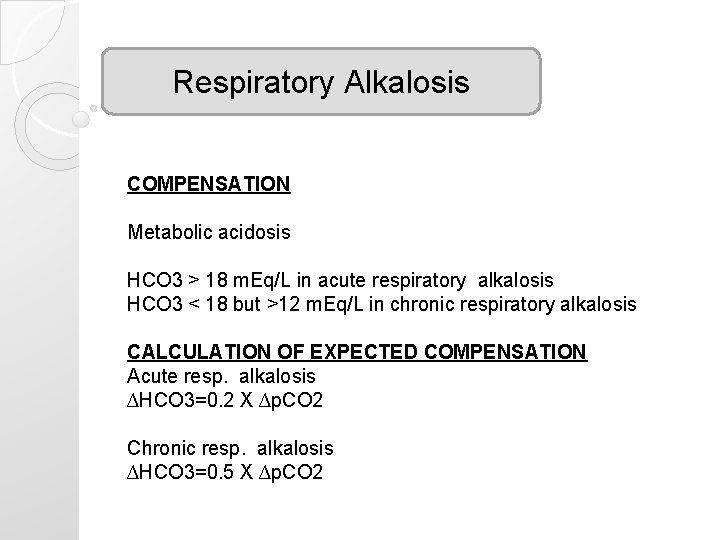
Respiratory Alkalosis COMPENSATION Metabolic acidosis HCO 3 > 18 m. Eq/L in acute respiratory alkalosis HCO 3 < 18 but >12 m. Eq/L in chronic respiratory alkalosis CALCULATION OF EXPECTED COMPENSATION Acute resp. alkalosis ∆HCO 3=0. 2 X ∆p. CO 2 Chronic resp. alkalosis ∆HCO 3=0. 5 X ∆p. CO 2
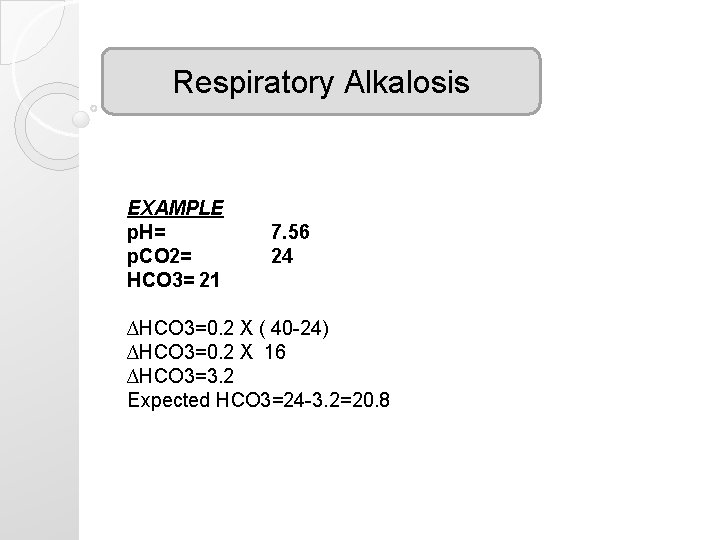
Respiratory Alkalosis EXAMPLE p. H= p. CO 2= HCO 3= 21 7. 56 24 ∆HCO 3=0. 2 X ( 40 -24) ∆HCO 3=0. 2 X 16 ∆HCO 3=3. 2 Expected HCO 3=24 -3. 2=20. 8
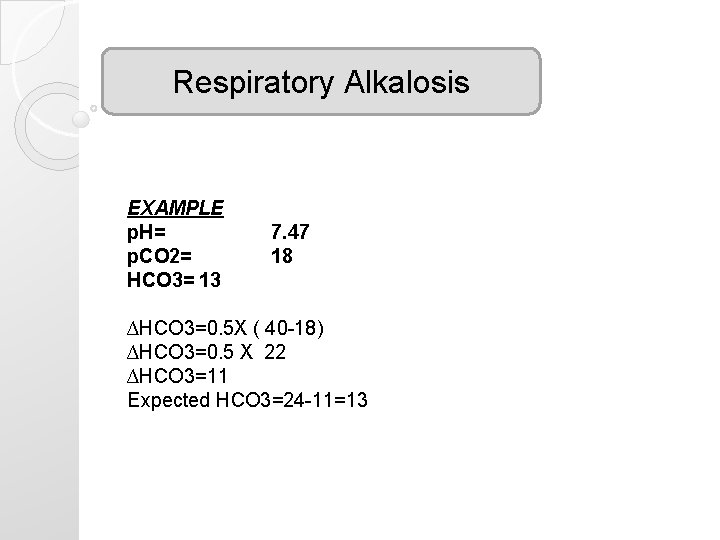
Respiratory Alkalosis EXAMPLE p. H= p. CO 2= HCO 3= 13 7. 47 18 ∆HCO 3=0. 5 X ( 40 -18) ∆HCO 3=0. 5 X 22 ∆HCO 3=11 Expected HCO 3=24 -11=13
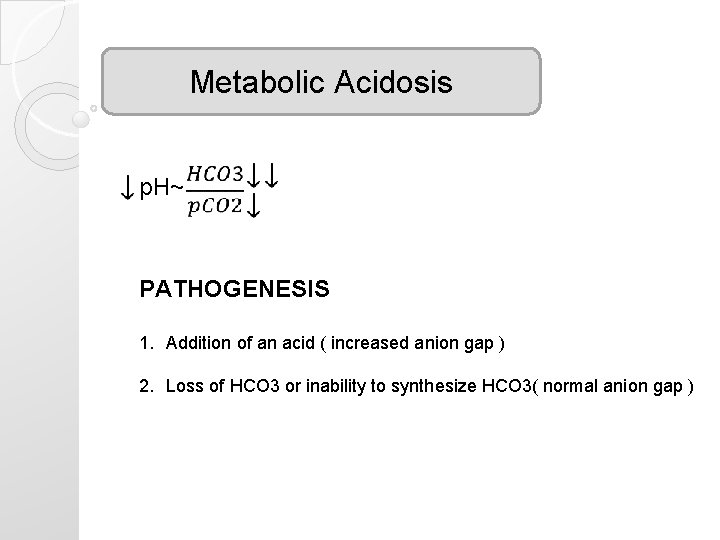
Metabolic Acidosis p. H~ PATHOGENESIS 1. Addition of an acid ( increased anion gap ) 2. Loss of HCO 3 or inability to synthesize HCO 3( normal anion gap )

Metabolic Acidosis COMPENSATION Resp. alkalosis CALCULATION OF EXPECTED COMPENSATION ∆p. CO 2 =1. 2 X ∆HCO 3 +/- 2
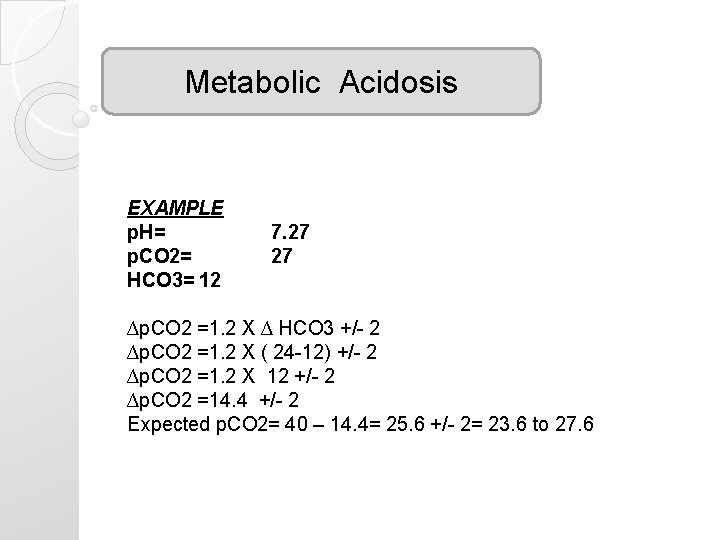
Metabolic Acidosis EXAMPLE p. H= p. CO 2= HCO 3= 12 7. 27 27 ∆p. CO 2 =1. 2 X ∆ HCO 3 +/- 2 ∆p. CO 2 =1. 2 X ( 24 -12) +/- 2 ∆p. CO 2 =1. 2 X 12 +/- 2 ∆p. CO 2 =14. 4 +/- 2 Expected p. CO 2= 40 – 14. 4= 25. 6 +/- 2= 23. 6 to 27. 6
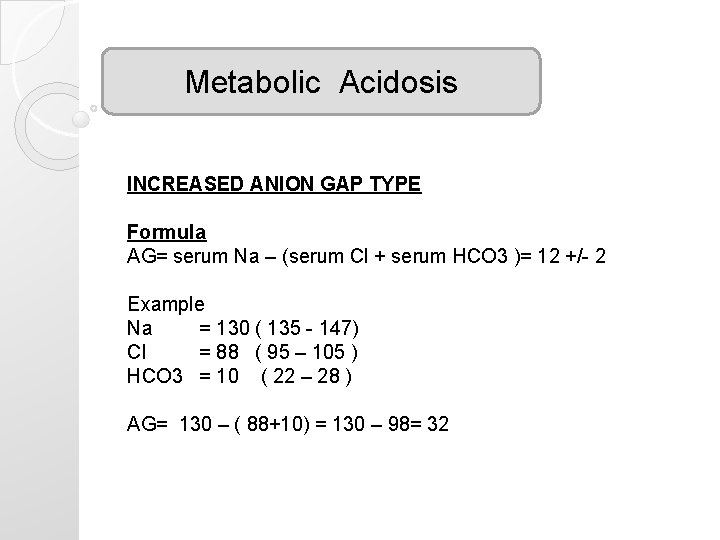
Metabolic Acidosis INCREASED ANION GAP TYPE Formula AG= serum Na – (serum Cl + serum HCO 3 )= 12 +/- 2 Example Na = 130 ( 135 - 147) Cl = 88 ( 95 – 105 ) HCO 3 = 10 ( 22 – 28 ) AG= 130 – ( 88+10) = 130 – 98= 32

Metabolic Acidosis INCREASED ANION GAP TYPE Causes Lactic acidosis Ketoacidosis Renal failure ( retention of organic acids) Salicylate poisoning Ethylene glycol poisoning Methyl alcohalpoisoning
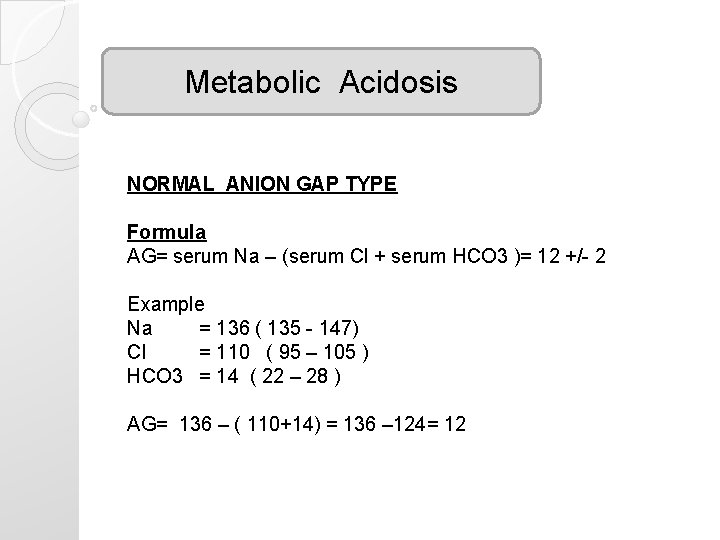
Metabolic Acidosis NORMAL ANION GAP TYPE Formula AG= serum Na – (serum Cl + serum HCO 3 )= 12 +/- 2 Example Na = 136 ( 135 - 147) Cl = 110 ( 95 – 105 ) HCO 3 = 14 ( 22 – 28 ) AG= 136 – ( 110+14) = 136 – 124= 12

Metabolic Acidosis NORMAL ANION GAP TYPE CAUSES Diarrhoea Cholestyramine Drainage of bile or pancreatic secretions Type 1 distal renal tubular acidosis Type 11 proximal renal tubular acidosis Type IV renal tubular acidosis
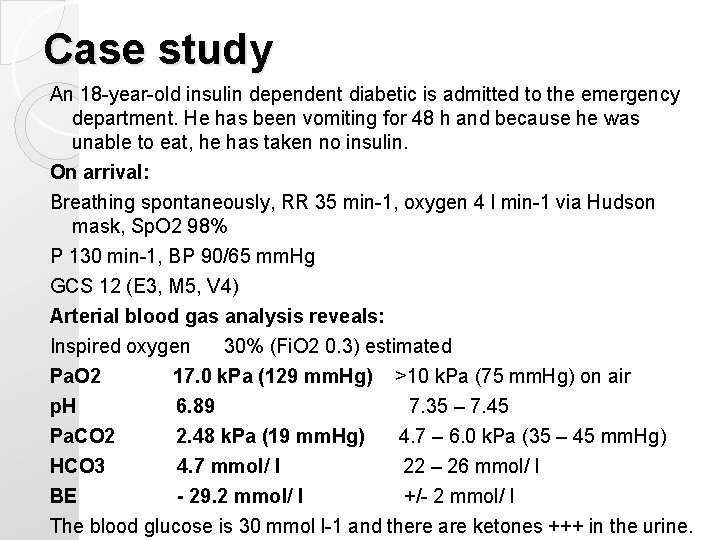
Case study An 18 -year-old insulin dependent diabetic is admitted to the emergency department. He has been vomiting for 48 h and because he was unable to eat, he has taken no insulin. On arrival: Breathing spontaneously, RR 35 min-1, oxygen 4 l min-1 via Hudson mask, Sp. O 2 98% P 130 min-1, BP 90/65 mm. Hg GCS 12 (E 3, M 5, V 4) Arterial blood gas analysis reveals: Inspired oxygen 30% (Fi. O 2 0. 3) estimated Pa. O 2 17. 0 k. Pa (129 mm. Hg) >10 k. Pa (75 mm. Hg) on air p. H 6. 89 7. 35 – 7. 45 Pa. CO 2 2. 48 k. Pa (19 mm. Hg) 4. 7 – 6. 0 k. Pa (35 – 45 mm. Hg) HCO 3 4. 7 mmol/ l 22 – 26 mmol/ l BE - 29. 2 mmol/ l +/- 2 mmol/ l The blood glucose is 30 mmol l-1 and there are ketones +++ in the urine.
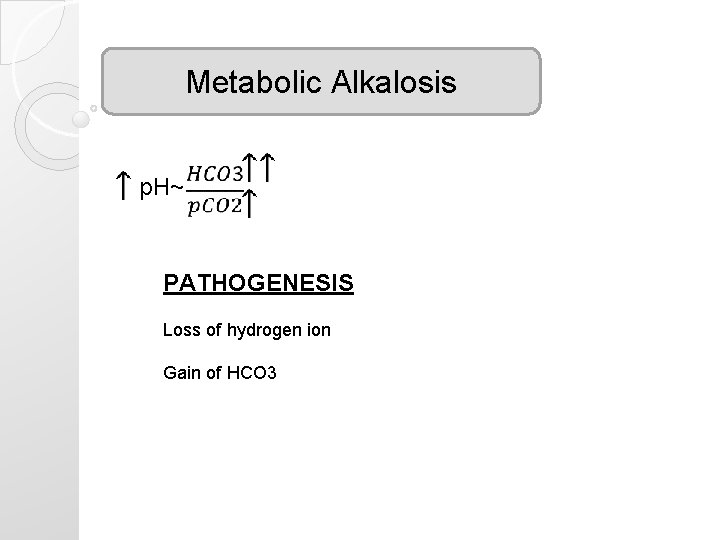
Metabolic Alkalosis p. H~ PATHOGENESIS Loss of hydrogen ion Gain of HCO 3

Metabolic Alkalosis COMPENSATION Resp. acidosis CALCULATION OF EXPECTED COMPENSATION ∆p. CO 2 =0. 7 X ∆ HCO 3 +/- 2
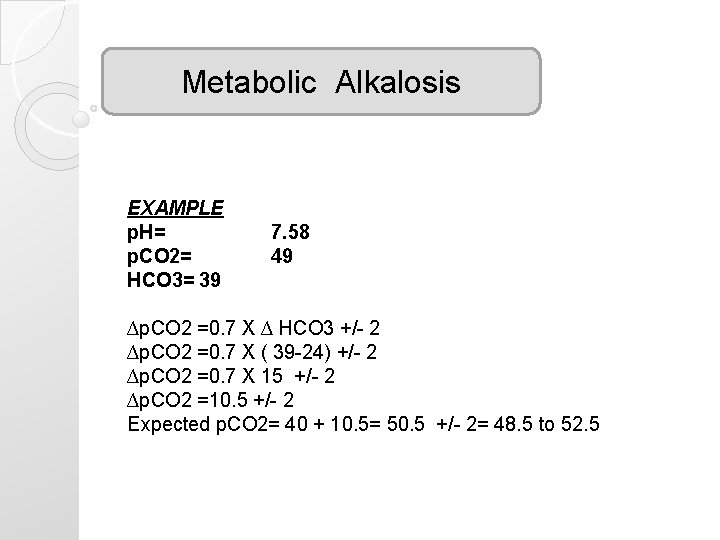
Metabolic Alkalosis EXAMPLE p. H= p. CO 2= HCO 3= 39 7. 58 49 ∆p. CO 2 =0. 7 X ∆ HCO 3 +/- 2 ∆p. CO 2 =0. 7 X ( 39 -24) +/- 2 ∆p. CO 2 =0. 7 X 15 +/- 2 ∆p. CO 2 =10. 5 +/- 2 Expected p. CO 2= 40 + 10. 5= 50. 5 +/- 2= 48. 5 to 52. 5

Metabolic Alkalosis CAUSES Vomiting Mineralocorticoid excess Thiazide and loop diuretics
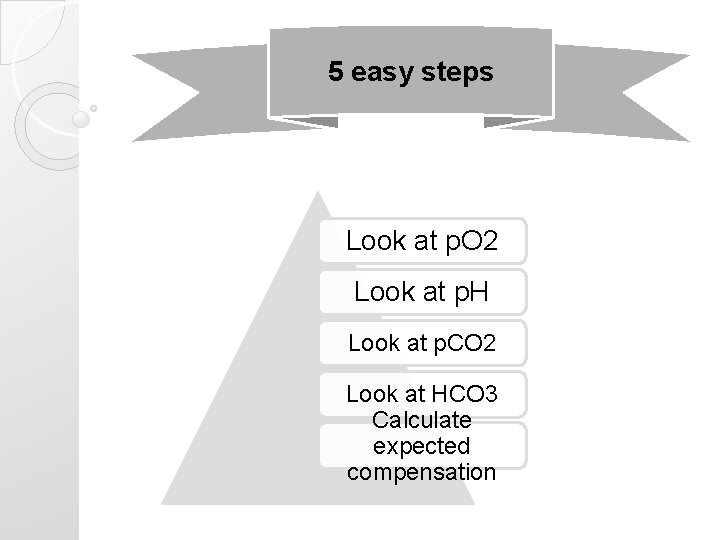
5 easy steps Look at p. O 2 Look at p. H Look at p. CO 2 Look at HCO 3 Calculate expected compensation
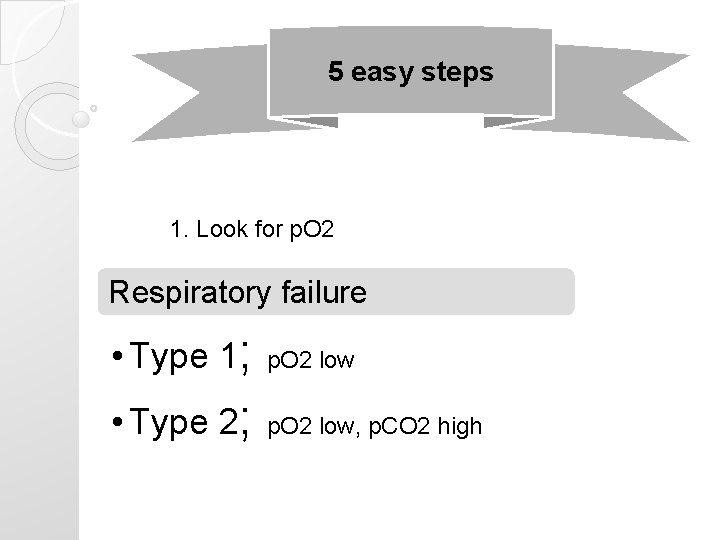
5 easy steps 1. Look for p. O 2 Respiratory failure • Type 1; p. O 2 low • Type 2; p. O 2 low, p. CO 2 high
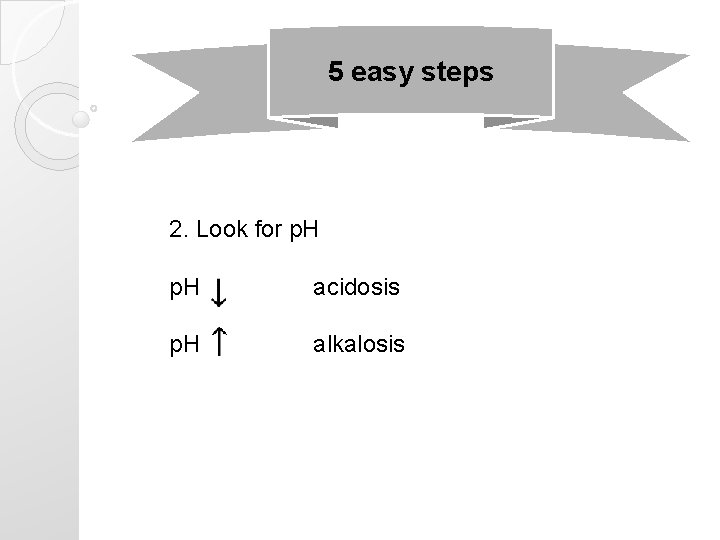
5 easy steps 2. Look for p. H acidosis p. H alkalosis
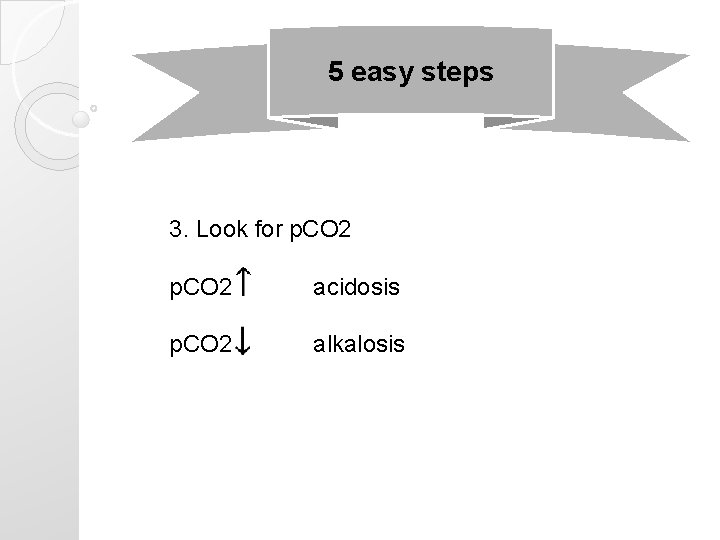
5 easy steps 3. Look for p. CO 2 acidosis p. CO 2 alkalosis
5 easy steps 4. Look for HCO 3 acidosis HCO 3 alkalosis
5 easy steps 5. Calculate for expected compensation If compensation within expected range one primary disorder If compensation outside normal range more than one primary disorder
5 easy steps 5. Compensation uncompensated disorder expected compensation in normal range partially compensated disorder Expected compensation moves outside the normal range, But does not bring p. H in normal range full compensation Expected compensation moves outside the normal range, and brings p. H in normal range
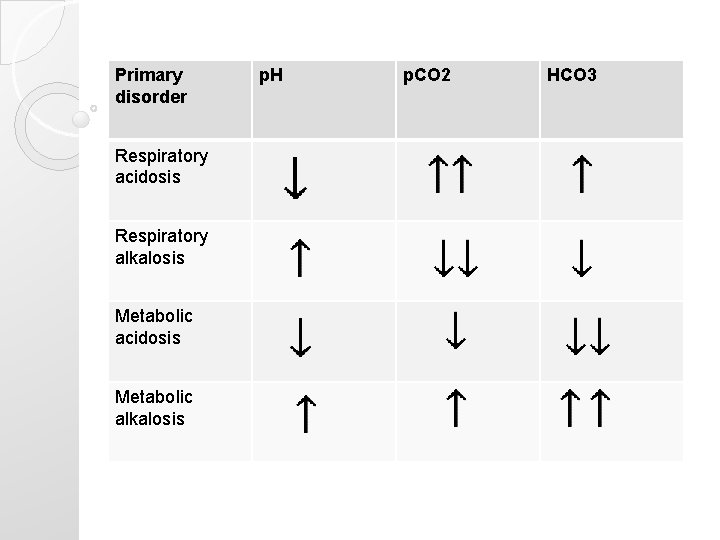
Primary disorder Respiratory acidosis Respiratory alkalosis Metabolic acidosis Metabolic alkalosis p. H p. CO 2 HCO 3
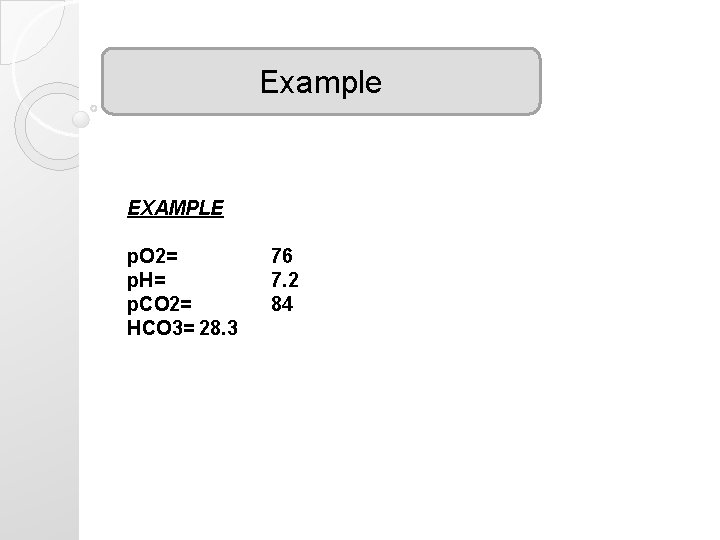
Example EXAMPLE p. O 2= p. H= p. CO 2= HCO 3= 28. 3 76 7. 2 84
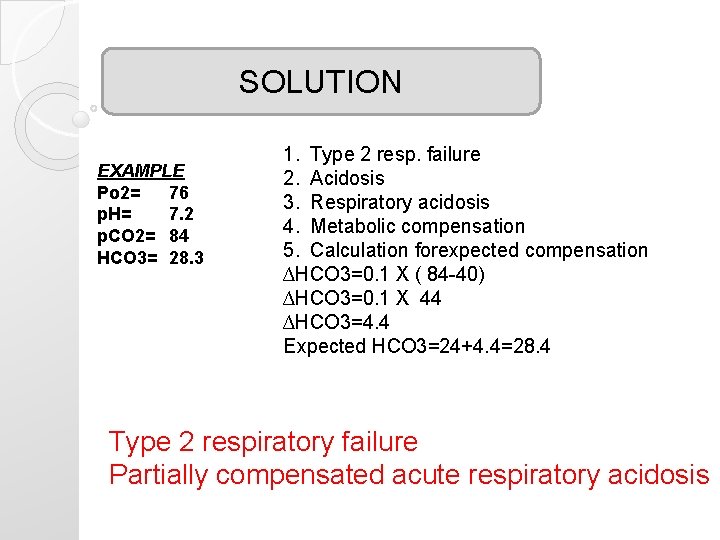
SOLUTION EXAMPLE Po 2= 76 p. H= 7. 2 p. CO 2= 84 HCO 3= 28. 3 1. Type 2 resp. failure 2. Acidosis 3. Respiratory acidosis 4. Metabolic compensation 5. Calculation forexpected compensation ∆HCO 3=0. 1 X ( 84 -40) ∆HCO 3=0. 1 X 44 ∆HCO 3=4. 4 Expected HCO 3=24+4. 4=28. 4 Type 2 respiratory failure Partially compensated acute respiratory acidosis
p 02 K N A H T U O Y p. H~
- Slides: 57