Lattice Energy Green Determined Inchworms Eat At Leaves
![Lattice Energy (k. J/Mol) [Mol = 6. 022 x 1023 # of particles=FW(g)] Lattice Lattice Energy (k. J/Mol) [Mol = 6. 022 x 1023 # of particles=FW(g)] Lattice](https://slidetodoc.com/presentation_image/3da0540939c825959f06f72bfc066c4f/image-3.jpg)

![k. J/mol ΔHof[Na. Cl (s)] = ΔHof[Na (g)] + ΔHof[Cl (g)] + I 1(Na) k. J/mol ΔHof[Na. Cl (s)] = ΔHof[Na (g)] + ΔHof[Cl (g)] + I 1(Na)](https://slidetodoc.com/presentation_image/3da0540939c825959f06f72bfc066c4f/image-7.jpg)

![ΔHof[Ca. Cl 2 (s)] = ΔHof[Ca (g)] + ΔHof[Cl (g)] + I 1(Ca) + ΔHof[Ca. Cl 2 (s)] = ΔHof[Ca (g)] + ΔHof[Cl (g)] + I 1(Ca) +](https://slidetodoc.com/presentation_image/3da0540939c825959f06f72bfc066c4f/image-12.jpg)
![Lattice Energy Problem 2. Calculate Heat of Formation of Ca. Cl 2. ΔHof[Ca (g)] Lattice Energy Problem 2. Calculate Heat of Formation of Ca. Cl 2. ΔHof[Ca (g)]](https://slidetodoc.com/presentation_image/3da0540939c825959f06f72bfc066c4f/image-13.jpg)
![ΔHof[Al. Cl 3 (s)] = ΔHof[Al (g)] + ΔHof[Cl (g)] + I 1(Al) + ΔHof[Al. Cl 3 (s)] = ΔHof[Al (g)] + ΔHof[Cl (g)] + I 1(Al) +](https://slidetodoc.com/presentation_image/3da0540939c825959f06f72bfc066c4f/image-14.jpg)
![Lattice Energy Problem 3. Calculate Lattice Energy of Al. Cl 3. ΔHof[Al (g)] ΔHof[Cl Lattice Energy Problem 3. Calculate Lattice Energy of Al. Cl 3. ΔHof[Al (g)] ΔHof[Cl](https://slidetodoc.com/presentation_image/3da0540939c825959f06f72bfc066c4f/image-15.jpg)
![ΔHof[Ca. O (s)] = ΔHof[Ca (g)] + ΔHof[O (g)] + I 1(Ca) + I ΔHof[Ca. O (s)] = ΔHof[Ca (g)] + ΔHof[O (g)] + I 1(Ca) + I](https://slidetodoc.com/presentation_image/3da0540939c825959f06f72bfc066c4f/image-16.jpg)
![Lattice Energy Problem 4. Calculate Heat of Formation of Ca. O. ΔHof[Ca (g)] = Lattice Energy Problem 4. Calculate Heat of Formation of Ca. O. ΔHof[Ca (g)] =](https://slidetodoc.com/presentation_image/3da0540939c825959f06f72bfc066c4f/image-17.jpg)

- Slides: 18

Lattice Energy Green Determined Inchworms Eat At Leaves Forever |E- -D C - |AG–C o o |G o A C D | E – D C| o Prem D. Sattsangi , Ph. D. © 2010 Christopher Byers, Programmer *Cheryl Farren Tkacs, M. Ed.

Table of Contents • • Lattice Energy Introduction Born-Haber Cycle Na. Cl Cycle Potential Energy Calculation Lattice Energy & Periodic Table Ca. Cl 2 Cycle Al. Cl 3 Cycle Ca. O Cycle 3 4 7 9 11 12 14 16 2
![Lattice Energy k JMol Mol 6 022 x 1023 of particlesFWg Lattice Lattice Energy (k. J/Mol) [Mol = 6. 022 x 1023 # of particles=FW(g)] Lattice](https://slidetodoc.com/presentation_image/3da0540939c825959f06f72bfc066c4f/image-3.jpg)
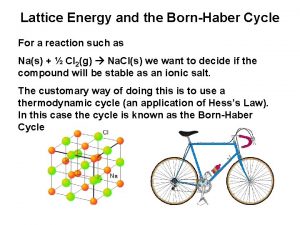
Lattice Energy (k. J/Mol) [Mol = 6. 022 x 1023 # of particles=FW(g)] Lattice Energy: The energy required to completely separate 1 mol of a solid ionic compound into its gaseous ions. Na. Cl(s) Na+(g) + Cl-(g) ΔHlattice = +788 k. J/mol (FW= 58. 45 g using the Periodic Table) + + - + - + - + - + +788 k. J/mol - + - + + + - + - Na. Cl (58. 5 g) solid ionic compound + - + - + - gaseous ions +

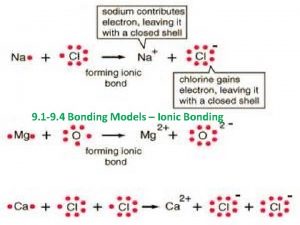
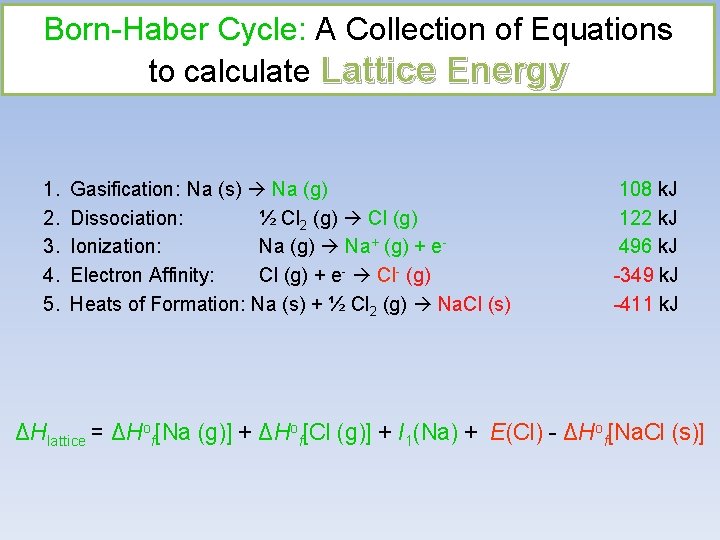
Born-Haber Cycle: A Collection of Equations to calculate Lattice Energy 1. 2. 3. 4. 5. Gasification: Na (s) Na (g) Dissociation: ½ Cl 2 (g) Cl (g) Ionization: Na (g) Na+ (g) + e. Electron Affinity: Cl (g) + e- Cl- (g) Heats of Formation: Na (s) + ½ Cl 2 (g) Na. Cl (s) 108 k. J 122 k. J 496 k. J -349 k. J -411 k. J ΔHlattice = ΔHof[Na (g)] + ΔHof[Cl (g)] + I 1(Na) + E(Cl) - ΔHof[Na. Cl (s)]

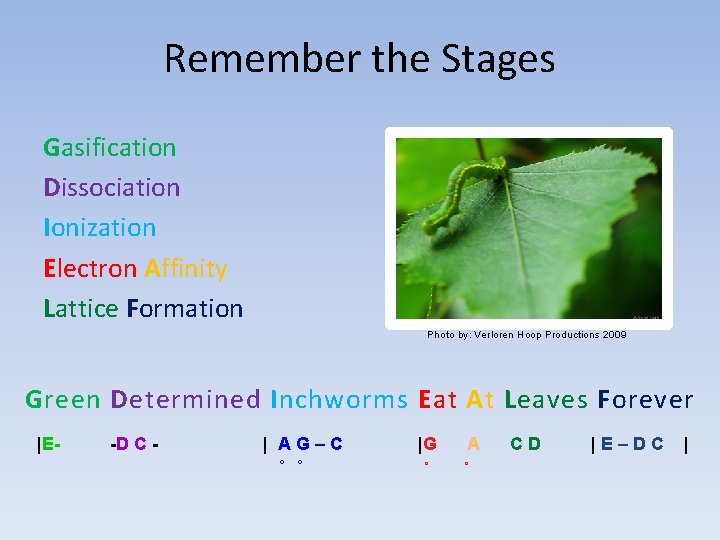
Remember the Stages Gasification Dissociation Ionization Electron Affinity Lattice Formation Photo by: Verloren Hoop Productions 2009 Green Determined Inchworms Eat At Leaves Forever |E- -D C - | AG–C o o |G o A o CD |E–DC |

Layout of Born-Haber Cycles k. J/mol The Lattice Energy Equation will be shown here Energy Meter Change of energy on the Born-Haber cycle. Increasing 0 k. J Decreasing An equation of the specific event will be shown here. Demonstration/Animation of the chemical process will be displayed here. ΔHof Calculated value - Lattice Energy .
![k Jmol ΔHofNa Cl s ΔHofNa g ΔHofCl g I 1Na k. J/mol ΔHof[Na. Cl (s)] = ΔHof[Na (g)] + ΔHof[Cl (g)] + I 1(Na)](https://slidetodoc.com/presentation_image/3da0540939c825959f06f72bfc066c4f/image-7.jpg)
k. J/mol ΔHof[Na. Cl (s)] = ΔHof[Na (g)] + ΔHof[Cl (g)] + I 1(Na) + E(Cl) – ΔHlattice Na+ (g) + e- + Cl (g) E(Cl) + I 1(Na) 0 Na+ (g) + Cl- (g) Na (g) + Cl (g) (atom) ΔHof[Cl (g)] ΔHof[Na (g)] 0 k. J Na (g) + ½ Cl 2 (g) Na (s) + ½ Cl 2 (g) ΔHof[Na. Cl (s)] -411 (-788) Na. Cl (s) - Lattice Energy of Na. Cl 377 (-349) 230 (122) 108 (108) k. J/mol - (-) Lattice Energy of Na. Cl 726 (496) 5. Na+ (g) + Cl- (g) Na. Cl (s) 788 4. Cl (g) + e- Cl- (g) -349 3. Na (g) Na+ (g) + e 496 2. ½ Cl 2 (g) Cl (g) 122 1. Na (s) Na (g) 108 Demonstration E N E R G Y + e- - Change of State Electron. Formation Ionization Dissociation Lattice Affinity + - e- + E N E R G Y

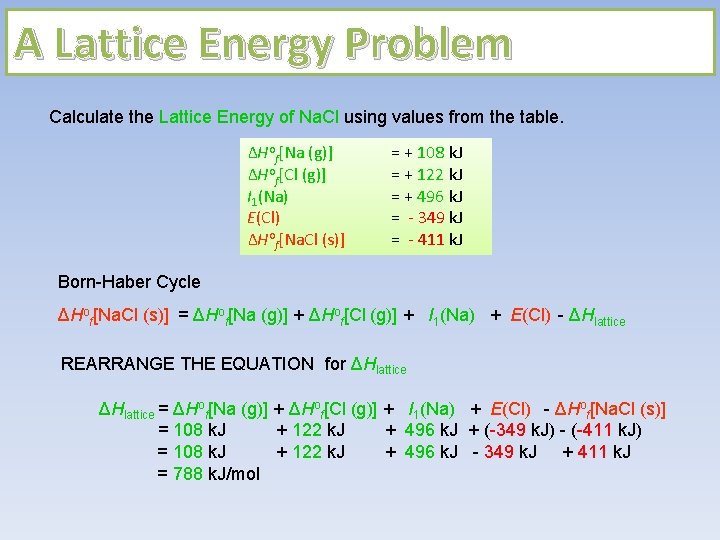
A Lattice Energy Problem Calculate the Lattice Energy of Na. Cl using values from the table. ΔHof[Na (g)] ΔHof[Cl (g)] I 1(Na) E(Cl) ΔHof[Na. Cl (s)] = + 108 k. J = + 122 k. J = + 496 k. J = - 349 k. J = - 411 k. J Born-Haber Cycle ΔHof[Na. Cl (s)] = ΔHof[Na (g)] + ΔHof[Cl (g)] + I 1(Na) + E(Cl) - ΔHlattice REARRANGE THE EQUATION for ΔHlattice = ΔHof[Na (g)] + ΔHof[Cl (g)] + I 1(Na) + E(Cl) - ΔHof[Na. Cl (s)] = 108 k. J + 122 k. J + 496 k. J + (-349 k. J) - (-411 k. J) = 108 k. J + 122 k. J + 496 k. J - 349 k. J + 411 k. J = 788 k. J/mol

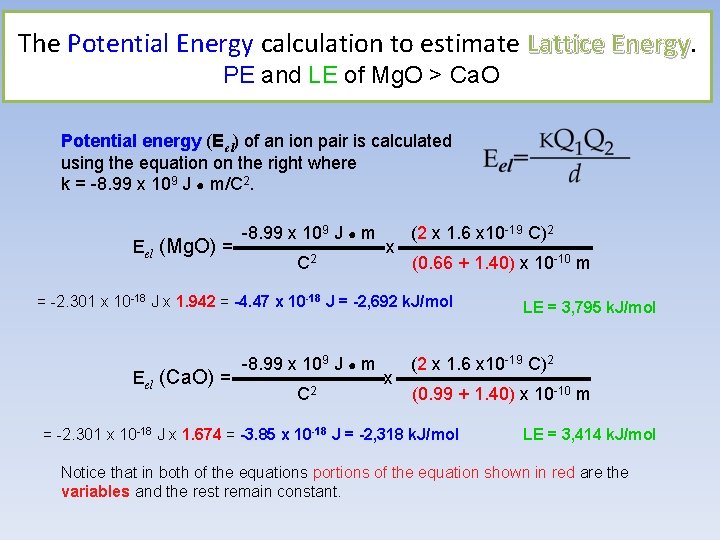
The Potential Energy calculation to estimate Lattice Energy PE and LE of Mg. O > Ca. O Potential energy (Eel) of an ion pair is calculated using the equation on the right where k = -8. 99 x 109 J ● m/C 2. -8. 99 x 109 J ● m (2 x 1. 6 x 10 -19 C)2 Eel (Mg. O) = ------------ x ---------------C 2 (0. 66 + 1. 40) x 10 -10 m = -2. 301 x 10 -18 J x 1. 942 = -4. 47 x 10 -18 J = -2, 692 k. J/mol LE = 3, 795 k. J/mol -8. 99 x 109 J ● m (2 x 1. 6 x 10 -19 C)2 Eel (Ca. O) = ------------ x ---------------C 2 (0. 99 + 1. 40) x 10 -10 m = -2. 301 x 10 -18 J x 1. 674 = -3. 85 x 10 -18 J = -2, 318 k. J/mol LE = 3, 414 k. J/mol Notice that in both of the equations portions of the equation shown in red are the variables and the rest remain constant.

Lattice Energy: Estimation It is related to the potential energy of an ion pair. Charges on the ions (from the Periodic Table) and sum of the ionic radii in crystals, may be used to compare Lattice Energies. R. D. Shannon, Acta Cryst. A 32 751 -767 (1976) Mg. O > Mg. Cl 2 Compound Q 1 Q 2 d (ionic radius) Eel predicted literature Mg. O 2+ 2 - [0. 86 + 1. 26]= 2. 12 larger 3795 Mg. Cl 2 2+ 1 - [0. 86 + 1. 67]= 2. 53 smaller 2326 Both, charges on ions and trends in ionic radii, can be estimated using the Periodic Table.

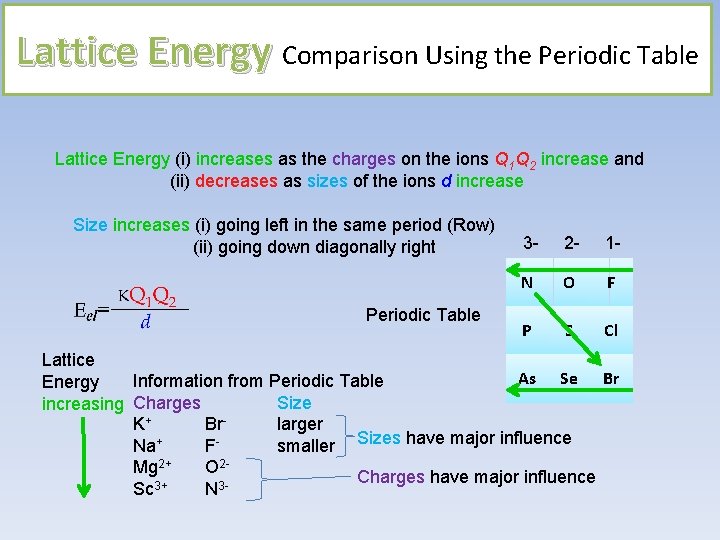
Lattice Energy Comparison Using the Periodic Table Lattice Energy (i) increases as the charges on the ions Q 1 Q 2 increase and (ii) decreases as sizes of the ions d increase Size increases (i) going left in the same period (Row) (ii) going down diagonally right Periodic Table 3 - 2 - 1 - N O F P S Cl Lattice As Se Br Information from Periodic Table Energy Size increasing Charges K+ Brlarger Na+ Fsmaller Sizes have major influence Mg 2+ O 2 Charges have major influence Sc 3+ N 3 -
![ΔHofCa Cl 2 s ΔHofCa g ΔHofCl g I 1Ca ΔHof[Ca. Cl 2 (s)] = ΔHof[Ca (g)] + ΔHof[Cl (g)] + I 1(Ca) +](https://slidetodoc.com/presentation_image/3da0540939c825959f06f72bfc066c4f/image-12.jpg)
ΔHof[Ca. Cl 2 (s)] = ΔHof[Ca (g)] + ΔHof[Cl (g)] + I 1(Ca) + I 2(Ca) + 2 E(Cl) – ΔHlattice k. J/mol Ca 2+ (g) + 2 e- + 2 Cl (g) 2158 (1145) 2 E(Cl) I 2(Ca) Ca 2+ (g) + 2 Cl- (g) 1460 (-698) 179 (179) Ca (g) + 2 Cl (g) (atom) ΔHof[Cl (g)] ΔHof[Ca (g)] 0 0 k. J Ca (g) + Cl 2 (g) Ca (s) + Cl 2 (g) ΔHof[Ca. Cl 2 (s)] -796 (- 2256) - Lattice Energy of Ca. Cl 2 I 1(Ca) (-) Lattice Energy of Ca. Cl 2 1013 (590) 423 (244) Demonstration Change of State Ionization Lattice Dissociation Electron Formation Affinity Ca+ (g) + e- + 2 Cl (g) e. E N E R G Y 2++ e- e E N E -R G Y + Δ = 2256 Ca. Cl 2 (s) 12
![Lattice Energy Problem 2 Calculate Heat of Formation of Ca Cl 2 ΔHofCa g Lattice Energy Problem 2. Calculate Heat of Formation of Ca. Cl 2. ΔHof[Ca (g)]](https://slidetodoc.com/presentation_image/3da0540939c825959f06f72bfc066c4f/image-13.jpg)
Lattice Energy Problem 2. Calculate Heat of Formation of Ca. Cl 2. ΔHof[Ca (g)] ΔHof[Cl (g)] I 1(Ca) I 2(Ca) E(Cl) ΔHlattice = + 179 k. J = + 122 k. J = + 590 k. J = + 1145 k. J = - 349 k. J = + 2256 k. J Born-Haber Cycle ΔHof[Ca. Cl 2 (s)] = ΔHof[Ca (g)] + 2ΔHof[Cl (g)] + I 1(Ca) + I 2(Ca) + 2 E(Cl) – ΔHlattice Choose the values that match the formula. Click to check answer. Correct Answer = + 179 + 2(122) + 590 + 1145 + 2(-349)- (+2256) = + 179 + 244 + 590 + 1145 + (-349) - (+2256) = + 179 + 244 = - 796 k. J/mol + 590 + 1145 - 698 - 2256 13
![ΔHofAl Cl 3 s ΔHofAl g ΔHofCl g I 1Al ΔHof[Al. Cl 3 (s)] = ΔHof[Al (g)] + ΔHof[Cl (g)] + I 1(Al) +](https://slidetodoc.com/presentation_image/3da0540939c825959f06f72bfc066c4f/image-14.jpg)
ΔHof[Al. Cl 3 (s)] = ΔHof[Al (g)] + ΔHof[Cl (g)] + I 1(Al) + I 2(Al) + I 3(Al) + 3 E(Cl) – ΔHlattice k. J/mol Al 3+ (g) + 3 e- + 3 Cl (g) 5799 (2745) 3 E(Cl) Al 3+ (g) + 3 Cl- (g) I 3(Al) I 2(Al) 293 (293) 0 -706 (- 5458) Al+ I 1(Al) ΔHof[Cl (g)] ΔHof[Al (g)] 0 k. J (g) + e- + 3 Cl (g) Al (g) + 3 Cl (g) (atom) Al (g) + 3/2 Cl 2 (g) Al (s) + 3/2 Cl 2 (g) ΔHof[Al. Cl 3 - Lattice Energy (s)] Al. Cl 3 (s) Lattice Energy of Al. Cl 3 3054 (1817) 1237 (578) 659 (366) Demonstration Change of State Ionization Lattice Dissociation Electron Formation Affinity Al 2+ (g) + 2 e- + 3 Cl (g) (-) Lattice Energy of Al. Cl 3 4752 (-1047) e- + 3+ 2+ ee-e- E N E R G Y - 2 ++ Δ = 5458 14 E N E R G Y
![Lattice Energy Problem 3 Calculate Lattice Energy of Al Cl 3 ΔHofAl g ΔHofCl Lattice Energy Problem 3. Calculate Lattice Energy of Al. Cl 3. ΔHof[Al (g)] ΔHof[Cl](https://slidetodoc.com/presentation_image/3da0540939c825959f06f72bfc066c4f/image-15.jpg)
Lattice Energy Problem 3. Calculate Lattice Energy of Al. Cl 3. ΔHof[Al (g)] ΔHof[Cl (g)] I 1(Al) I 2(Al) I 3(Al) E(Cl) ΔHof[Al. Cl 3 (s)] = + 293 k. J = + 122 k. J = + 578 k. J = + 1817 k. J = + 2745 k. J = - 349 k. J = - 706 k. J Born-Haber Cycle ΔHof[Al. Cl 3 (s)] = ΔHof[Al (g)] + 3ΔHof[Cl (g)] + I 1(Al) + I 2(Al) + I 3(Al) + 3 E(Cl) – ΔHlattice Choose the correctly rearranged equation. Click to check answer. ΔHlattice =Answer: ΔHof[Al (g)] + 3ΔHof[Cl (g)] + I 1(Al) + I 2(Al) + I 3(Al) + 3 E(Cl) + ΔHof[Al. Cl 3 (s)] Correct ΔHlattice = ΔHof[Al (g)] + 3ΔHof[Cl (g)] + I 1(Al) + I 2(Al) + I 3(Al) + 3 E(Cl) – ΔHof[Al. Cl 3 (s)] = + 293 + 3(122) + 578 + 1817 + 2745 + 3(-349) – (-706) = + 293 + 366 + 578 + 1817 + 2745 - 1047 + 706 = 5458 k. J/mol 15
![ΔHofCa O s ΔHofCa g ΔHofO g I 1Ca I ΔHof[Ca. O (s)] = ΔHof[Ca (g)] + ΔHof[O (g)] + I 1(Ca) + I](https://slidetodoc.com/presentation_image/3da0540939c825959f06f72bfc066c4f/image-16.jpg)
ΔHof[Ca. O (s)] = ΔHof[Ca (g)] + ΔHof[O (g)] + I 1(Ca) + I 2(Ca) + E 1(O) + E 2(O) – ΔHlattice k. J/mol Ca 2+ (g) + O 2 - (g) 2878 (845) Ca 2+ (g) + e- + O- (g) I 2(Ca) Demonstration Change of State Lattice Electron. Formation Ionization Dissociation Affinity Ca+ (g) + e- + O (g) 1029 (590) I 1(Ca) 439 (247) Ca (g) + O (g) (atom) 192 (192) Ca (g) + ½ O 2 (g) ΔHof[O (g)] 0 ΔHof[Ca (g)] 0 k. J E N E R G Y 2 -E 2+ + ee- e- + e- Ca (s) + ½ O 2 (g) - - N E R G Y Lattice Energy of Ca. O E 1(O) (-) Lattice Energy of Ca. O 2033 (-141) E 2(O) Ca 2+ (g) + 2 e- + O (g) 2174 (1145) Δ = 3514 ΔHof[Ca. O (s)] -636 (- 3514) - Lattice Energy Ca. O (s) 16
![Lattice Energy Problem 4 Calculate Heat of Formation of Ca O ΔHofCa g Lattice Energy Problem 4. Calculate Heat of Formation of Ca. O. ΔHof[Ca (g)] =](https://slidetodoc.com/presentation_image/3da0540939c825959f06f72bfc066c4f/image-17.jpg)
Lattice Energy Problem 4. Calculate Heat of Formation of Ca. O. ΔHof[Ca (g)] = +192 k. J ΔHof[O (g)] = +247 k. J I 1(Ca) = +590 k. J I 2(Ca) = +1145 k. J E 1(O) = -141 Born-Haber Cycle k. J ΔHof[Ca. O (s)] = ΔHof[Ca (g)] ΔHof[O (g)] + I 1(Ca) + I 2(Ca) + E 1(O) + E 2(O) – ΔHlattice E 2+(O) = +845 = + 192 k. J+ 247 + 590 + 1145 + (-141) + 845 - (3514) = +3514 = + 192 ΔH + lattice 247 + 590 + 1145 - 141 + 845 - 3514 k. J = - 636 k. J/mol 17

Questions? 18
 Suatu poset merupakan lattice jika hanya jika...
Suatu poset merupakan lattice jika hanya jika... Red green show
Red green show Write four five sentences about the baby elephant nandu
Write four five sentences about the baby elephant nandu Leaves we eat as vegetables
Leaves we eat as vegetables I would rather eat potatoes than to eat rice answer
I would rather eat potatoes than to eat rice answer They eat or eats
They eat or eats People buy me to eat but never eat me what am i
People buy me to eat but never eat me what am i What is an energy role
What is an energy role Pnp seal logo
Pnp seal logo Photosynthesis is important to animals because flocabulary
Photosynthesis is important to animals because flocabulary Bigger lattice energy
Bigger lattice energy Lattice energy
Lattice energy Lattice energy units
Lattice energy units Och lewis structure
Och lewis structure Thermal decomposition of group 1 nitrates
Thermal decomposition of group 1 nitrates Lattice energy trends
Lattice energy trends Increasing lattice energy trend
Increasing lattice energy trend Lattice energy of kcl
Lattice energy of kcl Coulomb's law and lattice energy
Coulomb's law and lattice energy