Laws of Thermodynamics 1 Disusun Oleh Ichwan Aryono












- Slides: 23

Laws of Thermodynamics 1 Disusun Oleh : Ichwan Aryono, S. Pd.

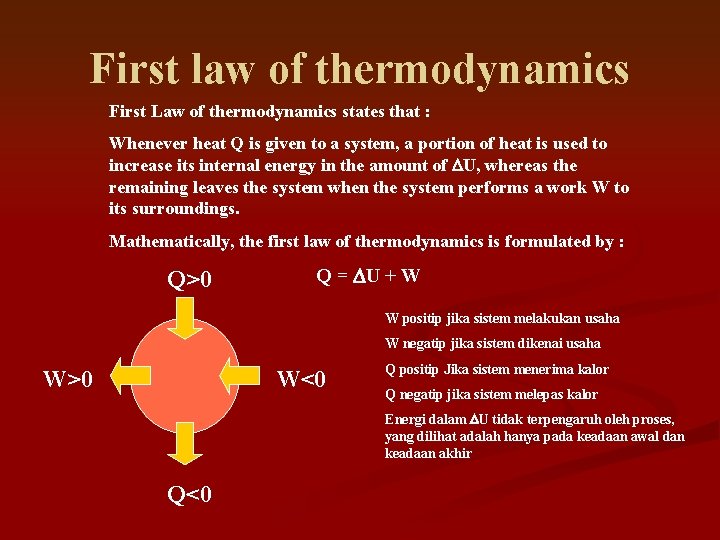
First law of thermodynamics First Law of thermodynamics states that : Whenever heat Q is given to a system, a portion of heat is used to increase its internal energy in the amount of DU, whereas the remaining leaves the system when the system performs a work W to its surroundings. Mathematically, the first law of thermodynamics is formulated by : Q>0 Q = DU + W W positip jika sistem melakukan usaha W negatip jika sistem dikenai usaha W>0 W<0 Q positip Jika sistem menerima kalor Q negatip jika sistem melepas kalor Energi dalam DU tidak terpengaruh oleh proses, yang dilihat adalah hanya pada keadaan awal dan keadaan akhir Q<0

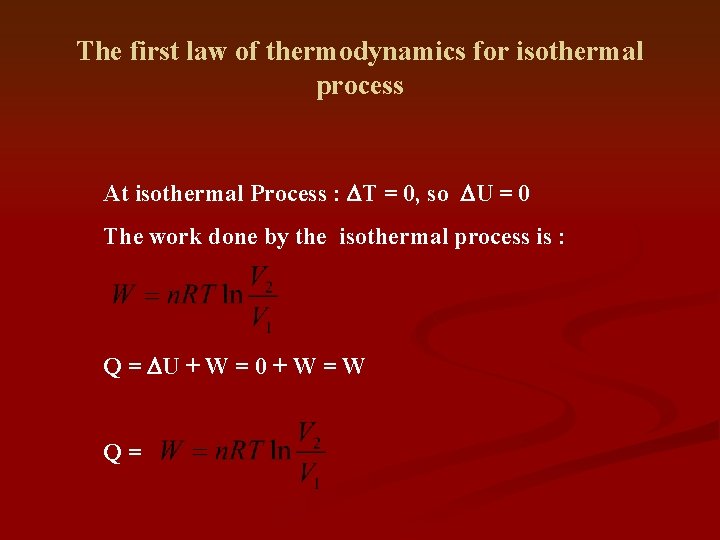
The first law of thermodynamics for isothermal process At isothermal Process : DT = 0, so DU = 0 The work done by the isothermal process is : Q = DU + W = 0 + W = W Q=

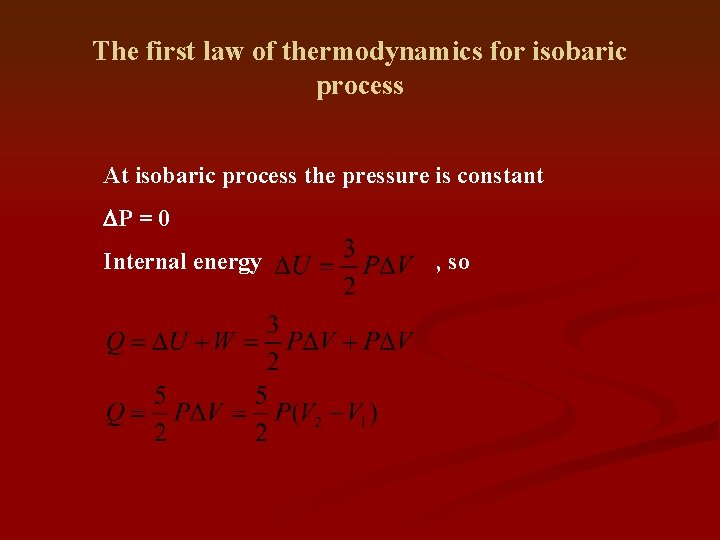
The first law of thermodynamics for isobaric process At isobaric process the pressure is constant DP = 0 Internal energy , so

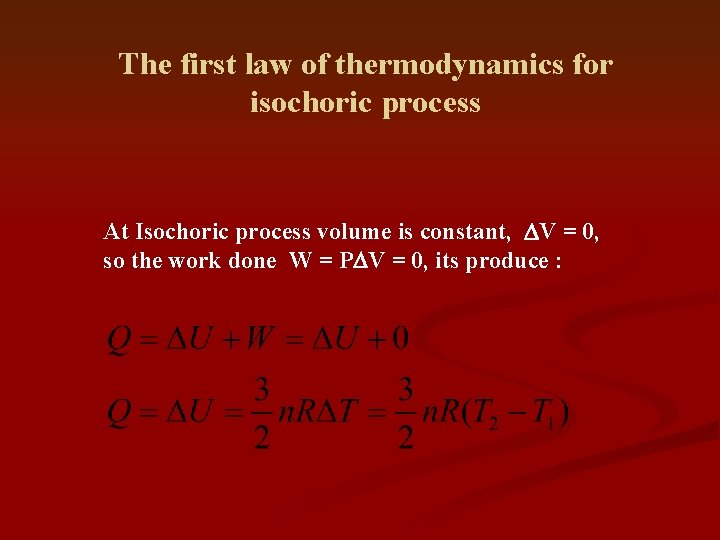
The first law of thermodynamics for isochoric process At Isochoric process volume is constant, DV = 0, so the work done W = PDV = 0, its produce :

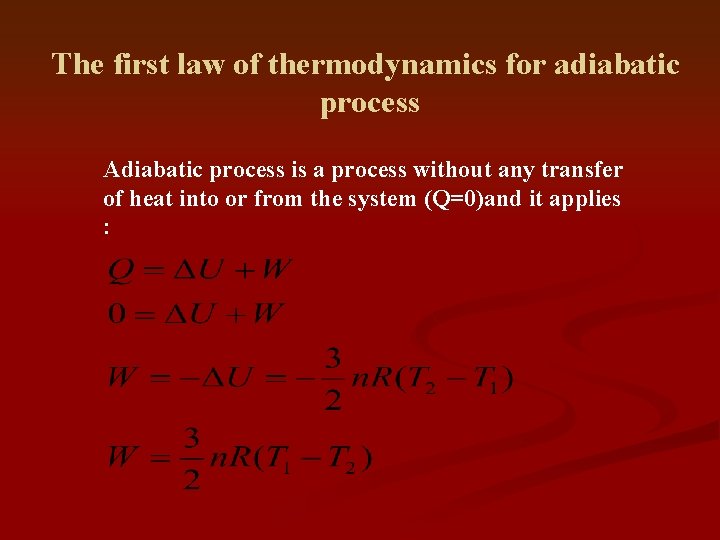
The first law of thermodynamics for adiabatic process Adiabatic process is a process without any transfer of heat into or from the system (Q=0)and it applies :

Student Activity #1 A system absorbs 1500 J of heat from surrounding and does work done 2200 J. Determine the change of internal energy system, increases or decreases ?

Student Activity n Mention three general variables of thermodynamics

n What is state function ?

n Why is the internal energy classified as a state function, meanwhile work and heat is not classified as a state function ?

n Can we warm up of a soap without giving any additional amount of heat to the soup ? Explain it

n A work can be done into a system at a constant pressure, heat can also be tranferred to a system at a constant volume. Is it possible to transfer heat to a system while maintaining the temperature of the system constant ? Explain it

n A diatomic in a closed container has an initial temperature of 250 o. C, a pressure of 105 N/m 2, and a volume of 5 L. The gas undergoes an isobaric process to a pressure of 2 x 105 N/m 2. a. Describes the process above in a P-V diagram b. Find the change in its internal energy c. find the total work done by the gas

Student Activity n n n Mention three general variables of thermodynamics What is state function ? Why is the internal energy classified as a state function, meanwhile work and heat is not classified as a state function ? Can we warm up of a soap without giving any additional amount of heat to the soup ? Explain it A work can be done into a system at a constant pressure, heat can also be tranferred to a system at a constant volume. Is it possible to transfer heat to a system while maintaining the temperature of the system constant ? Explain it

Student Activity #2 System absorbs 1500 J of energy from surrounding. At the same time 2200 J work done is given to the system. Determine the change of internal energy of the system. Is temperature decreses or increases ?

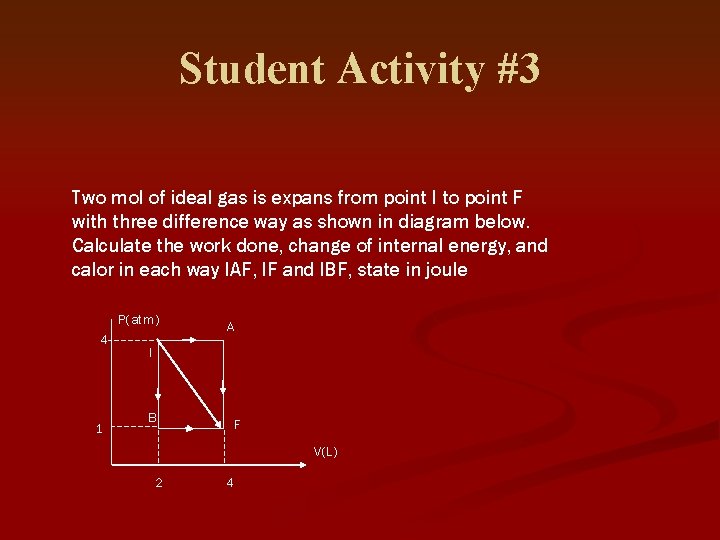
Student Activity #3 Two mol of ideal gas is expans from point I to point F with three difference way as shown in diagram below. Calculate the work done, change of internal energy, and calor in each way IAF, IF and IBF, state in joule P(atm) 4 1 A I B F V(L) 2 4

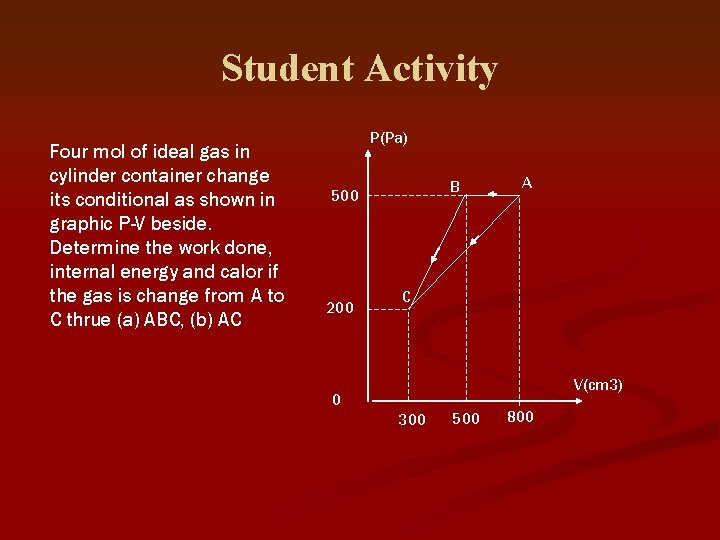
Student Activity Four mol of ideal gas in cylinder container change its conditional as shown in graphic P-V beside. Determine the work done, internal energy and calor if the gas is change from A to C thrue (a) ABC, (b) AC P(Pa) B 500 200 A C V(cm 3) 0 300 500 800

Quiz (work in pairs) Three different processes act on system. a. In process A, 42 J of work are done on the system and 77 J of heat are added to the system. Find the change in the system’s internal energy. b. b. In process B. the system does 42 J of work and 77 J of heat are added to the system. What is the change in the system’s internal energy ? c. c. In process C, the system’s internal energy decreases by 120 J while the system performs 120 J of work on its surroundings. How much heat was added to the system ?

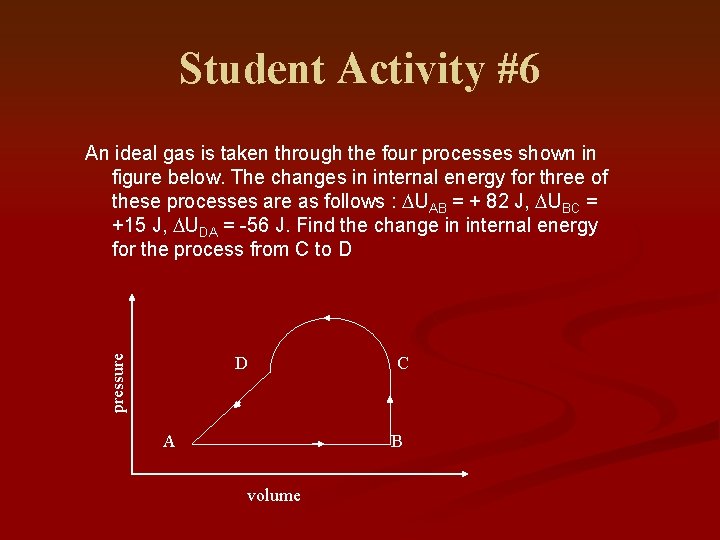
Student Activity #6 pressure An ideal gas is taken through the four processes shown in figure below. The changes in internal energy for three of these processes are as follows : DUAB = + 82 J, DUBC = +15 J, DUDA = -56 J. Find the change in internal energy for the process from C to D D A C B volume

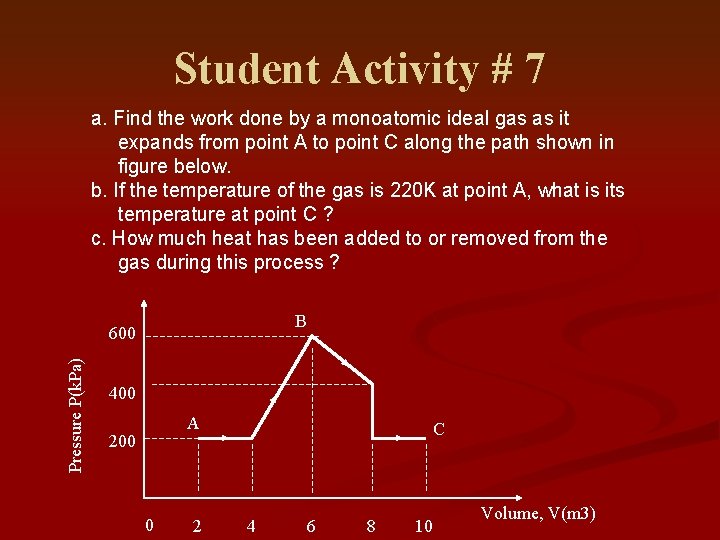
Student Activity # 7 a. Find the work done by a monoatomic ideal gas as it expands from point A to point C along the path shown in figure below. b. If the temperature of the gas is 220 K at point A, what is its temperature at point C ? c. How much heat has been added to or removed from the gas during this process ? B Pressure P(k. Pa) 600 400 A 200 0 2 C 4 6 8 10 Volume, V(m 3)

Student Activity #8 During an adiabatic process, the temperature of 3. 52 moles of monoatomic ideal gas drops from 485 o. C to 205 o. C. For this gas, find a. The work it does b. The heat it exchanges with its surroundings, and c. The changes in internal energy

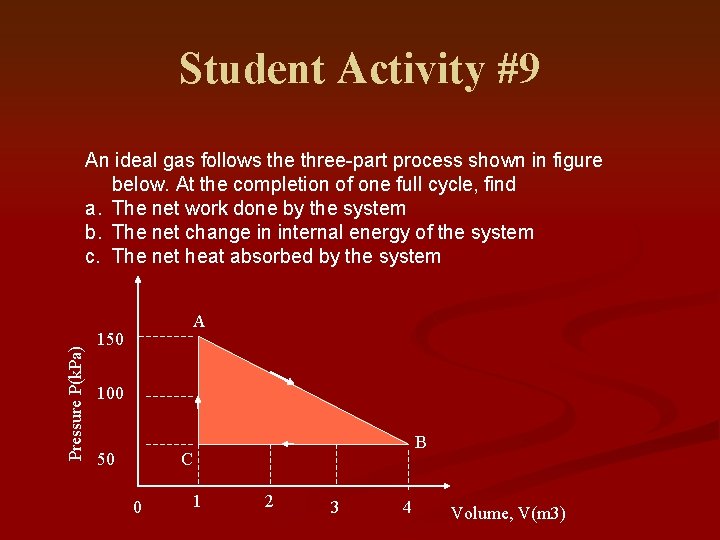
Student Activity #9 Pressure P(k. Pa) An ideal gas follows the three-part process shown in figure below. At the completion of one full cycle, find a. The net work done by the system b. The net change in internal energy of the system c. The net heat absorbed by the system A 150 100 50 B C 0 1 2 3 4 Volume, V(m 3)

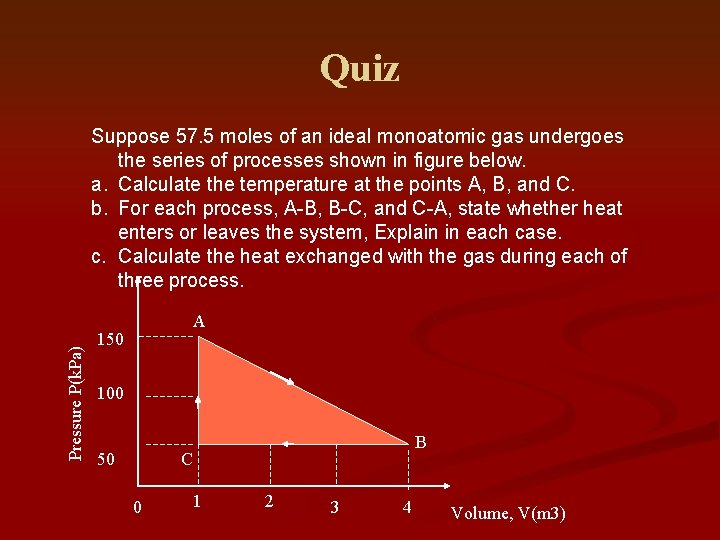
Quiz Pressure P(k. Pa) Suppose 57. 5 moles of an ideal monoatomic gas undergoes the series of processes shown in figure below. a. Calculate the temperature at the points A, B, and C. b. For each process, A-B, B-C, and C-A, state whether heat enters or leaves the system, Explain in each case. c. Calculate the heat exchanged with the gas during each of three process. A 150 100 50 B C 0 1 2 3 4 Volume, V(m 3)