Le Chteliers Principle If a change is imposed



- Slides: 8

Le Châtelier’s Principle • If a change is imposed on a system at equilibrium, the position of the equilibrium will shift in a direction that tends to reduce that change • If a component (reactant or product) is added to a reaction system at equilibrium (constant T and P or constant T and V), the equilibrium position will shift in the direction that lowers the concentration of that component. If a component is removed, the opposite effect occurs.

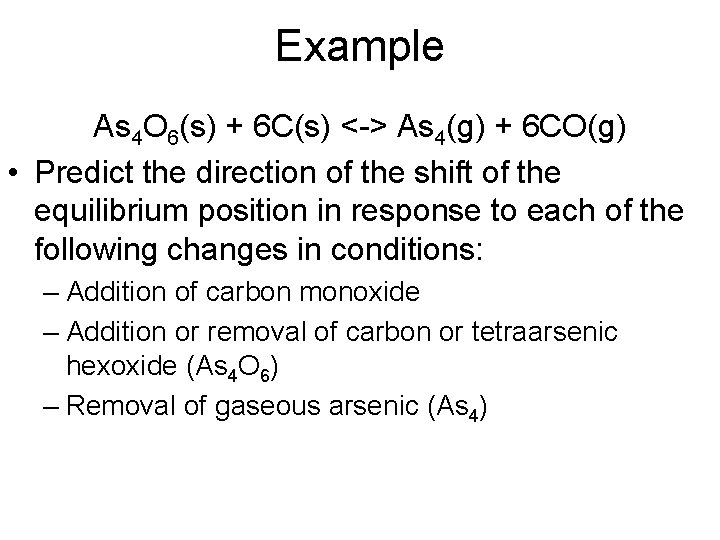
Example As 4 O 6(s) + 6 C(s) <-> As 4(g) + 6 CO(g) • Predict the direction of the shift of the equilibrium position in response to each of the following changes in conditions: – Addition of carbon monoxide – Addition or removal of carbon or tetraarsenic hexoxide (As 4 O 6) – Removal of gaseous arsenic (As 4)

Changing Pressure 1. Add or remove a gaseous reactant or product 2. Add an inert gas (one not involved in the reaction) - won’t affect equilibrium 3. Change the volume of the container**

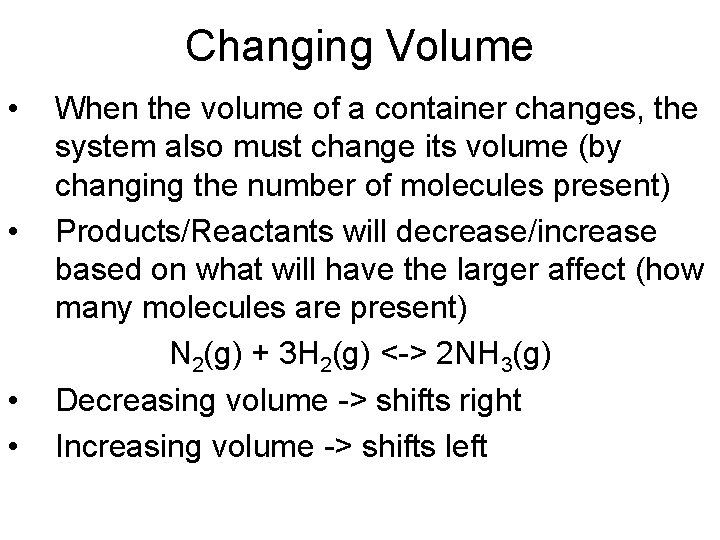
Changing Volume • • When the volume of a container changes, the system also must change its volume (by changing the number of molecules present) Products/Reactants will decrease/increase based on what will have the larger affect (how many molecules are present) N 2(g) + 3 H 2(g) <-> 2 NH 3(g) Decreasing volume -> shifts right Increasing volume -> shifts left

Example • If the volume is reduced, what will happen to the equilibrium? – P 4(s) + 6 Cl 2(g) <-> 4 PCl 3(l) – Answer: Shifts RIGHT – PCl 3(g) + Cl 2(g) <-> PCl 5(g) – Answer: Shifts RIGHT – PCl 3(g) + 3 NH 3(g) <-> P(NH 2)3(g) + 3 HCl(g) – Answer: No Shift

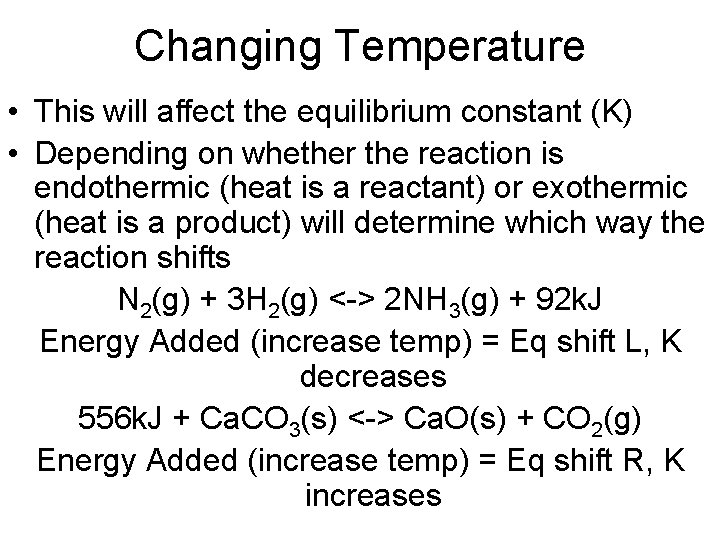
Changing Temperature • This will affect the equilibrium constant (K) • Depending on whether the reaction is endothermic (heat is a reactant) or exothermic (heat is a product) will determine which way the reaction shifts N 2(g) + 3 H 2(g) <-> 2 NH 3(g) + 92 k. J Energy Added (increase temp) = Eq shift L, K decreases 556 k. J + Ca. CO 3(s) <-> Ca. O(s) + CO 2(g) Energy Added (increase temp) = Eq shift R, K increases

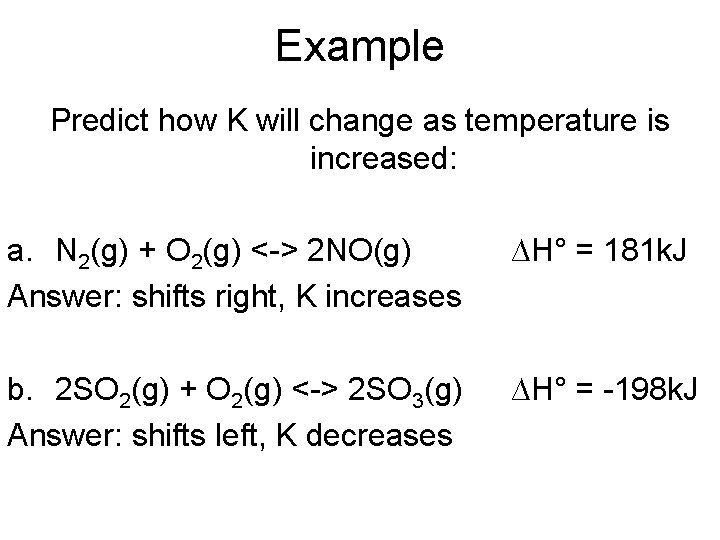
Example Predict how K will change as temperature is increased: a. N 2(g) + O 2(g) <-> 2 NO(g) Answer: shifts right, K increases ∆H° = 181 k. J b. 2 SO 2(g) + O 2(g) <-> 2 SO 3(g) Answer: shifts left, K decreases ∆H° = -198 k. J

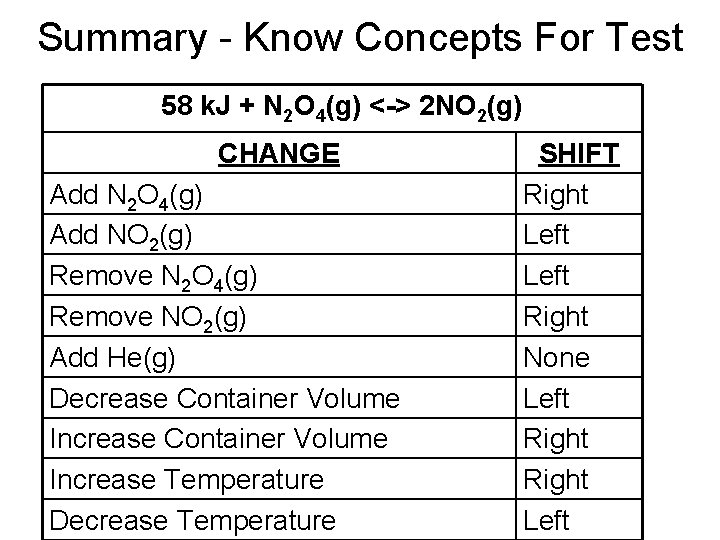
Summary - Know Concepts For Test 58 k. J + N 2 O 4(g) <-> 2 NO 2(g) CHANGE Add N 2 O 4(g) Add NO 2(g) Remove N 2 O 4(g) Remove NO 2(g) Add He(g) Decrease Container Volume Increase Temperature Decrease Temperature SHIFT Right Left Right None Left Right Left