Le Chateliers Principle Chapter 11 n n Le





- Slides: 10

Le Chatelier’s Principle Chapter 11

n n Le Chatelier’s Principle states that a system at equilibrium will stay at equilibrium UNLESS it is acted upon externally. If there is an external action, then the equilibrium has to be re-established.

Let us examine the following reaction: a. A + b. B c. C + d. D • At equilibrium, there is a certain amount of A, B, C, D present. • These amounts will remain unchanged UNLESS some external stress is applied. • Let’s see how equilibrium is affected when a stress is applied.

a. A + b. B n n n c. C + d. D Addition of some B If you add more B, then there will be more molecules of B able to react. The forward reaction will be favored and equilibrium will move towards the right. With time, there will be less A, more B, more C and more D present than at the original equilibrium.

a. A + b. B n n c. C + d. D Removal of some A If you remove some A this will cause the reaction to shift towards the left (reactant side). The amount of A will go down, the amount of B will go up and the amount of C and D will also go down.

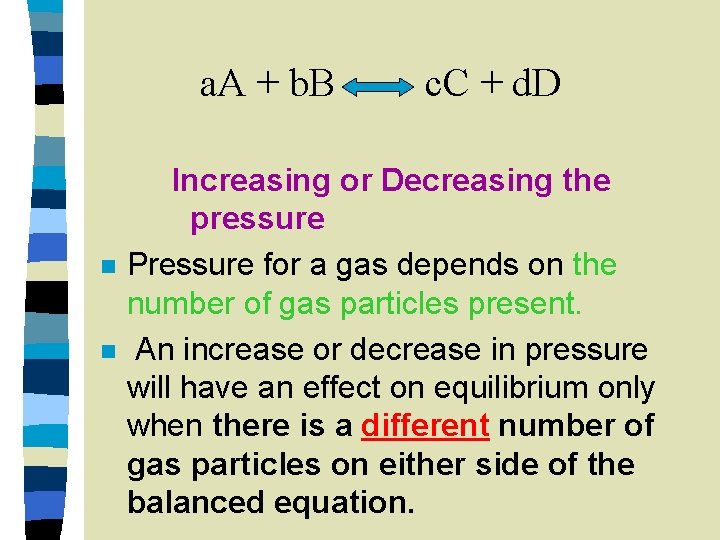
a. A + b. B n n c. C + d. D Increasing or Decreasing the pressure Pressure for a gas depends on the number of gas particles present. An increase or decrease in pressure will have an effect on equilibrium only when there is a different number of gas particles on either side of the balanced equation.

n n An increase in pressure will favor the side with the fewer gas particles. A decrease in pressure will favor the side with the larger number of gas particles.

a. A + b. B c. C + d. D + Energy Increasing or Decreasing the temperature For an exothermic reaction, an increase in temperature will favor the reverse reaction. (equilibrium shifts to the left) A decrease in temperature will have the opposite effect.

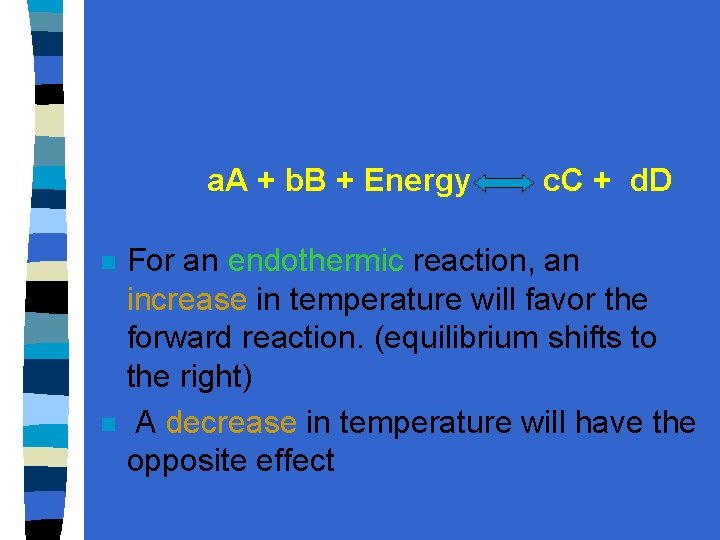
a. A + b. B + Energy n n c. C + d. D For an endothermic reaction, an increase in temperature will favor the forward reaction. (equilibrium shifts to the right) A decrease in temperature will have the opposite effect

n Pressure (due to decreased volume): increase in pressure favors side with fewer molecules. shift right n Catalysts: do not influence the reaction