LEACHING TECHNIQUE Leaching Techniques In situ and modified








































- Slides: 63

LEACHING TECHNIQUE

Leaching Techniques • • • In situ and modified in situ Leaching Heap and Dump Leaching Vat Leaching Agitation Leaching Pressure Leaching Bacterial Leaching

In situ and modified in situ leaching A. Insitu leaching Also called solution mining Its about dissolution of metal values from minerals present in the undisturbed ore body in place. The leaching is done where the ore is located. Hence, the term in situ leaching. – The cost to carry out in situ leaching is lower compared to other leaching schemes.

§ The process initially involves drilling of holes into the ore deposit. § Explosive or hydraulic fracturing may be used to create open pathways in the deposit for solution to penetrate. § Leaching solution is pumped into the deposit where it makes contact with the ore. § The solution bearing the dissolved ore content is then pumped to the surface and processed. § This process allows the extraction of metals and salts from an ore body without the need for conventional mining involving drill-and-blast, open-cut or underground mining.

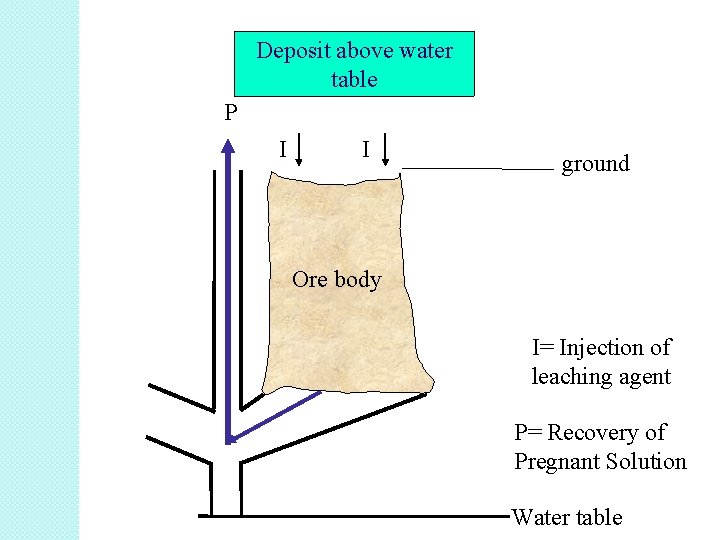
Steps in in situ leaching: 1. Injection of the leaching agent into the ore body 2. Dissolution of the metal values by the leaching agent 3. Recovery of the pregnant solutions for processing above ground

Deposit above water table P I I ground Ore body I= Injection of leaching agent P= Recovery of Pregnant Solution Water table


Successful application of in situ leaching requires: The ore body should be permeable to the solution • The ore body should be bounded by relatively impermeable strata to avoid loss of solution • The deposit must be below water table so that the leach liquor does not mix with natural ground water (modified in situ leaching) •

• If mixing occurs, this leads to metal loss and contamination of ground water. • This leaching techique requires the drilling of a monitor well around the ore body periphery. • The wells are used during and after mining to check on the possible ground water contamination by the mining solutions.

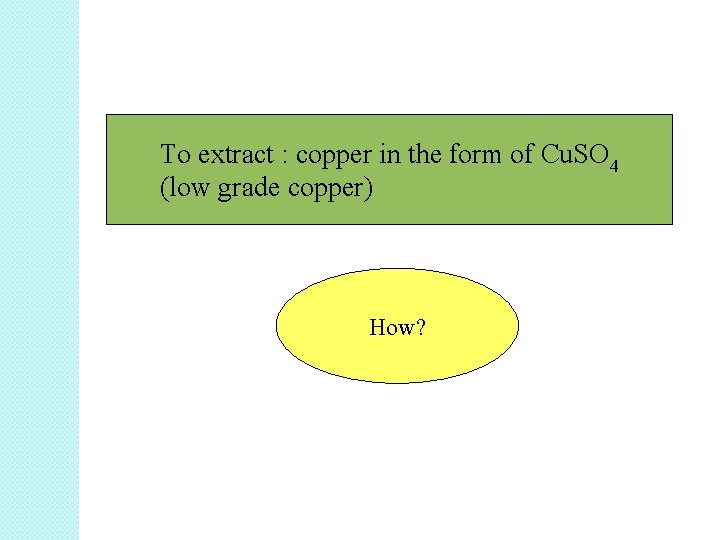
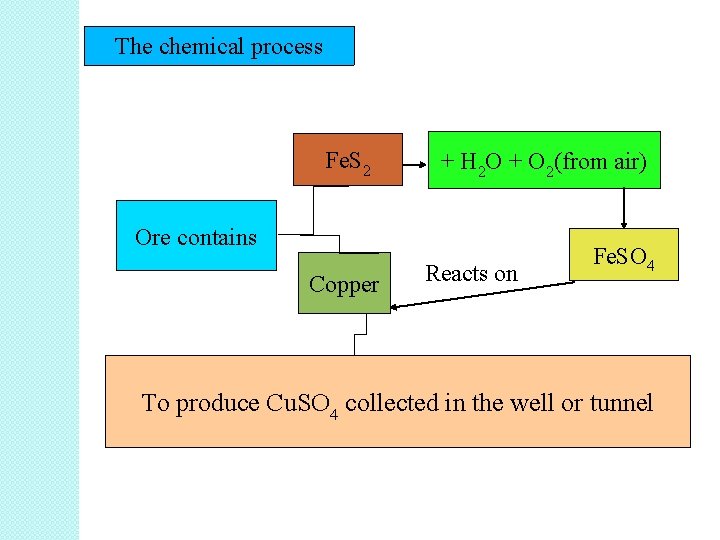
• In situ leaching is suitable for the extraction of low-grade ores i. e copper, gold, silver and uranium. • Example of in situ leaching ore: copper ore • Other compound inside the ore: iron sulfide, Fe. S 2

To extract : copper in the form of Cu. SO 4 (low grade copper) How?

The chemical process Fe. S 2 + H 2 O + O 2(from air) Ore contains Copper Reacts on Fe. SO 4 To produce Cu. SO 4 collected in the well or tunnel

• This process occurs over weeks or years to complete especially the oxidation reaction.

B. Modified In Situ Leaching Prerequisites for modified in situ leaching: • If the ore body is not permeable enough to the solution, it has to be fractured with explosives to increase permeability. • Hence , it is being termed modified in situ leaching. • Blasting with explosives increases surface area of the ore action.

5 cases of in situ leaching: 4 modified in situ leaching and 1 true in situ leaching 1. 2. Deposits above water table. For case 2 A and 2 B, the deposits are rubblized using explosives or mining, hydrofractured or chemical-induced porosity.

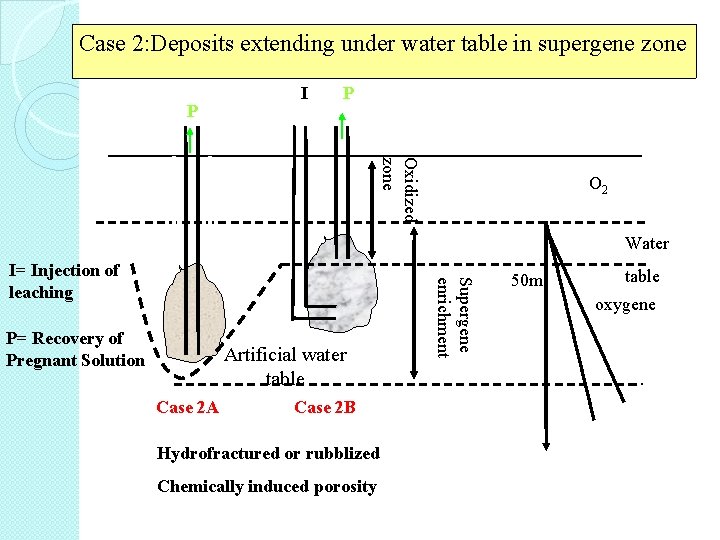
Case 2: Deposits extending under water table in supergene zone I P P Oxidized zone Water I P= Recovery of Pregnant Solution Artificial water table Case 2 A Case 2 B Hydrofractured or rubblized Chemically induced porosity Supergene enrichment I= Injection of leaching agent I O 2 table 50 m oxygene air

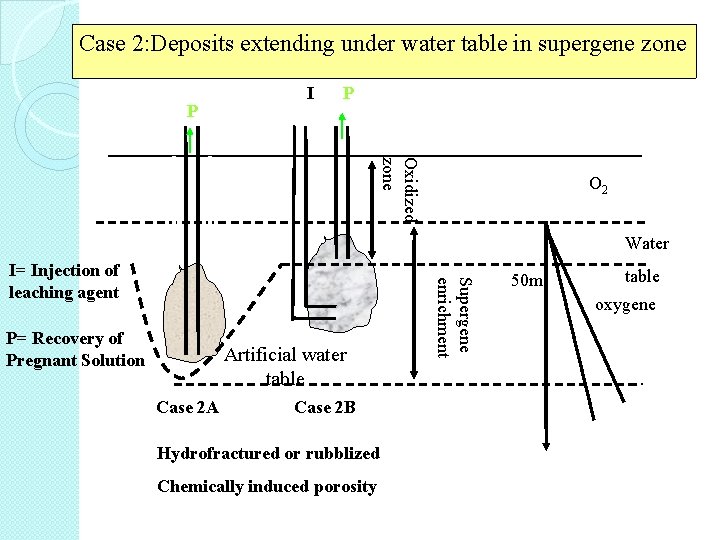
Case 2 A • an artificially depressed water table produced by pumping, with or without using grouting to determine water inflow. • this deposit if sufficiently rubblized, due to percolation leached. �

Case 2 B • deposit has been rubblized by partial mining or fracturing by using either explosives, hydraulic pressures (hydrofracturing) or chemicals • leaching solutions are being injected through a well into the bottom of the deposit and rise upward. �

Case 2: Deposits extending under water table in supergene zone I P P Oxidized zone Water I P= Recovery of Pregnant Solution Artificial water table Case 2 A Case 2 B Hydrofractured or rubblized Chemically induced porosity Supergene enrichment I= Injection of leaching agent I O 2 table 50 m oxygene air

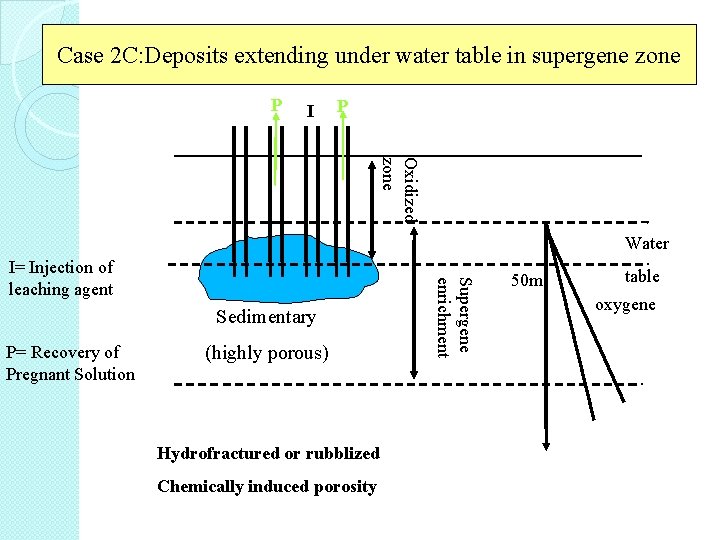
Case 2 C • highly porous deposit with high natural porosity • the presence of natural porosity permits flow of leaching solution • this requires many years to complete the leaching of an ore body, during which time numerous bore holes would be drilled, operated and finally closed. �

Case 2 C: Deposits extending under water table in supergene zone I P Oxidized zone Water Sedimentary P= Recovery of Pregnant Solution (highly porous) Hydrofractured or rubblized Chemically induced porosity Supergene enrichment I= Injection of leaching agent table 50 m oxygene air

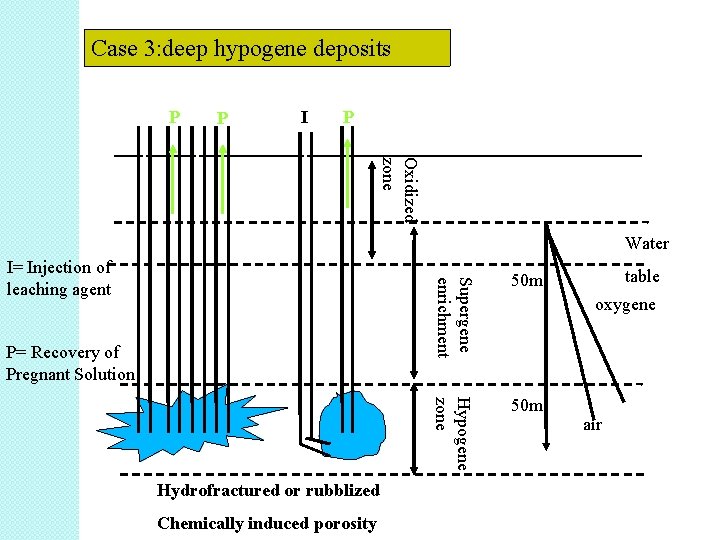
• Case 3 – represent deep hypogene deposit. – is a variation of case 2 B. – deposit is well below the water table. – deposit is not accesible by mining. – therefore, nuclear devices are used to fracture deep-seated deposits.

Case 3: deep hypogene deposits I P I P Oxidized zone Water Hydrofractured or rubblized Chemically induced porosity 50 m Hypogene zone P= Recovery of Pregnant Solution Supergene enrichment I= Injection of leaching agent 50 m table oxygene air

• Major problems of modified in situ leaching: blockage of the well. 2. loss of permeability of the ore body due to the precipitation of insoluble compounds. 1.

For example, • During in situ leaching of uranium ore with sulfuric acid, the precipitation of calcium sulfate can result in a significant drop in the permeability of the ore. • Therefore, ammonium carbonate is preferred to sulfuric acid as it is selective towards uranium minerlas and does not generate impermeable deposits. •

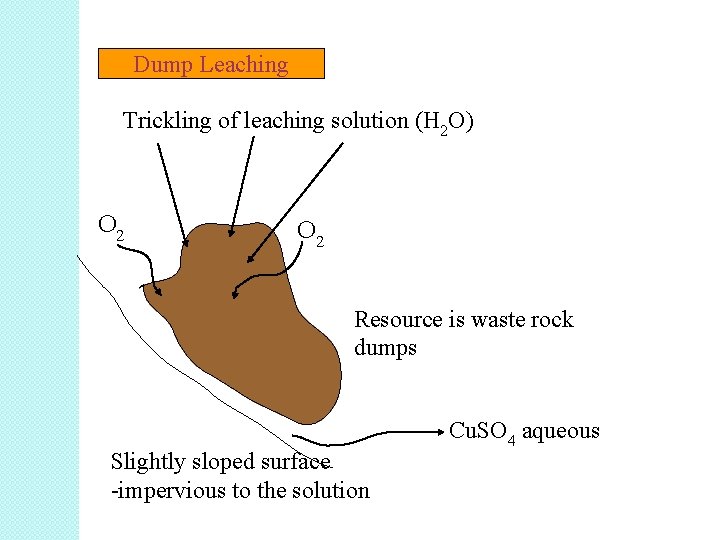
• 2. 3. 6. 2 Heap and Dump Leaching A. Dump Leaching � Dump leaching is similar to heap leaching, however in the case of dump leaching ore is taken directly from the mine and stacked on the leach pad without crushing. � In the case of gold and silver, the dump is irrigated with a dilute cyanide solution that percolates through the ore to dissolve gold and silver. � The solution containing gold and silver exits the base of the dump, is collected and precious metals extracted. The resultant barren solution is recharged with additional cyanide and returned to the dump.

� This method of leaching is usually suitable for low grade ores because it is very low cost. However, it operates with slow kinetics and may take up about 1 to 2 years to extract 50% of the desired mineral. � The resource: The waste rock dump originating from the mining and milling operations of the ore. The particle size of the material is generally big and the ore is processed for many years by sprinkling acidified water on the dump surface.

Example of dump leaching: Recovery of copper from huge dump of mined waste. • Cu. S + 2 O 2 Cu. SO 4 • As the leaching agent trickles down through the dump, copper passes into solution as copper sulfate. • The oxygen passes into the dump via the cracks between the particles. • The dump assembles on an impermeable (resistant) surface to the solution and slightly sloped from one side. • Or the dump is moved to such surface.

Dump Leaching Trickling of leaching solution (H 2 O) O 2 Resource is waste rock dumps Slightly sloped surface -impervious to the solution Cu. SO 4 aqueous

• The leach solution percolates through the dump and is collected in channels at the base of the dump. • It can be considered to be a crude version of heap leaching where no special arrangements are made to increase the leaching rate. Therefore, metal recoveries from dump leaching operations are low and the duration of the operation is relatively long. • Problems may arise if the base of the dump is not properly prepared, causing losses of solution. This might become a source of environmental pollution if leachate escapes collection and flows into natural water-supplies. • The initial plants for copper recovery by dump leaching from oxide ore were a success and led to the development of heap leaching for ores with higher grade.

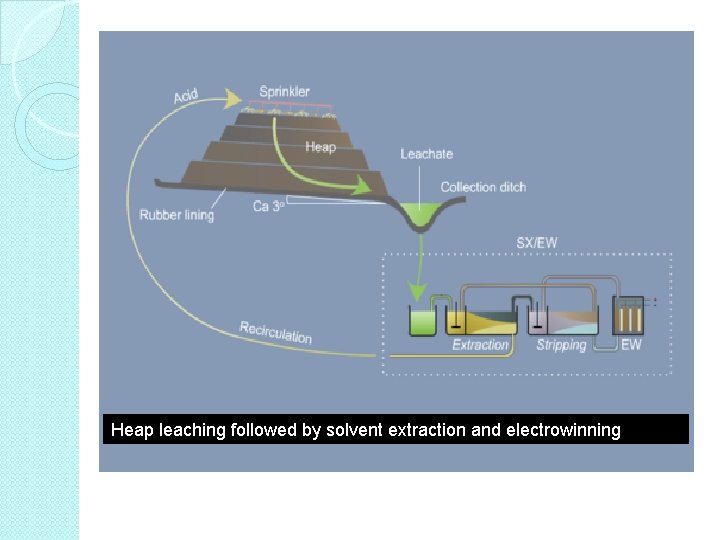
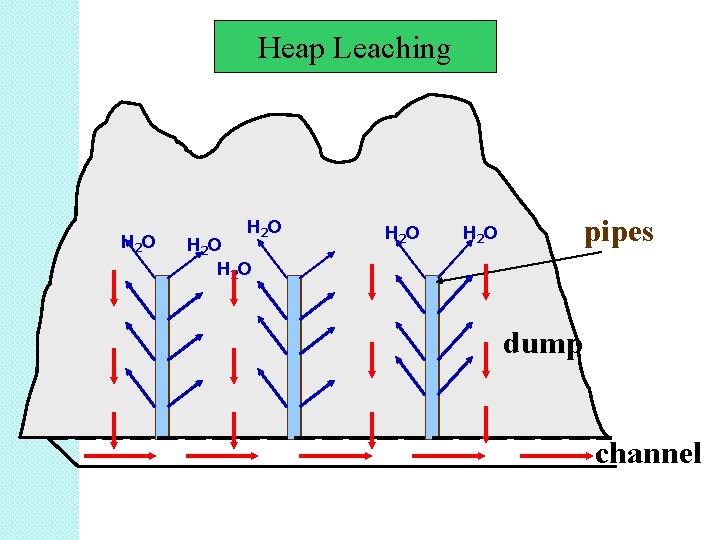
B. Heap Leaching � Heap leaching is, in contrast to many dump leaching operations, a pre-planned process where arrangements are made to optimize conditions for leaching. � In brief, the mineral ore or concentrate is piled in a heap and lixiviate fluid is distributed over the surface to leach metal from the heap. � Heap leaching is used to leach low-grade ores. � Low-grade ores are broken and piled into small heaps on impervious ground/asphalted surface/concrete.

• Heap leaching takes months rather than years as for dump leaching. • The reaction conditions in a heap varies from top to bottom, from core to surface and sometimes also locally in the heap. • The surface has drainage channels and pipes to carry away the pregnant solution to a collection pond. • Good application in the processing of gold, silver, uranium and copper ores.

Heap leaching followed by solvent extraction and electrowinning

Heap Leaching H 2 O H 2 O pipes dump channel

Steps in Heap Leaching 1. 2. 3. 4. 5. The soil on a slightly sloping ground is first compacted and then covered with an impermeable pad like an asphalt layer or a flexible plastic sheet. Crushed ore is stacked in big heaps on the pad. Fine particles are agglomerated to increase permeability. The heap is sprayed with leaching reagent. As the reagent percolates through the heap the wanted metals are solubilized. The leachate (metal containing solution) drained from the heap is collected in a pond and the solution is subsequently sent for metal recovery.

Summary operation of heap leaching 1. 2. 3. 4. 5. The construction of leaching pads The formation of the heaps The distribution of the lixiviants The collection of the leach liquor in the solution pond The recirculation of the barren solution to the heaps after the recovery of the metal values.

Important factors for a successful heap leaching operation are: �Maintain a good permeability. Precipitates like gypsum, ferric hydroxides and clay minerals might cause clogging and flooding. �A good water balance. Sprinklers or drippers buried in the heap are used to deliver the leaching solution over the heap. �p. H control �A steady supply of leaching reagents.

Mine truck dumps ore on leach pad. The Pregnant Solution Pond, collecting the liquid from the leach pad Impermeable lining will prevent any leach liquid from penetrating ground.

Heap leaching is done widely in Spain to leach Cu and Zn from pyrite ore. So, what happens to the pyrite ore?

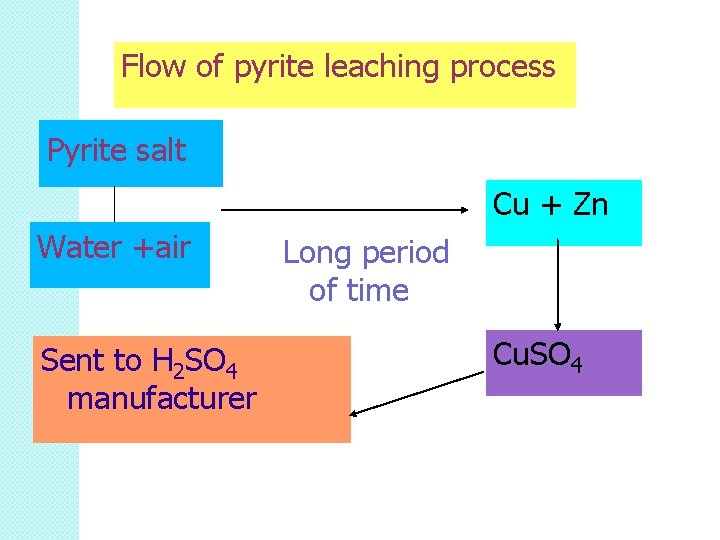
Pyrite ore is sprayed with water and left for a long time. This allows chemical reaction between water, air and pyrite salt.

Reaction occurs until almost all Cu is changed to Cu. SO 4 Once leaching completed, the product: Cu. SO 4 is transferred to a tank and sent to H 2 SO 4 manufacturer.

Flow of pyrite leaching process Pyrite salt Cu + Zn Water +air Sent to H 2 SO 4 manufacturer Long period of time Cu. SO 4

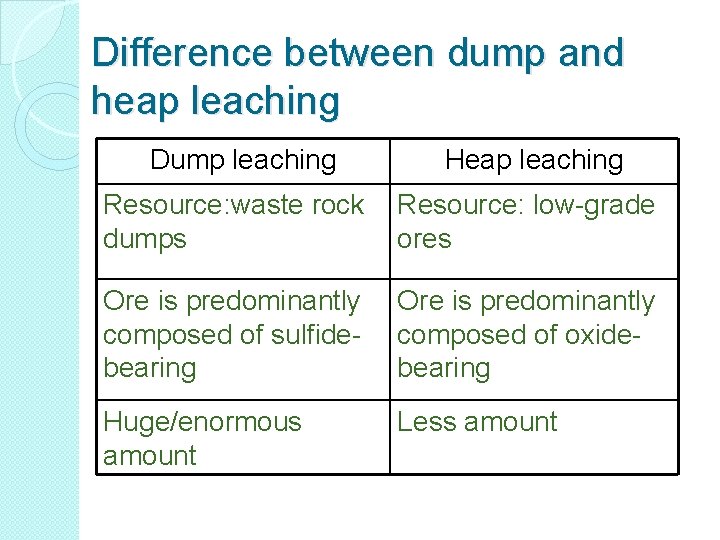
Difference between dump and heap leaching Dump leaching Heap leaching Resource: waste rock dumps Resource: low-grade ores Ore is predominantly composed of sulfidebearing Ore is predominantly composed of oxidebearing Huge/enormous amount Less amount

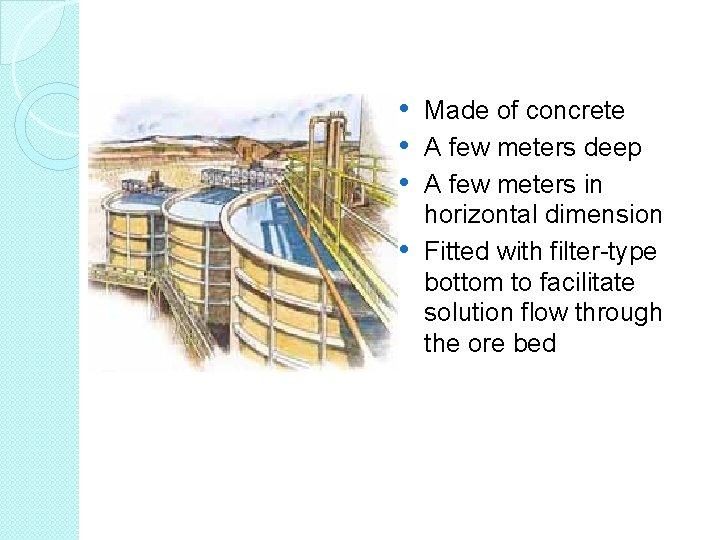
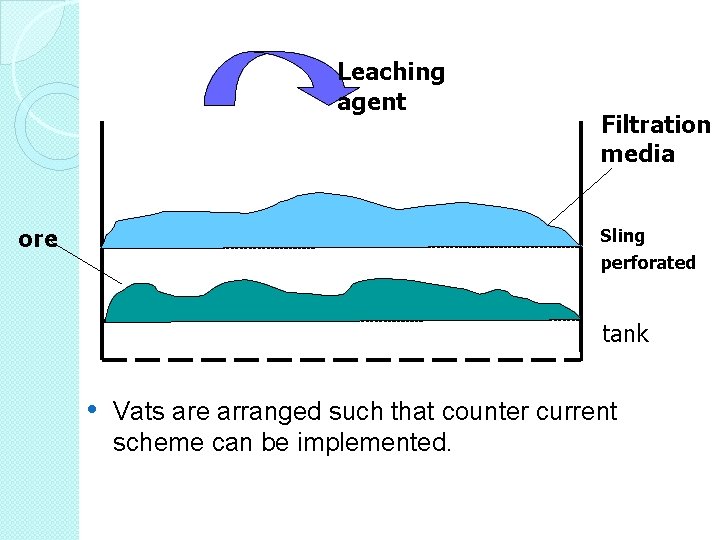
Vat Leaching A more complex leaching technique � Ore to leached is loaded into vats. � Vat leach units are rectangular containers (drums, barrels, tanks or vats), usually very big and made of wood or concrete, lined with material resistant to the leaching media. � The treated ore is usually coarse. The leaching reagents may be added to the leaching object in different ways. � What are the vats?

• Made of concrete • A few meters deep • A few meters in • horizontal dimension Fitted with filter-type bottom to facilitate solution flow through the ore bed

• It involves placing ore, usually after size reduction and classification, in large tanks or vats containing a leaching solution. • Often the vats are equipped with agitators to keep the solids in suspension in the vats and improve the solid to liquid contact. • This is further assisted by the use of tank baffles to increase the efficiency of agitation and prevent centrifuging of slurries in circular tanks. • After vat leaching, the leached solids and pregnant solution are usually separated prior to further processing. •

Leaching agent ore Filtration media Sling perforated tank • Vats are arranged such that counter current scheme can be implemented.

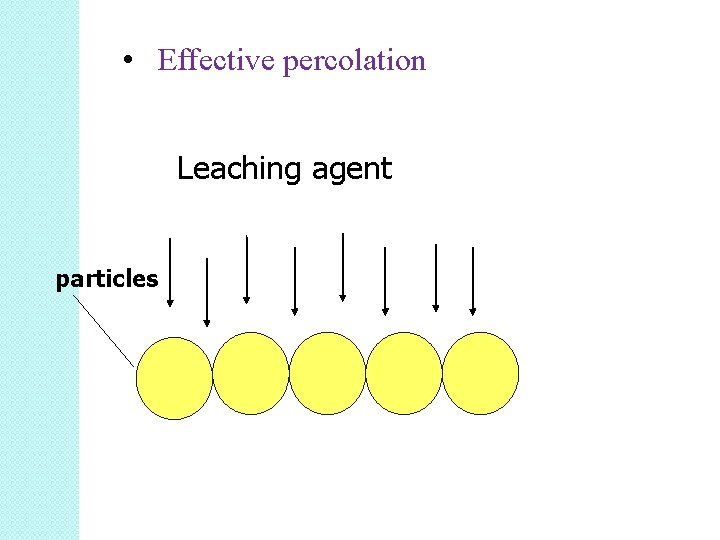
Vat leaching is classified into agitation leaching. • Vat leaching is not suitable for materials which have the tendency to pack into impervious masses • Good percolation results from regularly sized particles •

• Effective percolation Leaching agent particles

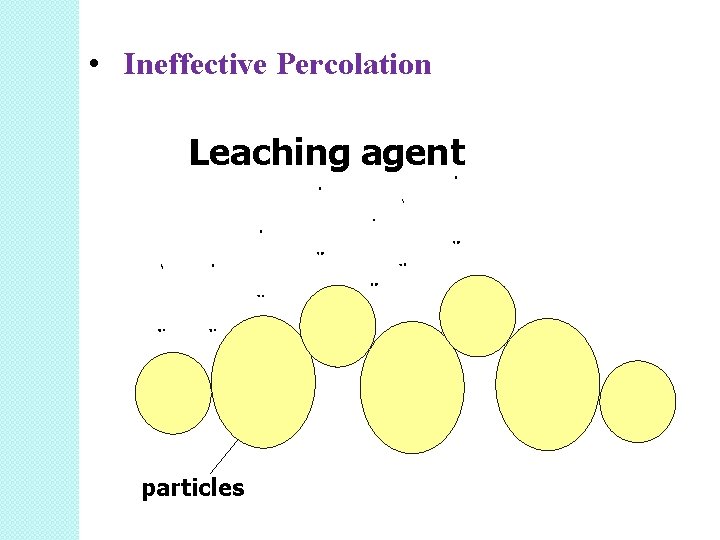
Good percolation is not achieved if the particles of unequal sizes. • This is because small particles tend to pack in the openings of the larger-sized particles. • This results in the clogging of the channels. • This slows down extraction •

• Ineffective Percolation Leaching agent particles

• Advantages of vat leaching: Low solvent consumption. q the production of good grade leach solution. q elimination of the use of thickener or filters. q

Example of vat leaching: q Ores: copper oxide q Leaching agent: dilute sulfuric acid q Product: Copper sulfate solution q

Agitation leaching �Agitation leaching is a process where the soil is slurried with the extraction fluid for a period of time. �When equilibrium between the metal on the soils surface and the metal contained by the solution is approached, the solubilization of the metal in the soil is slowed, and the extraction is considered to be complete.

�At equilibrium, additional metal will not be extracted from the soils surface unless the soil is subjected to fresh extraction solution. �Once the process is considered to be at equilibrium, the soil is separated from the extraction fluid using sedimentation, thickening, or clarification. �The extraction process may be continued in a separate extraction vat with clean extraction solution to enhance extraction. �An agitation vat coupled with a solid-liquid separation vessel (sedimentation or clarification) is considered to be a single stage


Pressure leaching � Pressure leaching is the chemical dissolution of soluble minerals within a solid ore or concentrate carried out at elevated pressures and giving rise to a solution containing metals to be recovered. � The process is carried out in closed autoclaves which permit higher temperatures (>220°C) and pressures (>20 atm) than are possible with open tanks. � The increased pressure improves the solubility rate of solids and increases the speed of dissolution into the leach solution. Because of the very fast kinetics, the duration of the leaching is short - from 30 minutes up to 24 hours depending on the concentrate being leached and conditions applied.

� The high pressure makes the method ideal for oxidative leaching of sulfides since the content of dissolved oxygen in the leaching solution is directly proportional to the pressure. � Also in acid leaching of sulfides it can be chosen if the sulfide should be converted to elemental sulfur (S°) or sulfate (SO 42 -). At temperatures below 120150°C elemental sulfur is formed which in many cases is a preferred form and also requires less oxidant to be added. � Pressure leaching is applied in the leaching of Co and Ni sulfides with ammonia as leaching reagent, in the so-called Sherrit Gordon process. � Another considerable application of pressure leaching is the Bayer process where bauxite is leached with sodium hydroxide to produce alumina (Al 2 O 3), which is used as raw material for aluminium production by smelt electrolysis.

Bacterial leaching �Bacterial leaching is the extraction of metals from their ores using microorganisms. �Microbial technology offers an economic alternative for the mining industry, at a time when high-grade mineral resources are being depleted.

Why bacterial leaching ? � Worldwide reserves of high-grade ores are diminishing at an alarming rate due to the rapid increase in the demand for metals. � However there exist large stockpiles of low and lean grade ores yet to be mined. � The problem is that the recovery of metals from low and lean grade ores using conventional techniques is very expensive due to high energy and capital inputs required. � Another major problem is environmental costs due to the high level of pollution from these technologies. � Environmental standards continue to stiffen, particularly regarding toxic wastes, so costs for ensuring environmental protection will continue to rise.

�Bacterial ore leaching can be applied to extract heavy metals from low grade ores, industrial wastes and other materials on an industrial scale by different procedures: dump leaching, in situ leaching, tank leaching, leaching in suspension. �Sulfidic copper and uranium ores are the principle ores leached in several countries. So 20% to 25% of the copper production in the U. S. A. and about 5% of the world copper production is obtained by bacterial leaching. �This process is a very slow one and needs a long time (years) for good recovery, but its main advantages are low investment costs and low operating costs.

Examples of bacteria used : �The principal bacterium in ore leaching is Thiobacillus ferrooxidans, which is capable of oxidizing ferrous iron as well as sulfur and sulfur compounds. �But there are some other bacteria which may also be involved. �For example thermophilic Sulfolobus plays a role in leaching at elevated temperatures. �Thiobacillus thiooxidans, which oxidizes merely sulfur and sulfur compounds but not iron. �Leptospirillum ferrooxidans, which contrarily oxidizes only ferrous iron, may play a role if they work together or with other bacteria.

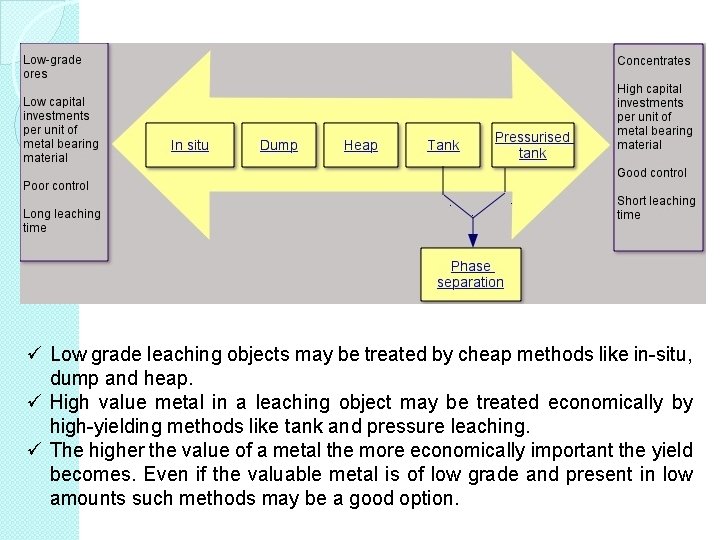
ü Low grade leaching objects may be treated by cheap methods like in-situ, dump and heap. ü High value metal in a leaching object may be treated economically by high-yielding methods like tank and pressure leaching. ü The higher the value of a metal the more economically important the yield becomes. Even if the valuable metal is of low grade and present in low amounts such methods may be a good option.