LEAD HALIDE PEROVSKITE COLLOIDAL NANOCRYSTALS Perovskite lattice structure














- Slides: 42

LEAD HALIDE PEROVSKITE COLLOIDAL NANOCRYSTALS

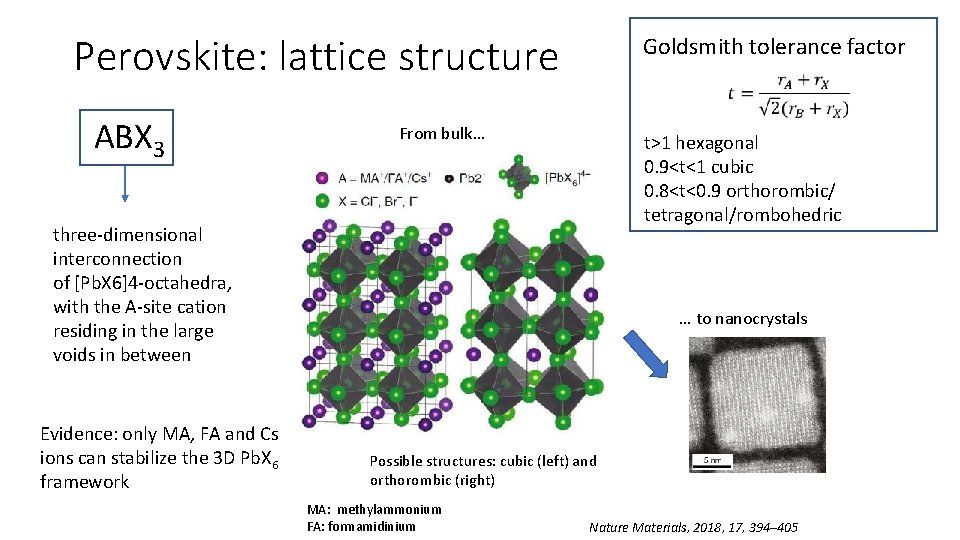
Perovskite: lattice structure ABX 3 Goldsmith tolerance factor From bulk… t>1 hexagonal 0. 9<t<1 cubic 0. 8<t<0. 9 orthorombic/ tetragonal/rombohedric three-dimensional interconnection of [Pb. X 6]4 -octahedra, with the A-site cation residing in the large voids in between Evidence: only MA, FA and Cs ions can stabilize the 3 D Pb. X 6 framework … to nanocrystals Possible structures: cubic (left) and orthorombic (right) MA: methylammonium FA: formamidinium Nature Materials, 2018, 17, 394– 405

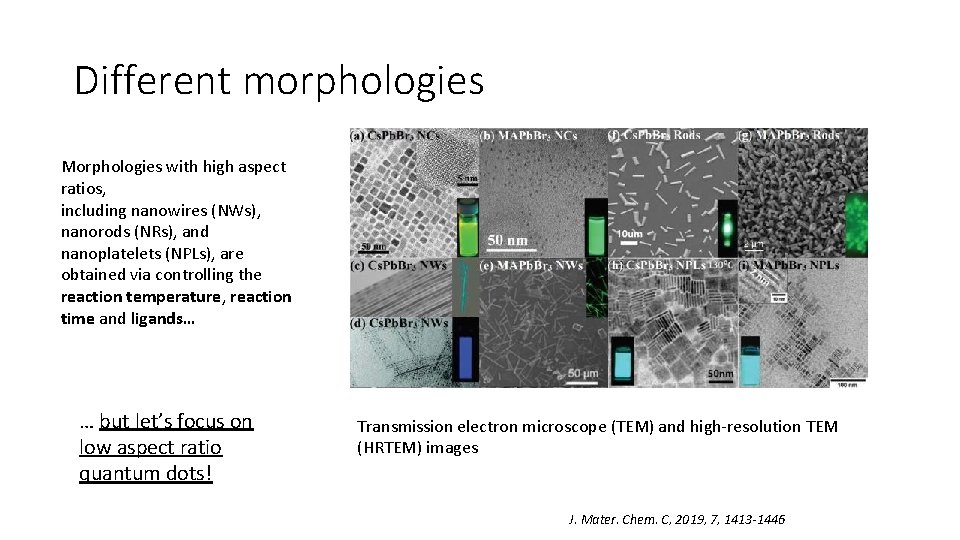
Different morphologies Morphologies with high aspect ratios, including nanowires (NWs), nanorods (NRs), and nanoplatelets (NPLs), are obtained via controlling the reaction temperature, reaction time and ligands… … but let’s focus on low aspect ratio quantum dots! Transmission electron microscope (TEM) and high-resolution TEM (HRTEM) images J. Mater. Chem. C, 2019, 7, 1413 -1446

Why LHP QDs? Pros & cons Cons Pros • Low Temperature of synthesis Instability • Low costs of fabrication Low defect formation energy Toxicity • Narrow emission • Very High Photoluminescence Quantum Yield • Excellent spectral versatily • In situ crystallization (PROCESSABILITY!)

Pros: 1) Low T of synthesis Covalent Si QDs In. P, Ga. N QDs Ionic Cd. Se, Cd. S, Zn. S QDs Perovskite QDs Energy … the more difficult to make The more covalent… Methods: • First attempts: ligand assisted hot-injection synthesis (RT 200°C) • Further attempts: ligand-assisted reprecipitation (LARP); ionic solution of the respective ions in a polar solvent is rapidly destabilized by mixing with a non -solvent

Pros: 2) Good color tunability Easy anion exchange: instead of size let’s tune the composition By adjusting the halide ratios in the colloidal nanocrystal solution, the PL can be tuned over the entire visible spectral region (410− 700 nm) while maintaining high quantum yields of 20− 80% and narrow emission line widths Exchange only for the Cl−Br and Br−I couples, never from Cl− to I− (and reverse): it would involve a structural stress on the lattice that cannot be tolerated without degradation of the NCs size: ∼ 10 nm Nano Lett. 2015, 5635− 5640

continuous formation of homogeneous Cs. Pb. Brx. I 3−x solid solutions: The anion-exchange reactions were conducted in octadecene (ODE) mixing a specific ratio of the halide source and Cs. Pb. X 3 NCs Halide source: oleylammonium halides (OAm. X), Pb. X 2 salts NCs themselves act as halide sources for each other Nano Lett. 2015, 5635− 5640

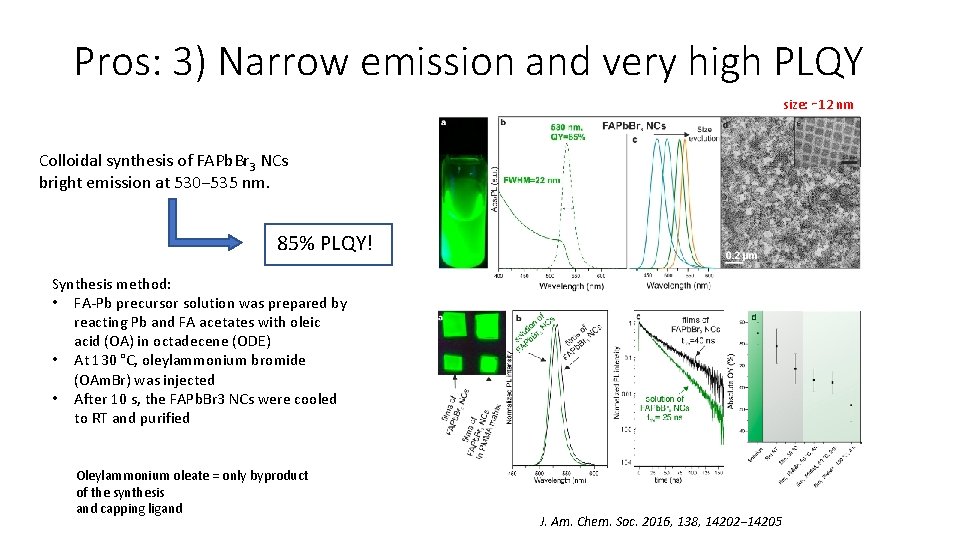
Pros: 3) Narrow emission and very high PLQY size: ∼ 12 nm Colloidal synthesis of FAPb. Br 3 NCs bright emission at 530− 535 nm. 85% PLQY! Synthesis method: • FA-Pb precursor solution was prepared by reacting Pb and FA acetates with oleic acid (OA) in octadecene (ODE) • At 130 °C, oleylammonium bromide (OAm. Br) was injected • After 10 s, the FAPb. Br 3 NCs were cooled to RT and purified Oleylammonium oleate = only byproduct of the synthesis and capping ligand J. Am. Chem. Soc. 2016, 138, 14202− 14205

… but why? Lots and lots of papers declare that perovskite NCs are bright emissive with high QY, even without focused strategies of shell passivation during the synthesis (whereas such passivation is mandatory to achieve a high PL QY from conventional QDs!)… The answer is the peculiar so called «defect tolerance» The system DOES HAVE large density of various structural defects but this DOES NOT AFFECT so much the optical and electronic properties

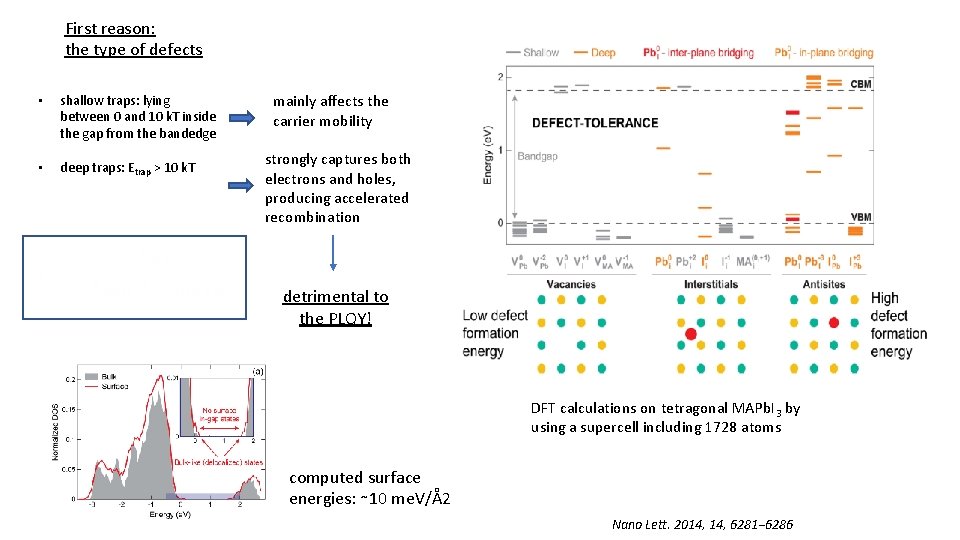
First reason: the type of defects • shallow traps: lying between 0 and 10 k. T inside the gap from the bandedge • deep traps: Etrap > 10 k. T mainly affects the carrier mobility strongly captures both electrons and holes, producing accelerated recombination detrimental to the PLQY! DFT calculations on tetragonal MAPb. I 3 by using a supercell including 1728 atoms computed surface energies: ∼ 10 me. V/Å2 Nano Lett. 2014, 6281− 6286

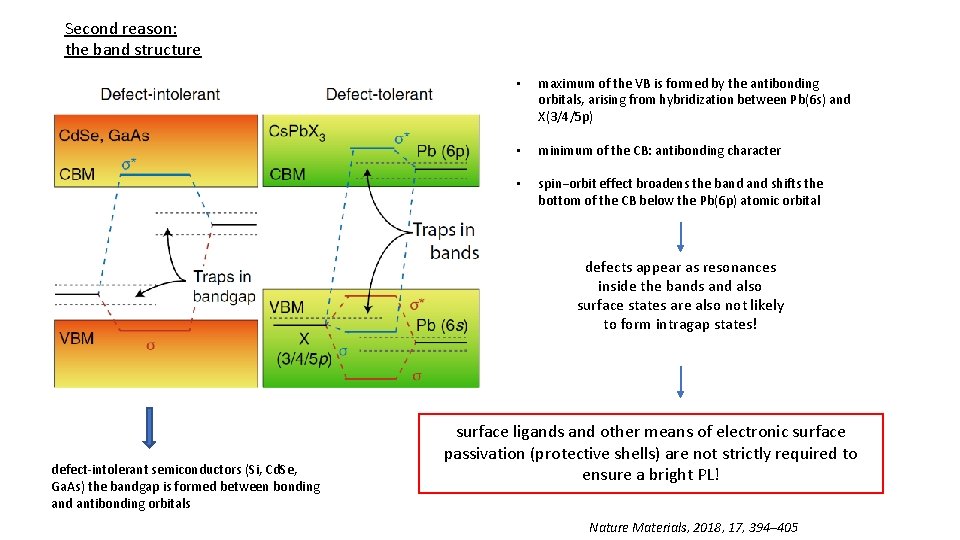
Second reason: the band structure • maximum of the VB is formed by the antibonding orbitals, arising from hybridization between Pb(6 s) and X(3/4/5 p) • minimum of the CB: antibonding character • spin−orbit effect broadens the band shifts the bottom of the CB below the Pb(6 p) atomic orbital defects appear as resonances inside the bands and also surface states are also not likely to form intragap states! defect-intolerant semiconductors (Si, Cd. Se, Ga. As) the bandgap is formed between bonding and antibonding orbitals surface ligands and other means of electronic surface passivation (protective shells) are not strictly required to ensure a bright PL! Nature Materials, 2018, 17, 394– 405

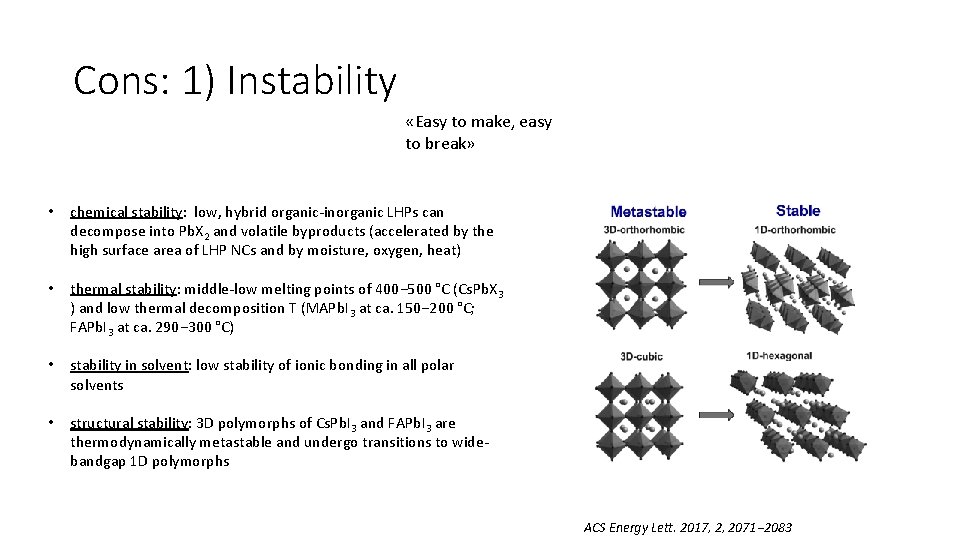
Cons: 1) Instability «Easy to make, easy to break» • chemical stability: low, hybrid organic-inorganic LHPs can decompose into Pb. X 2 and volatile byproducts (accelerated by the high surface area of LHP NCs and by moisture, oxygen, heat) • thermal stability: middle-low melting points of 400− 500 °C (Cs. Pb. X 3 ) and low thermal decomposition T (MAPb. I 3 at ca. 150− 200 °C; FAPb. I 3 at ca. 290− 300 °C) • stability in solvent: low stability of ionic bonding in all polar solvents • structural stability: 3 D polymorphs of Cs. Pb. I 3 and FAPb. I 3 are thermodynamically metastable and undergo transitions to widebandgap 1 D polymorphs ACS Energy Lett. 2017, 2, 2071− 2083

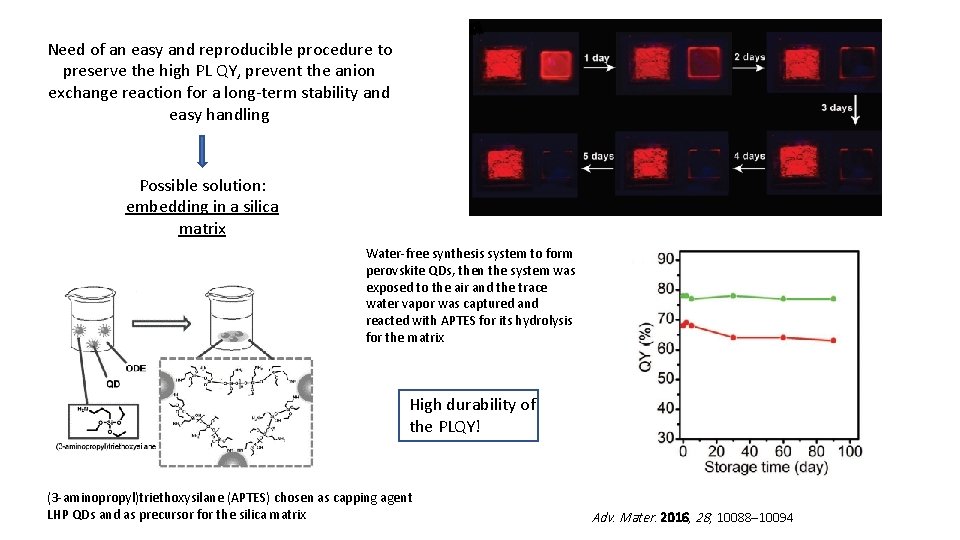
Need of an easy and reproducible procedure to preserve the high PL QY, prevent the anion exchange reaction for a long-term stability and easy handling Possible solution: embedding in a silica matrix Water-free synthesis system to form perovskite QDs, then the system was exposed to the air and the trace water vapor was captured and reacted with APTES for its hydrolysis for the matrix High durability of the PLQY! (3 -aminopropyl)triethoxysilane (APTES) chosen as capping agent LHP QDs and as precursor for the silica matrix Adv. Mater. 2016, 28, 10088– 10094

Cons: 2) Toxicity, hot topic The European Restriction of Hazardous Substances Directive (Ro. HS) limits the use of heavy-metalbased substances in electrical and electronic equipment. The current limit for lead is 1, 000 ppm by weight in any continuous solid component of the device Defect-tolerant semiconductors are those in which both the s and p electrons of a metal hybridize to form the valence and conduction bands. • Sn 2+ and Ge 2+ compounds (Cs. MX 3): analogues of LHPs do exhibit bright emissions but are highly unstable • Bi 3+ and In 3+ compounds (Cs 3 M 2 X 9): typically crystallize into 0 D or 2 D networks of M–X polyhedral and have indirect bandgaps • double perovskite such as Cs 2 Ag. Bi. Cl 6 and Cs 2 Ag. In. Cl 6 (A 2 M 1+M 3+X 6): retain the 3 D interconnection of octahedra but have poor photophysical characteristics

Possible applications • LEDs • LCD downconverters • Single photon emitters • Solar cells • Photodetectors Nature Materials, 2018, 17, 394– 405

Challenge Fundamental Science Gap Industrial Requirements PL Display Precursor High Temperature reaction Purified QD solution Roll to roll coating QDEF Inkjet printing 索尼 EL Display 16 Key issues: stability, high cost and complicated process

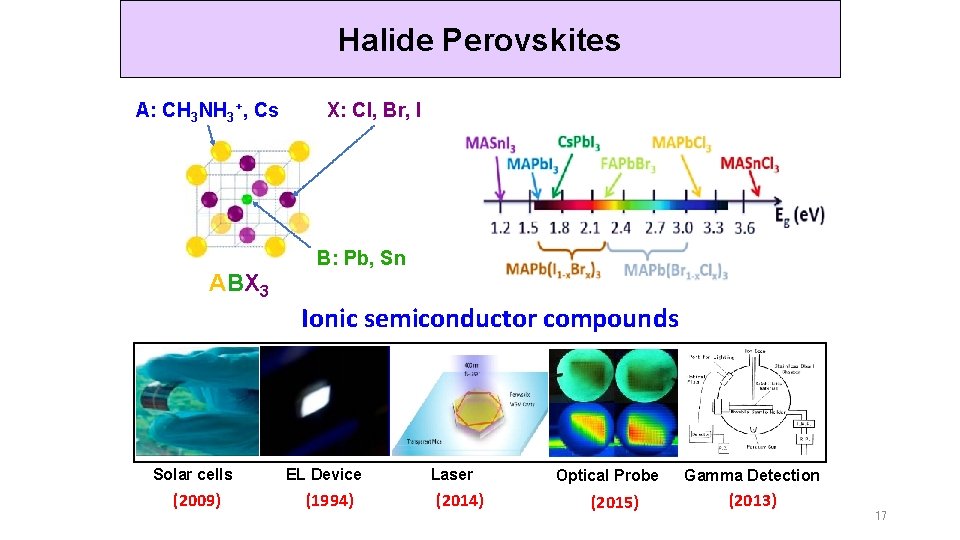
Halide Perovskites A: CH 3 NH 3+, Cs ABX 3 X: Cl, Br, I B: Pb, Sn Ionic semiconductor compounds Solar cells (2009) EL Device (1994) Laser (2014) Optical Probe (2015) Gamma Detection (2013) 17

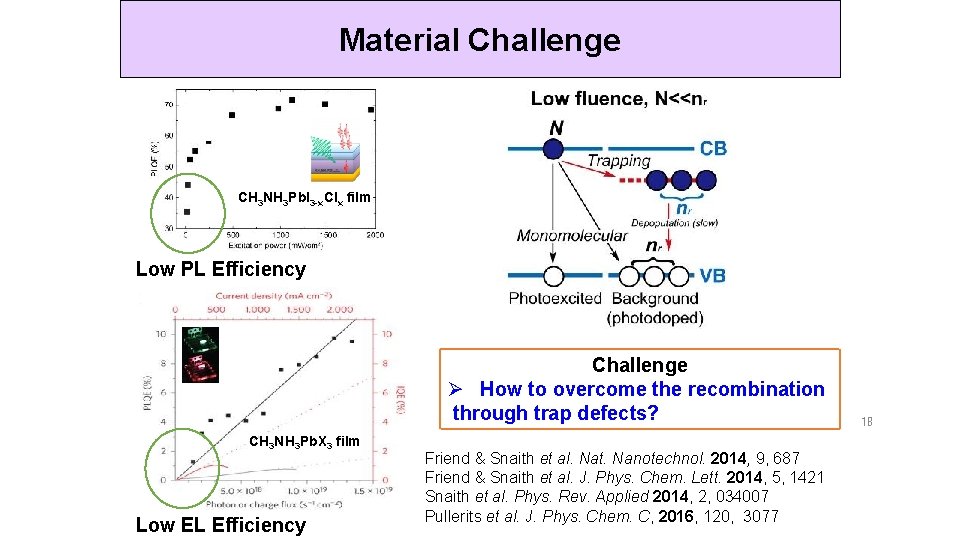
Material Challenge CH 3 NH 3 Pb. I 3 -x. Clx film Low PL Efficiency Challenge Ø How to overcome the recombination through trap defects? CH 3 NH 3 Pb. X 3 film Low EL Efficiency Friend & Snaith et al. Nat. Nanotechnol. 2014, 9, 687 Friend & Snaith et al. J. Phys. Chem. Lett. 2014, 5, 1421 Snaith et al. Phys. Rev. Applied 2014, 2, 034007 Pullerits et al. J. Phys. Chem. C, 2016, 120, 3077 18

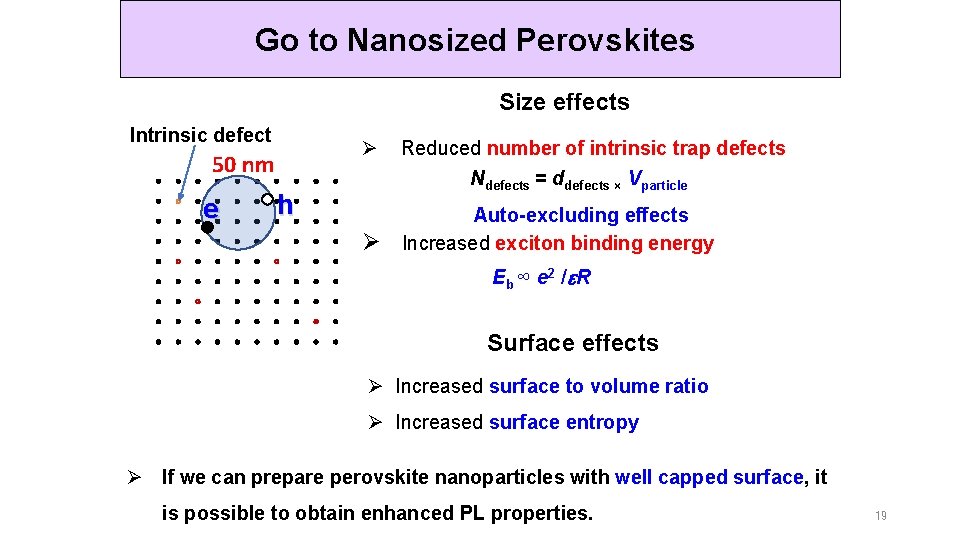
Go to Nanosized Perovskites Size effects Intrinsic defect Ø 50 nm e Reduced number of intrinsic trap defects Ndefects = ddefects × Vparticle h Ø Auto-excluding effects Increased exciton binding energy E b ∞ e 2 / e R Surface effects Ø Increased surface to volume ratio Ø Increased surface entropy Ø If we can prepare perovskite nanoparticles with well capped surface, it is possible to obtain enhanced PL properties. 19

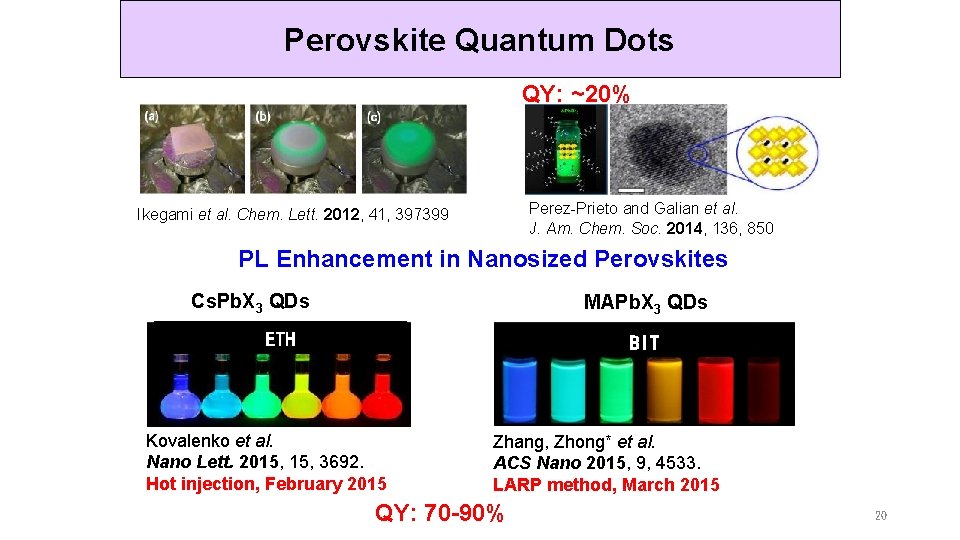
Perovskite Quantum Dots 3、国内外研究现状 QY: ~20% Perez-Prieto and Galian et al. J. Am. Chem. Soc. 2014, 136, 850 Ikegami et al. Chem. Lett. 2012, 41, 397399 PL Enhancement in Nanosized Perovskites Cs. Pb. X 3 QDs MAPb. X 3 QDs ETH BIT Kovalenko et al. Nano Lett. 2015, 3692. Hot injection, February 2015 Zhang, Zhong* et al. ACS Nano 2015, 9, 4533. LARP method, March 2015 QY: 70 -90% 20

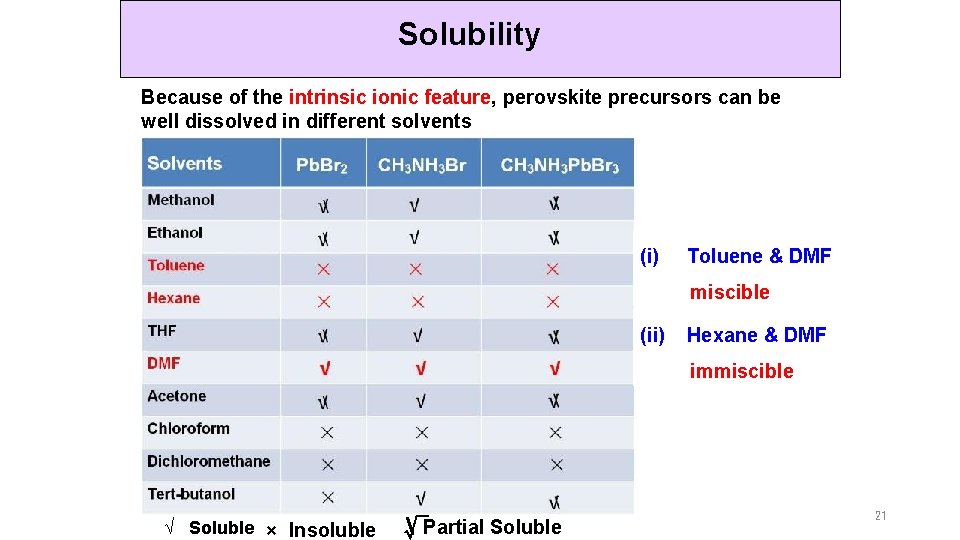
Solubility Because of the intrinsic ionic feature, perovskite precursors can be well dissolved in different solvents (i) Toluene & DMF miscible (ii) Hexane & DMF immiscible √ Soluble × Insoluble √Partial Soluble 21

LARP Synthesis LARP technique: Ligand-assisted Reprecipitation Synthesis By Feng Zhang and Xian-gang Wu Precursor in DMF ① Toluene CH NH 3 3 I Pb. I 2 ② CH 3 NH octyla 3 I , Pb. I 2 m oleic PLQY: < 0. 1, Size: 2 -8 mm ine acid Good solvent: DMF Poor solvent: Toluene Zhang, Zhong* et al. ACS Nano, 2015, 9, 4533 March 2015 Google Citation: 903 times PLQY: 70 -90%, Size: 3. 3 nm 22

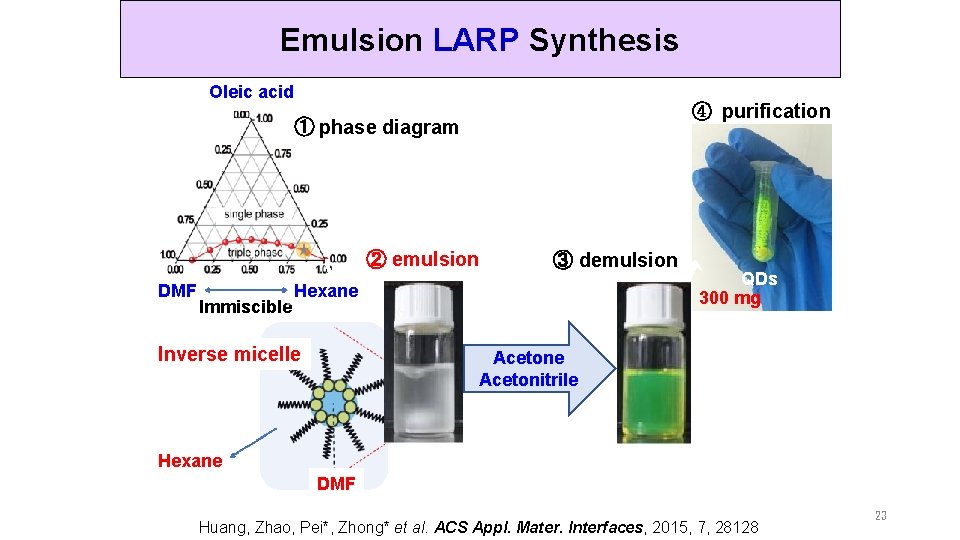
Emulsion LARP Synthesis Oleic acid ④ purification ① phase diagram ② emulsion DMF Immiscible ③ demulsion Hexane Inverse micelle QDs 300 mg Acetone Acetonitrile Hexane DMF Huang, Zhao, Pei*, Zhong* et al. ACS Appl. Mater. Interfaces, 2015, 7, 28128 23

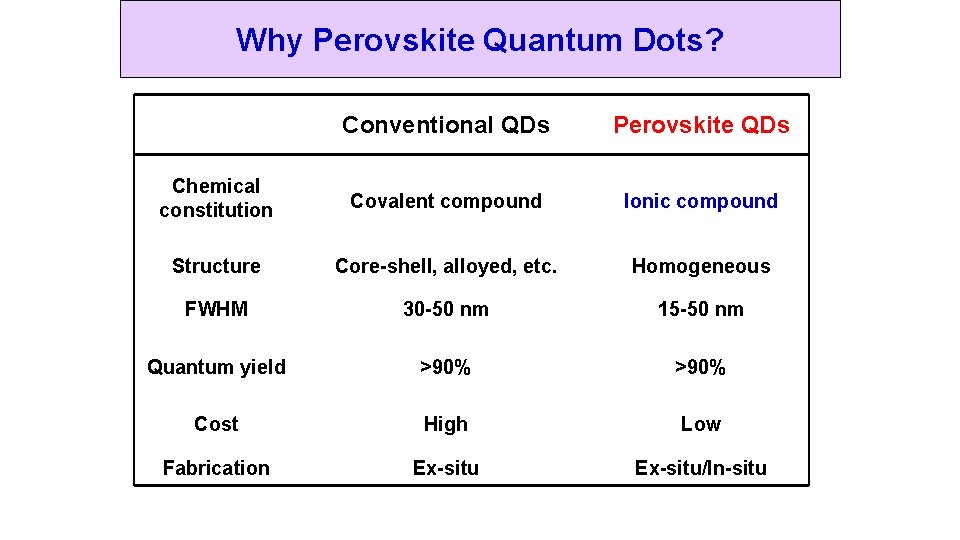
Why Perovskite Quantum Dots? Conventional QDs Perovskite QDs Chemical constitution Covalent compound Ionic compound Structure Core-shell, alloyed, etc. Homogeneous FWHM 30 -50 nm 15 -50 nm Quantum yield >90% Cost High Low Fabrication Ex-situ/In-situ

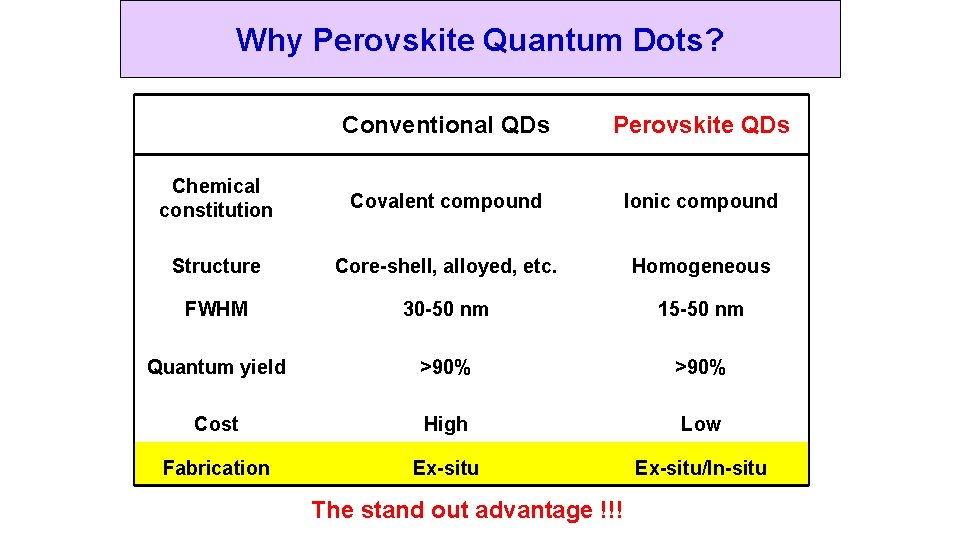
Why Perovskite Quantum Dots? Conventional QDs Perovskite QDs Chemical constitution Covalent compound Ionic compound Structure Core-shell, alloyed, etc. Homogeneous FWHM 30 -50 nm 15 -50 nm Quantum yield >90% Cost High Low Fabrication Ex-situ/In-situ The stand out advantage !!!

In-Situ Fabrication Chang, Bai and Zhong* et al. Advanced Optical Materials 2018, 6, 1800380 26

Progress 1. In-situ Fabrication of Perovskite Quantum Dots Film for Electroluminescence Applications 2. In-situ Fabrication of Perovskite Quantum Dots embedded Composite Films (PQDCF) for Photonic Applications 3. In-situ Formation of Cs. Pb. Br 3 Quantum Dots embedded Cs 4 Pb. Br 6 Single Crystals for Photonic Applications 27

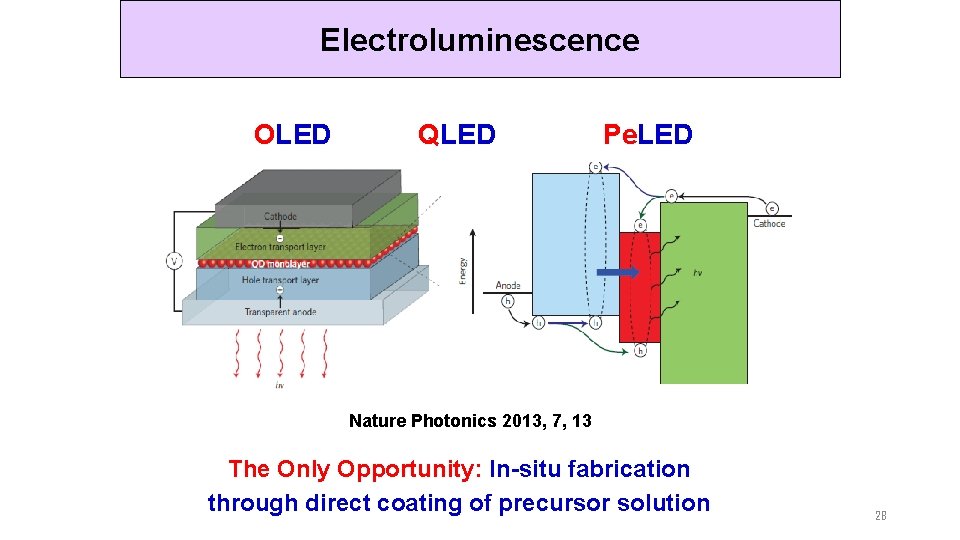
Electroluminescence OLED QLED Pe. LED Nature Photonics 2013, 7, 13 The Only Opportunity: In-situ fabrication through direct coating of precursor solution 28

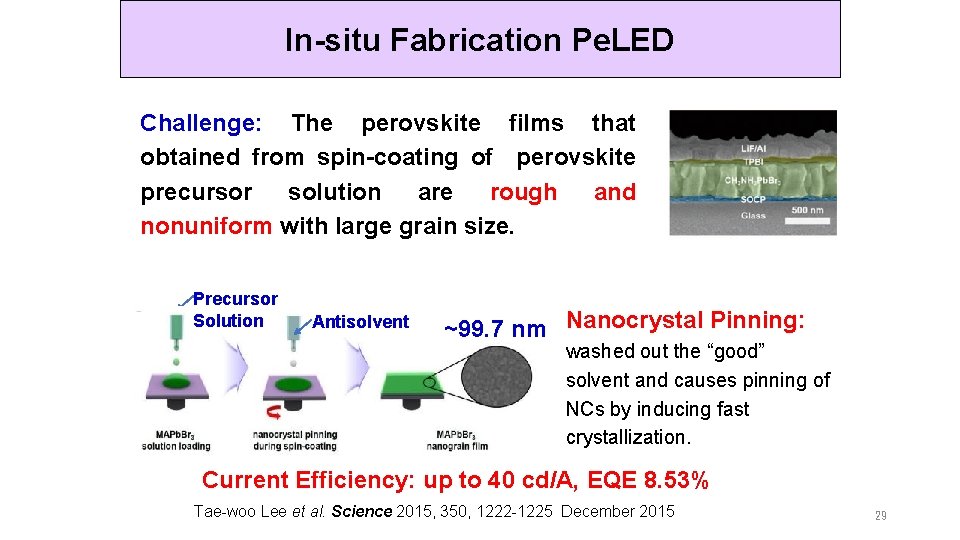
In-situ Fabrication Pe. LED Challenge: The perovskite films that obtained from spin-coating of perovskite precursor solution are rough and nonuniform with large grain size. Precursor Solution Antisolvent ~99. 7 nm Nanocrystal Pinning: washed out the “good” solvent and causes pinning of NCs by inducing fast crystallization. Current Efficiency: up to 40 cd/A, EQE 8. 53% Tae-woo Lee et al. Science 2015, 350, 1222 -1225 December 2015 29

Nanocrystal Pinning Precursor Solution: MABr, Pb. Br 2, Ligand in DMF Anti-solvent: Toluene Green PL Liquid Precursor Film Nucleation & Growth 30

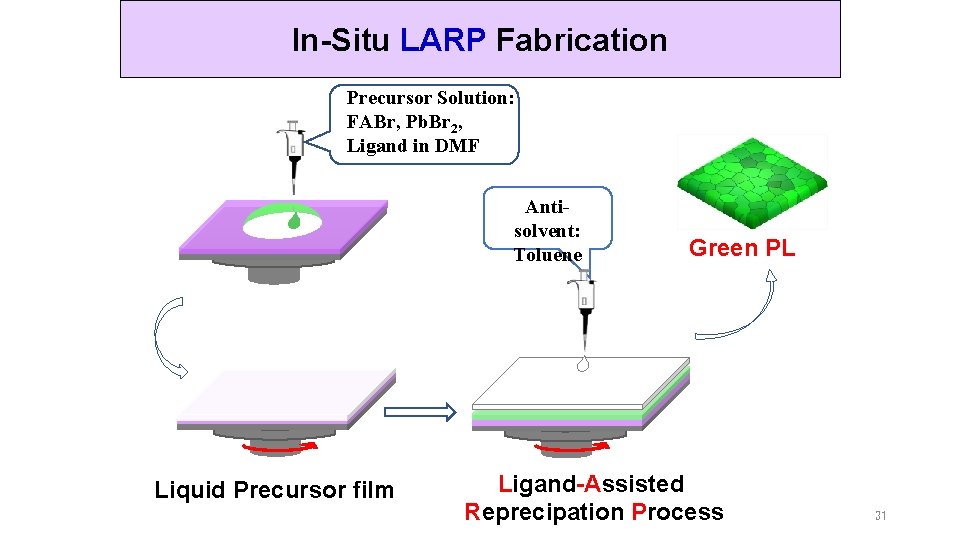
In-Situ LARP Fabrication Precursor Solution: FABr, Pb. Br 2, Ligand in DMF Antisolvent: Toluene Liquid Precursor film Green PL Ligand-Assisted Reprecipation Process 31

In-Situ Fabricated Perovskite Films Size: 10 -20 nm PL QYs: Up to 78% S 1 LARP S 2 S 3 NCP S 4 RMS: 1. 6 nm Han, Muhammad, Wang*, Zou* and Zhong* et al. ACS Nano 2018, 12, 8808− 8816 32

Electroluminescence Devices Max Current Efficiency : up to 66 Cd/A Max EQE : 16. 3% ITO/PEDOT: PSS/TFB/PQD/TPBi/Al Han, Muhammad, Wang*, Zou* and Zhong* et al. ACS Nano 2018, 12, 8808− 8816 33

TEM Characterization 14. 4 nm 19. 7 nm 60. 6 nm Zhang, Han, Chang* and Zhong* et al. Advanced Optical Materials 2019, 1900774

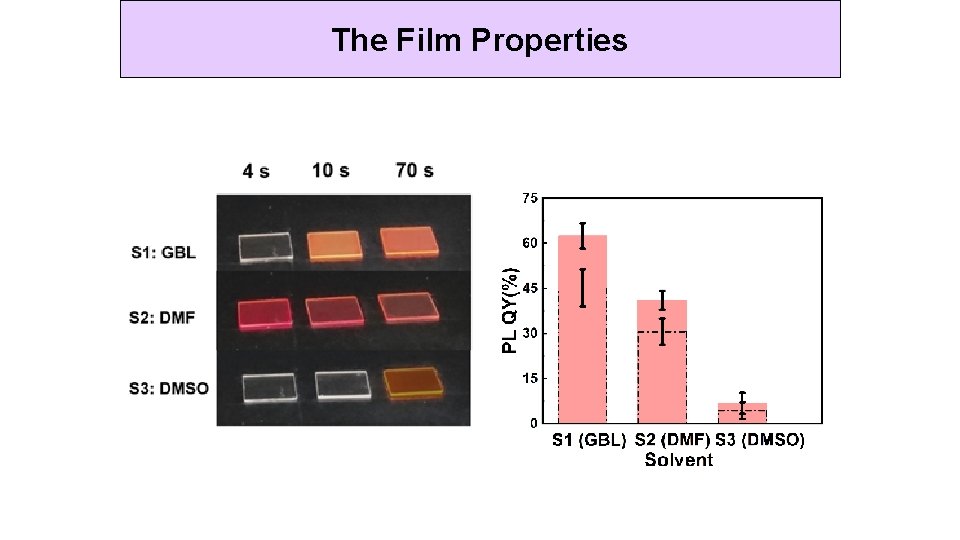
The Film Properties

In-situ Fabricated FAPb. I 3 PNCs Max EQE 15. 8% Zhang, Han, Chang* and Zhong* et al. Advanced Optical Materials 2019, 1900774 36

Display Backlights QDs in Glass Tube QDs in Films QDs on Chips Quantum Dots Enhanced Films ECS J. Solid State Sci. Technol. 2013, 2, R 3026 37

QDs Enhanced Color Convention Films By Qingchao Zhou High cost !!! “in-situ” fabrication Strategy Zhou and Zhong* et al. Adv. Mater. 2016, 28, 9163 -9168 38

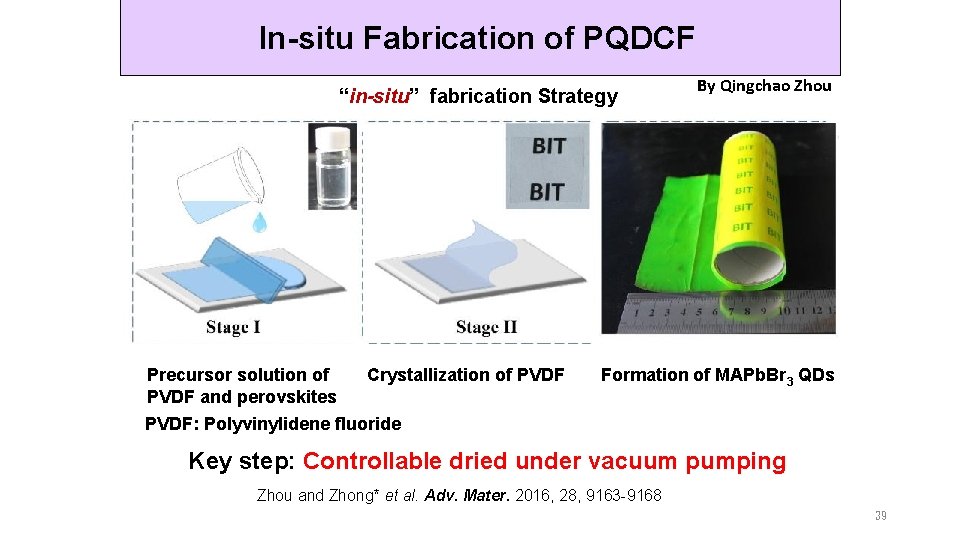
In-situ Fabrication of PQDCF “in-situ” fabrication Strategy Precursor solution of PVDF and perovskites Crystallization of PVDF By Qingchao Zhou Formation of MAPb. Br 3 QDs PVDF: Polyvinylidene fluoride Key step: Controllable dried under vacuum pumping Zhou and Zhong* et al. Adv. Mater. 2016, 28, 9163 -9168 39

Scale-up Fabrication 1. 5 r e t e m Developed by Zhijing Nanotech & Hefei Lucky Co. Ltd. 40

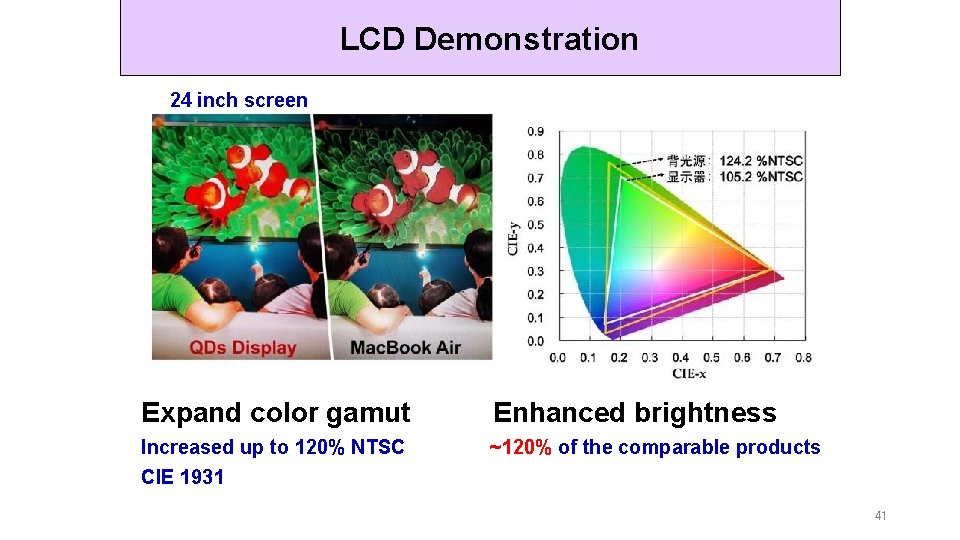
LCD Demonstration 24 inch screen Expand color gamut Enhanced brightness Increased up to 120% NTSC CIE 1931 ~120% of the comparable products 41

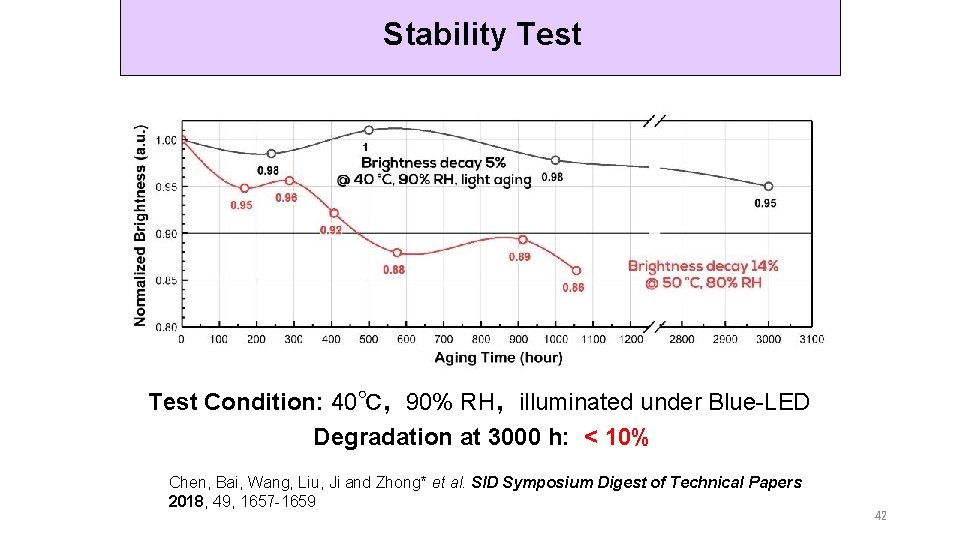
Stability Test Condition: 40℃,90% RH,illuminated under Blue-LED Degradation at 3000 h: < 10% Chen, Bai, Wang, Liu, Ji and Zhong* et al. SID Symposium Digest of Technical Papers 2018, 49, 1657 -1659 42