LEARNING AREA PERIODIC TABLE OF ELEMENT ANALYSING GROUP

LEARNING AREA: PERIODIC TABLE OF ELEMENT ‘ANALYSING GROUP 18 ELEMENT’ CREATED BY : THIRU KUMARI

INTRODUCTION PHYSICAL PROPERTIES GOING DOWN THE GROUP THE INERT PROPERTY APPLICATION TUTORIAL
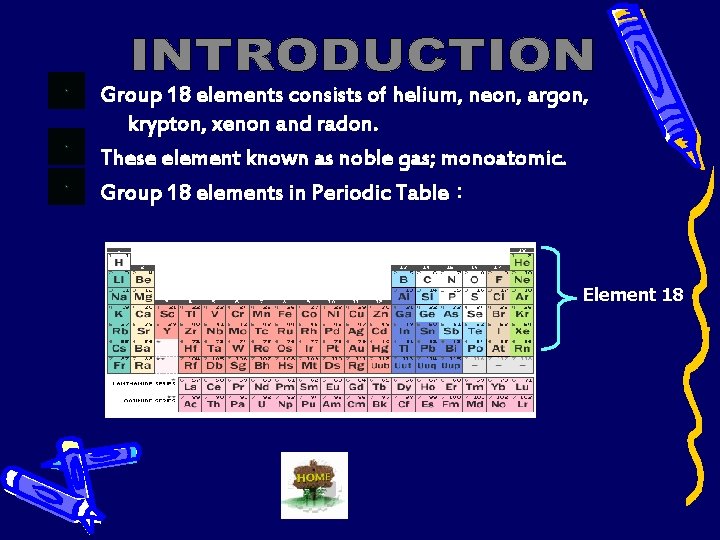
Group 18 elements consists of helium, neon, argon, krypton, xenon and radon. These element known as noble gas; monoatomic. Group 18 elements in Periodic Table : Element 18

Small atomic sizes. Colourless (at room temperature & pressure). Low melting point & boiling point. Low density. Insoluble in water. Cannot conduct electricity. Poor conductor.

Physical Atomic size Melting & Boiling Effect Reason Because the number of occupied shell in the atom increase from helium to radon Atomic size increases, the forces of attraction between the atom become stronger. So more heat energy is required to overcome the stronger force of attraction during melting or boiling.
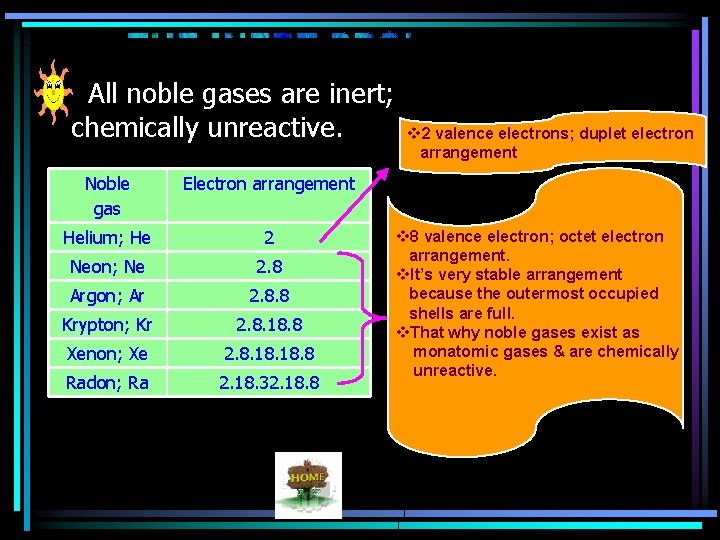
All noble gases are inert; chemically unreactive. Noble gas Electron arrangement Helium; He 2 Neon; Ne 2. 8 Argon; Ar 2. 8. 8 Krypton; Kr 2. 8. 18. 8 Xenon; Xe 2. 8. 18. 8 Radon; Ra 2. 18. 32. 18. 8 v 2 valence electrons; duplet electron arrangement v 8 valence electron; octet electron arrangement. v. It’s very stable arrangement because the outermost occupied shells are full. v. That why noble gases exist as monatomic gases & are chemically unreactive.
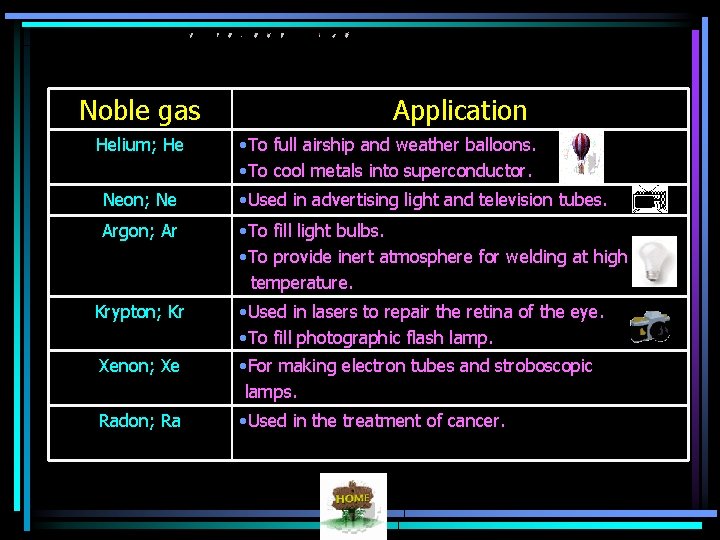
Noble gas Helium; He Application • To full airship and weather balloons. • To cool metals into superconductor. Neon; Ne • Used in advertising light and television tubes. Argon; Ar • To fill light bulbs. • To provide inert atmosphere for welding at high temperature. Krypton; Kr • Used in lasers to repair the retina of the eye. • To fill photographic flash lamp. Xenon; Xe • For making electron tubes and stroboscopic lamps. Radon; Ra • Used in the treatment of cancer.

Activity : In group of 5, discuss the answer of this exercises. At the end of the class, each group will present their answer. 1 - Explain each of the following statements. a) Argon gas is used in light bulbs but not air. b) Neon exists as a monoatomic gas. c) Helium gas is used in weather balloons but not hydrogen.
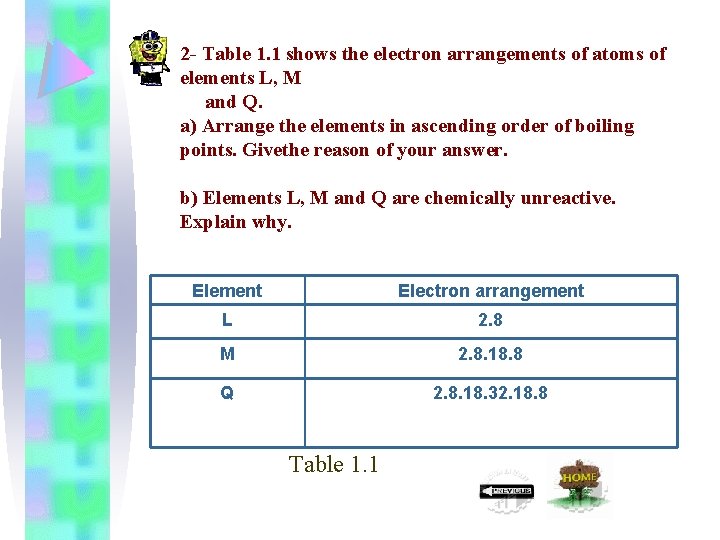
2 - Table 1. 1 shows the electron arrangements of atoms of elements L, M and Q. a) Arrange the elements in ascending order of boiling points. Givethe reason of your answer. b) Elements L, M and Q are chemically unreactive. Explain why. Element Electron arrangement L 2. 8 M 2. 8. 18. 8 Q 2. 8. 18. 32. 18. 8 Table 1. 1
- Slides: 9