Latimer Diagrams O S N Descriptive Chemistry Cl

- Slides: 7

Latimer Diagrams O, S, N Descriptive Chemistry

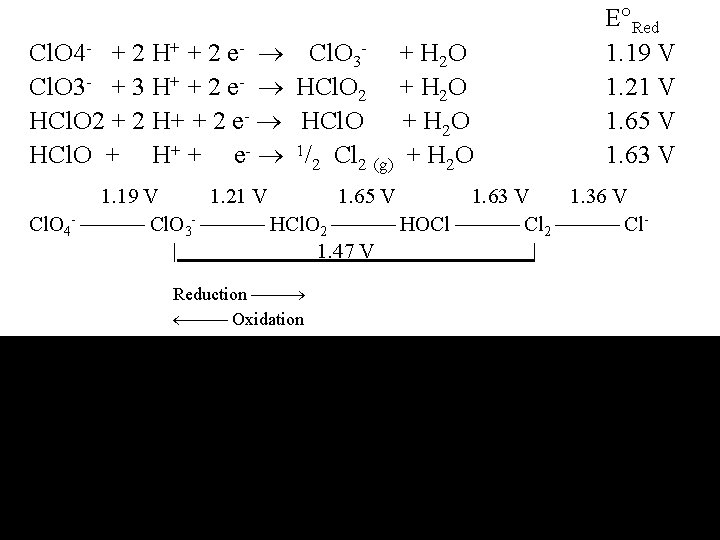
Cl. O 4 - + 2 H+ + 2 e- Cl. O 3 - + H 2 O Cl. O 3 - + 3 H+ + 2 e- HCl. O 2 + H 2 O HCl. O 2 + 2 H+ + 2 e- HCl. O + H 2 O HCl. O + H+ + e- 1/2 Cl 2 (g) + H 2 O E Red 1. 19 V 1. 21 V 1. 65 V 1. 63 V 1. 19 V 1. 21 V 1. 65 V 1. 63 V 1. 36 V Cl. O 4 - Cl. O 3 - HCl. O 2 HOCl Cl 2 Cl| 1. 47 V | Reduction Oxidation 0. 36 V 0. 35 V 0. 65 V 0. 40 V 1. 36 V Cl. O 4 - Cl. O 3 - Cl. O 2 - OCl- Cl 2 Cl | 0. 88 V |

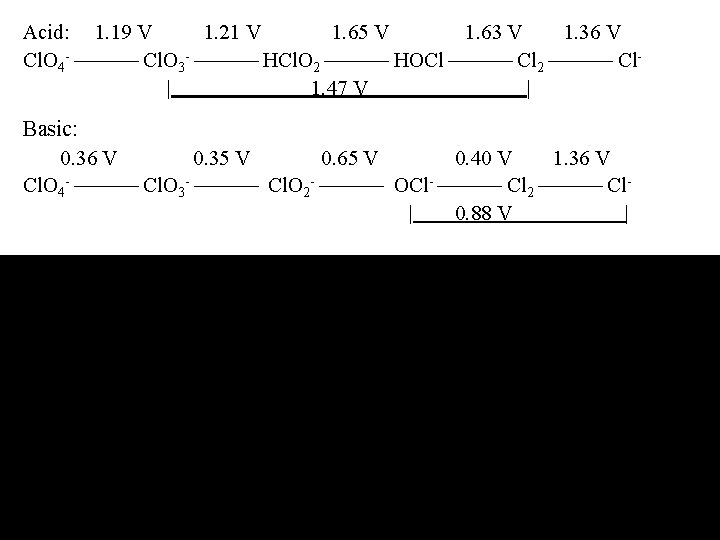
Acid: 1. 19 V 1. 21 V 1. 65 V 1. 63 V 1. 36 V Cl. O 4 - Cl. O 3 - HCl. O 2 HOCl Cl 2 Cl| 1. 47 V | Basic: 0. 36 V 0. 35 V 0. 65 V 0. 40 V 1. 36 V Cl. O 4 - Cl. O 3 - Cl. O 2 - OCl- Cl 2 Cl | 0. 88 V |

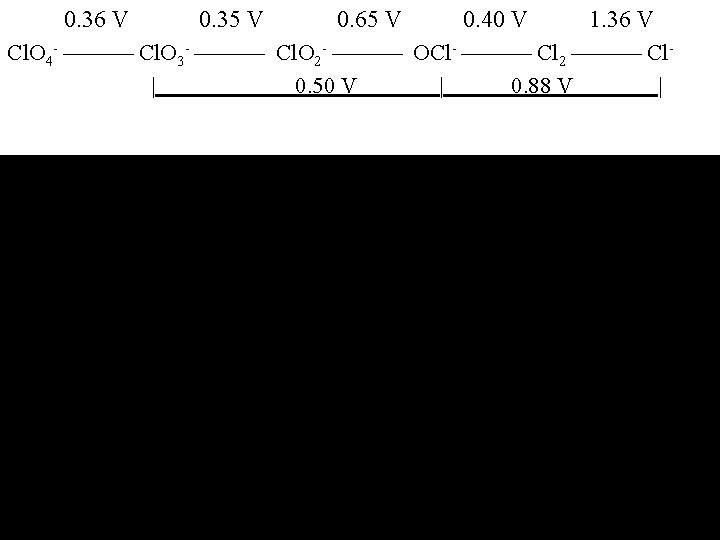
0. 36 V 0. 35 V 0. 65 V 0. 40 V 1. 36 V Cl. O 4 - Cl. O 3 - Cl. O 2 - OCl- Cl 2 Cl| 0. 50 V | 0. 88 V |

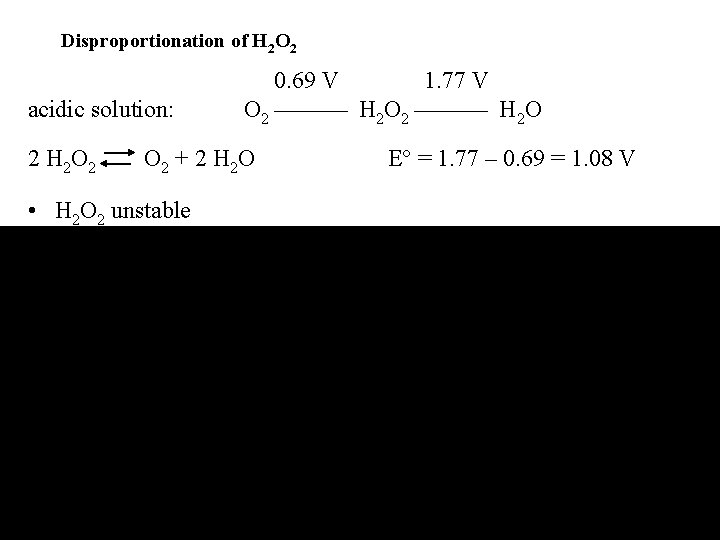
Disproportionation of H 2 O 2 acidic solution: 0. 69 V 1. 77 V O 2 H 2 O 2 O 2 + 2 H 2 O • H 2 O 2 unstable E = 1. 77 – 0. 69 = 1. 08 V

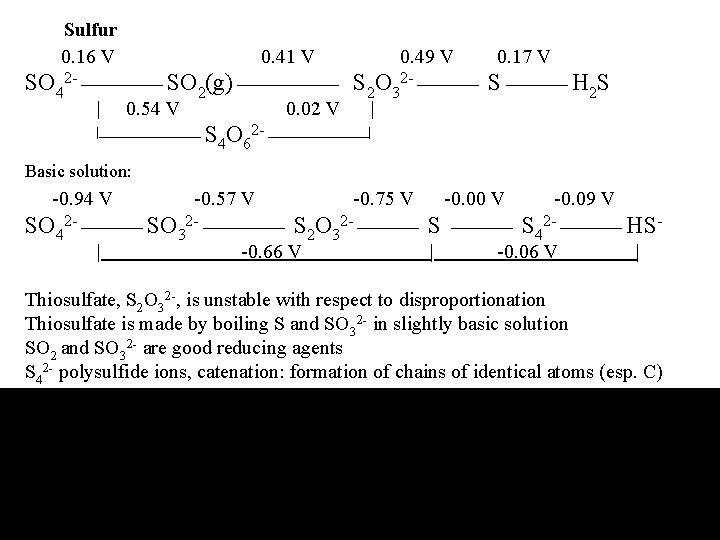
Sulfur 0. 16 V 0. 41 V 0. 49 V 0. 17 V SO 42 - SO 2(g) S 2 O 32 - S H 2 S | 0. 54 V 0. 02 V | | S O 2 - | 4 6 Basic solution: -0. 94 V -0. 57 V -0. 75 V -0. 00 V -0. 09 V SO 42 - SO 32 - S 2 O 32 - S S 42 - HS| -0. 66 V | -0. 06 V | Thiosulfate, S 2 O 32 -, is unstable with respect to disproportionation Thiosulfate is made by boiling S and SO 32 - in slightly basic solution SO 2 and SO 32 - are good reducing agents S 42 - polysulfide ions, catenation: formation of chains of identical atoms (esp. C)

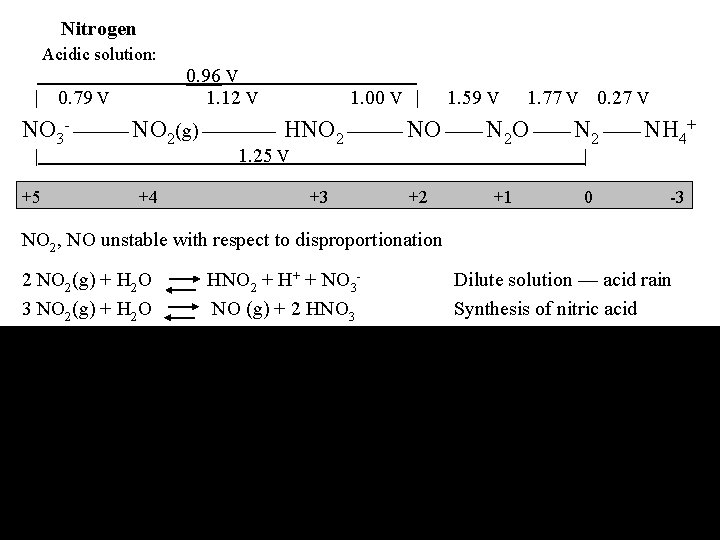
Nitrogen Acidic solution: | 0. 79 V 0. 96 V 1. 12 V . 1. 00 V | 1. 59 V 1. 77 V 0. 27 V NO 3 - NO 2(g) HNO 2 NO N 2 NH 4+ 1. 25 V | +5 +4 | +3 +2 +1 0 -3 NO 2, NO unstable with respect to disproportionation 2 NO 2(g) + H 2 O HNO 2 + H+ + NO 33 NO 2(g) + H 2 O NO (g) + 2 HNO 3 Dilute solution — acid rain Synthesis of nitric acid