Late Effects of PreOperative ImagedGuided Radiation Therapy IGRT

- Slides: 19

Late Effects of Pre-Operative Imaged-Guided Radiation Therapy (IGRT) in Extremity Sarcoma Patients: Results OF RTOG 0630 Dian Wang, MD. , Ph. D. ; Qiang Zhang, Ph. D. ; Burton L. Eisenberg, MD. ; John Kane III, MD. ; X Allen Li, Ph. D. ; David Lucas, MD. ; Carolyn R. Freeman, MD. ; Andy Trotti, MD. ; Ying Hitchcock, MD. , ; David G. Kirsch, MD. , Ph. D.

Objectives • Primary objective: – Determine the effect of reduced RT volume through IGRT on late radiation morbidity at 2 years from the start of RT • Late morbidity defined as Grade 2 or higher: (1) edema scored by Stern’s scale (2) subcutaneous fibrosis scored by RTOG/EORTC criteria (3) joint stiffness scored by RTOG/EORTC criteria • Secondary objectives: – Determine the pattern of failure 2

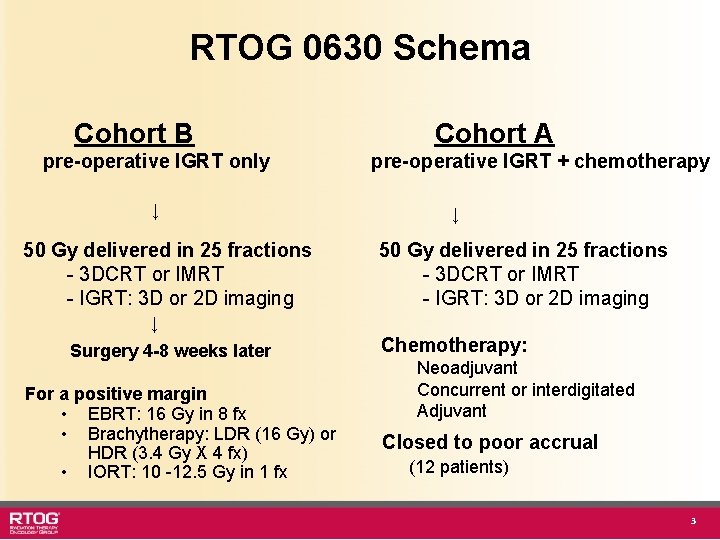
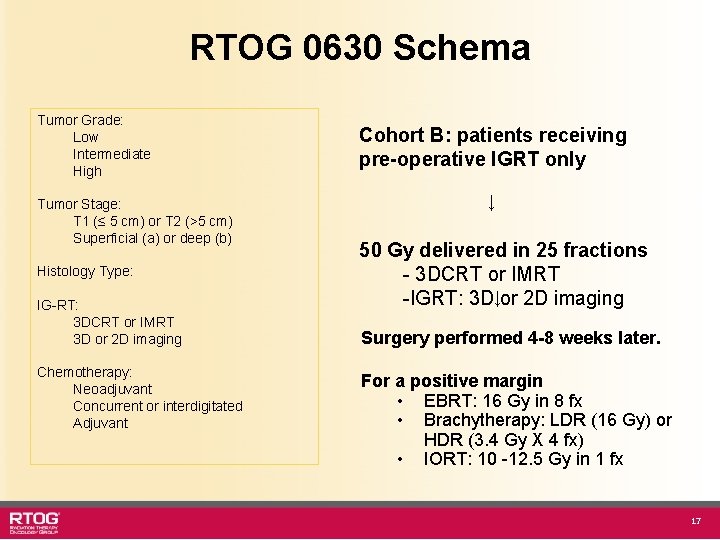
RTOG 0630 Schema Cohort B pre-operative IGRT only ↓ 50 Gy delivered in 25 fractions - 3 DCRT or IMRT - IGRT: 3 D or 2 D imaging Cohort A pre-operative IGRT + chemotherapy ↓ 50 Gy delivered in 25 fractions - 3 DCRT or IMRT - IGRT: 3 D or 2 D imaging ↓ Surgery 4 -8 weeks later For a positive margin • EBRT: 16 Gy in 8 fx • Brachytherapy: LDR (16 Gy) or HDR (3. 4 Gy X 4 fx) • IORT: 10 -12. 5 Gy in 1 fx Chemotherapy: Neoadjuvant Concurrent or interdigitated Adjuvant Closed to poor accrual (12 patients) 3

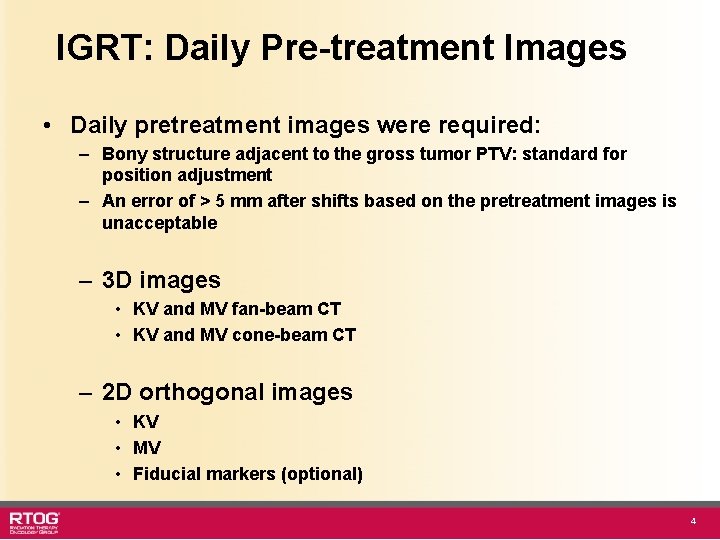
IGRT: Daily Pre-treatment Images • Daily pretreatment images were required: – Bony structure adjacent to the gross tumor PTV: standard for position adjustment – An error of > 5 mm after shifts based on the pretreatment images is unacceptable – 3 D images • KV and MV fan-beam CT • KV and MV cone-beam CT – 2 D orthogonal images • KV • MV • Fiducial markers (optional) 4

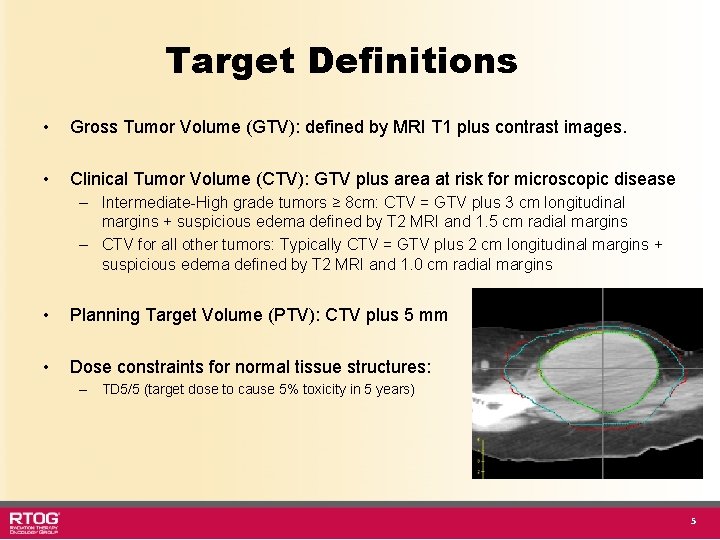
Target Definitions • Gross Tumor Volume (GTV): defined by MRI T 1 plus contrast images. • Clinical Tumor Volume (CTV): GTV plus area at risk for microscopic disease – Intermediate-High grade tumors ≥ 8 cm: CTV = GTV plus 3 cm longitudinal margins + suspicious edema defined by T 2 MRI and 1. 5 cm radial margins – CTV for all other tumors: Typically CTV = GTV plus 2 cm longitudinal margins + suspicious edema defined by T 2 MRI and 1. 0 cm radial margins • Planning Target Volume (PTV): CTV plus 5 mm • Dose constraints for normal tissue structures: – TD 5/5 (target dose to cause 5% toxicity in 5 years) 5

Protocol Summary • Patient accrual: March 2008 through September 2010 • Cohort B: 86 patients – 7 patients excluded from analysis: • 6 ineligible – 2 not extremity sarcoma, 2 MRI>8 weeks prior to study entry, 1 no labs prior to study entry, 1 started protocol tx prior to study entry) • 1 no protocol treatment – 79 patients eligible: analyzed for this late toxicity report • Sample size of 66 needed to detect a 15% absolute improvement in late toxicity compared to pre-operative RT arm of the NCIC SR 2 trial 37% (27/73) to 22% 6

Patient Characteristics • Location – Upper leg 58. 3% – Lower leg 20. 3% • Median Size 10. 5 cm (3. 5 -30 cm) • Histology – – UPS 21. 5% Myxofibrosarcoma 20. 3% Myxoid Liposarcoma 12. 7% LMS 10. 2% • Grade: G 3 46. 8%, G 2 24. 1% 7

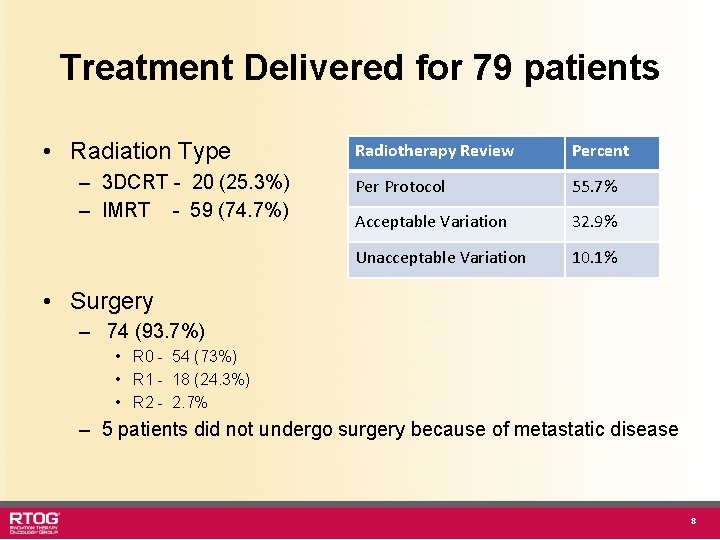
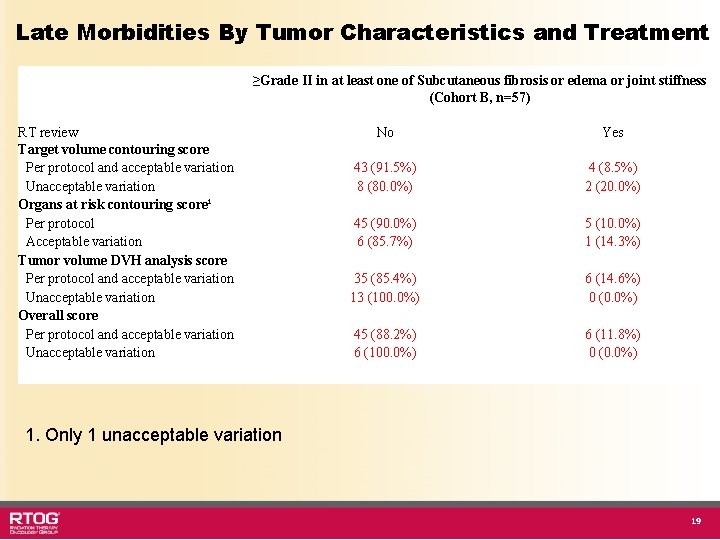
Treatment Delivered for 79 patients • Radiation Type – 3 DCRT - 20 (25. 3%) – IMRT - 59 (74. 7%) Radiotherapy Review Percent Per Protocol 55. 7% Acceptable Variation 32. 9% Unacceptable Variation 10. 1% • Surgery – 74 (93. 7%) • R 0 - 54 (73%) • R 1 - 18 (24. 3%) • R 2 - 2. 7% – 5 patients did not undergo surgery because of metastatic disease 8

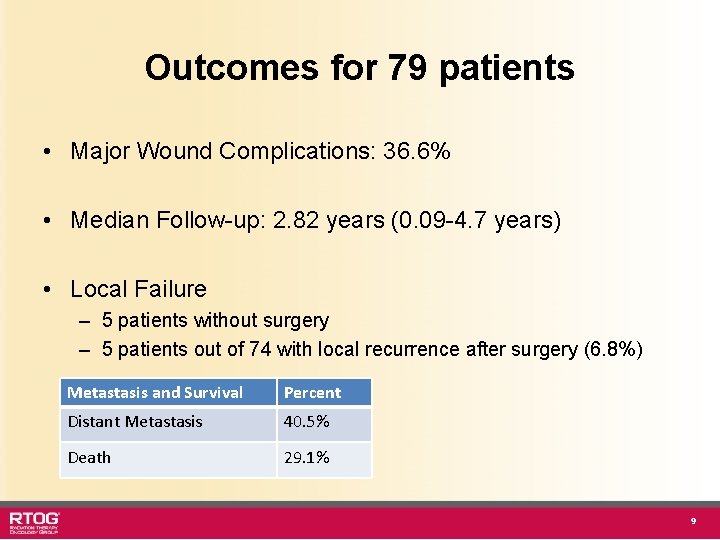
Outcomes for 79 patients • Major Wound Complications: 36. 6% • Median Follow-up: 2. 82 years (0. 09 -4. 7 years) • Local Failure – 5 patients without surgery – 5 patients out of 74 with local recurrence after surgery (6. 8%) Metastasis and Survival Percent Distant Metastasis 40. 5% Death 29. 1% 9

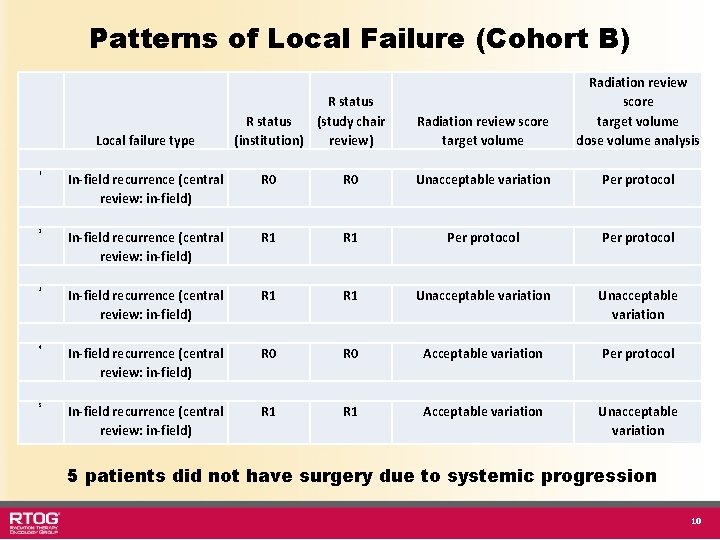
Patterns of Local Failure (Cohort B) Local failure type R status (study chair (institution) review) Radiation review score target volume dose volume analysis 1 In-field recurrence (central review: in-field) R 0 Unacceptable variation Per protocol 2 In-field recurrence (central review: in-field) R 1 Per protocol 3 In-field recurrence (central review: in-field) R 1 Unacceptable variation 4 In-field recurrence (central review: in-field) R 0 Acceptable variation Per protocol 5 In-field recurrence (central review: in-field) R 1 Acceptable variation Unacceptable variation 5 patients did not have surgery due to systemic progression 10

Toxicities Definitively, Probably or Possibly Related to Protocol • Grade 3/4 by CTCAE: mainly due to skin and bleeding • Grade 3: 20 (25. 3%) • Grade 4: 4 (5. 1%) • For the 2 -year late toxicities (subcutaneous fibrosis, edema and joint stiffness) • 22 patients excluded: – – – 13 died<1. 75 years 3 <1. 75 years F/U 6 have at least 1. 75 year F/U but without 2 -year toxicity assessment • 57 patients are evaluable for the 2 -year late toxicity endpoints (between 1. 75 and 2. 25 years). Subjects analyzed for this report 11

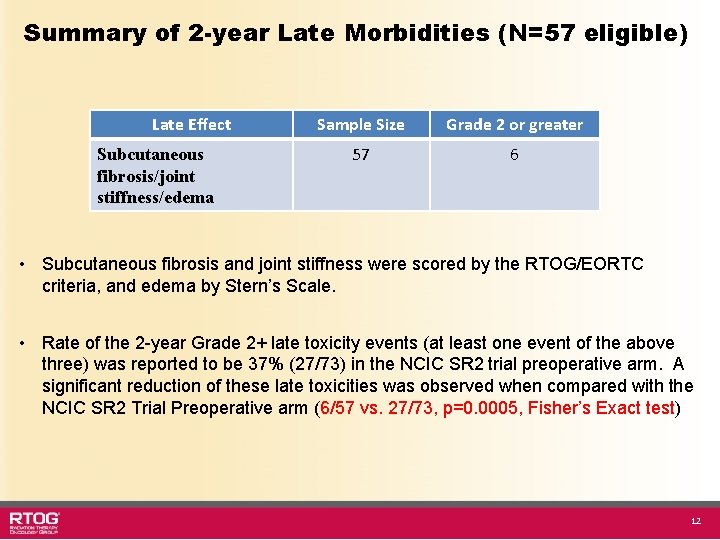
Summary of 2 -year Late Morbidities (N=57 eligible) Late Effect Subcutaneous fibrosis/joint stiffness/edema Sample Size Grade 2 or greater 57 6 • Subcutaneous fibrosis and joint stiffness were scored by the RTOG/EORTC criteria, and edema by Stern’s Scale. • Rate of the 2 -year Grade 2+ late toxicity events (at least one event of the above three) was reported to be 37% (27/73) in the NCIC SR 2 trial preoperative arm. A significant reduction of these late toxicities was observed when compared with the NCIC SR 2 Trial Preoperative arm (6/57 vs. 27/73, p=0. 0005, Fisher’s Exact test) 12

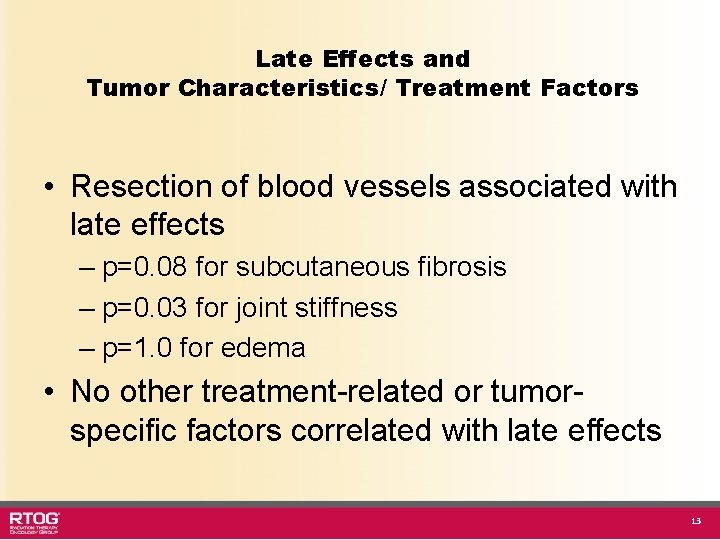
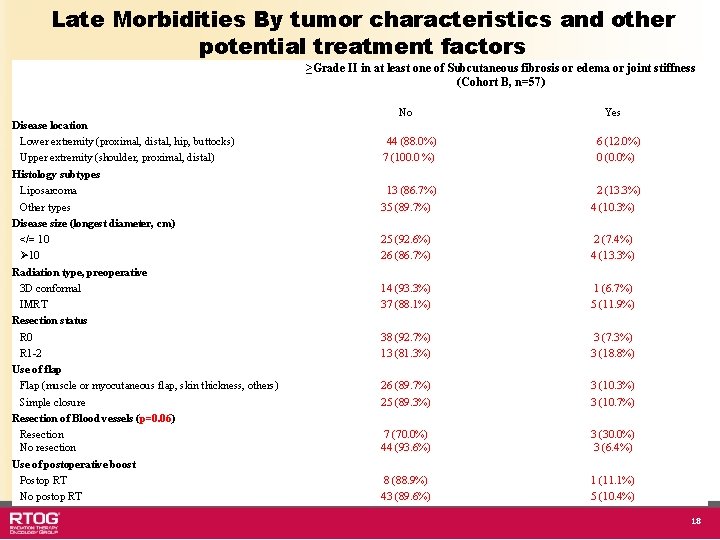
Late Effects and Tumor Characteristics/ Treatment Factors • Resection of blood vessels associated with late effects – p=0. 08 for subcutaneous fibrosis – p=0. 03 for joint stiffness – p=1. 0 for edema • No other treatment-related or tumorspecific factors correlated with late effects 13

Summary • RTOG 0630 Target Definitions and IGRT: Significant reduction of late radiation morbidities (Grade 2+ subcutaneous fibrosis, joint stiffness and edema) when compared to NCIC SR 2 Trial Preoperative RT Arm • 10. 5% RTOG 0630 vs. 37% NCIC SR 2 (p=0. 0005) • Resection of blood vessels has a borderline significance on late effects • Rate of Local Failure after surgery (6. 8% at 2. 8 years) is within expected range and all are in-field failures • Absence of marginal-field local failures reported to-date and the favorable late effect profile indicates that the parameters of radiotherapy in RTOG 0630 can be utilized for pre-operative IGRT of extremity STS 14

Acknowlegement • RTOG HQ • All investigators and patients from participating institutions • Colleagues in the RTOG sarcoma working group • This project was supported by RTOG grant U 10 CA 21661, CCOP grant U 10 CA 37422, and ATC grant U 24 CA 81647 from the National Cancer Institute (NCI) 15

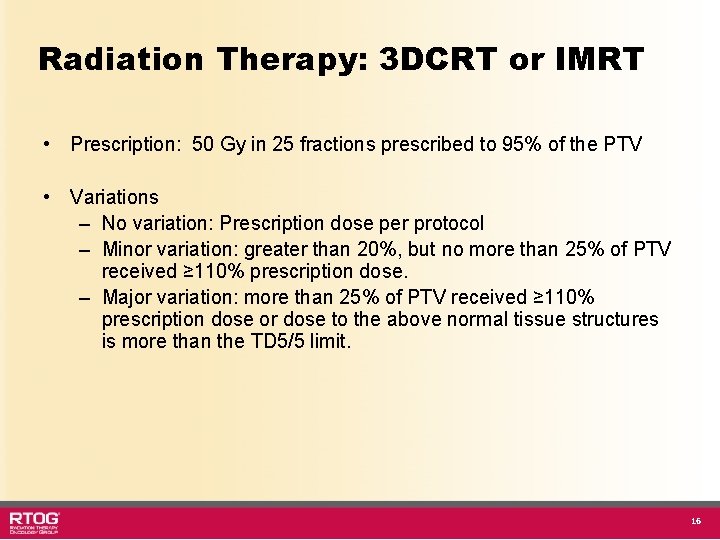
Radiation Therapy: 3 DCRT or IMRT • Prescription: 50 Gy in 25 fractions prescribed to 95% of the PTV • Variations – No variation: Prescription dose per protocol – Minor variation: greater than 20%, but no more than 25% of PTV received ≥ 110% prescription dose. – Major variation: more than 25% of PTV received ≥ 110% prescription dose or dose to the above normal tissue structures is more than the TD 5/5 limit. 16

RTOG 0630 Schema Tumor Grade: Low Intermediate High Tumor Stage: T 1 (≤ 5 cm) or T 2 (>5 cm) Superficial (a) or deep (b) Histology Type: IG-RT: 3 DCRT or IMRT 3 D or 2 D imaging Chemotherapy: Neoadjuvant Concurrent or interdigitated Adjuvant Cohort B: patients receiving pre-operative IGRT only ↓ 50 Gy delivered in 25 fractions - 3 DCRT or IMRT -IGRT: 3 D↓or 2 D imaging Surgery performed 4 -8 weeks later. For a positive margin • EBRT: 16 Gy in 8 fx • Brachytherapy: LDR (16 Gy) or HDR (3. 4 Gy X 4 fx) • IORT: 10 -12. 5 Gy in 1 fx 17

Late Morbidities By tumor characteristics and other potential treatment factors ≥Grade II in at least one of Subcutaneous fibrosis or edema or joint stiffness (Cohort B, n=57) No Disease location Lower extremity (proximal, distal, hip, buttocks) Upper extremity (shoulder, proximal, distal) Histology subtypes Liposarcoma Other types Disease size (longest diameter, cm) </= 10 Ø 10 Radiation type, preoperative 3 D conformal IMRT Resection status R 0 R 1 -2 Use of flap Flap (muscle or myocutaneous flap, skin thickness, others) Simple closure Resection of Blood vessels (p=0. 06) Resection No resection Use of postoperative boost Postop RT No postop RT Yes 44 (88. 0%) 7 (100. 0 %) 6 (12. 0%) 0 (0. 0%) 13 (86. 7%) 35 (89. 7%) 2 (13. 3%) 4 (10. 3%) 25 (92. 6%) 26 (86. 7%) 2 (7. 4%) 4 (13. 3%) 14 (93. 3%) 37 (88. 1%) 1 (6. 7%) 5 (11. 9%) 38 (92. 7%) 13 (81. 3%) 3 (7. 3%) 3 (18. 8%) 26 (89. 7%) 25 (89. 3%) 3 (10. 7%) 7 (70. 0%) 44 (93. 6%) 3 (30. 0%) 3 (6. 4%) 8 (88. 9%) 43 (89. 6%) 1 (11. 1%) 5 (10. 4%) 18

Late Morbidities By Tumor Characteristics and Treatment ≥Grade II in at least one of Subcutaneous fibrosis or edema or joint stiffness (Cohort B, n=57) RT review Target volume contouring score Per protocol and acceptable variation Unacceptable variation Organs at risk contouring score¹ Per protocol Acceptable variation Tumor volume DVH analysis score Per protocol and acceptable variation Unacceptable variation Overall score Per protocol and acceptable variation Unacceptable variation No Yes 43 (91. 5%) 8 (80. 0%) 4 (8. 5%) 2 (20. 0%) 45 (90. 0%) 6 (85. 7%) 5 (10. 0%) 1 (14. 3%) 35 (85. 4%) 13 (100. 0%) 6 (14. 6%) 0 (0. 0%) 45 (88. 2%) 6 (100. 0%) 6 (11. 8%) 0 (0. 0%) 1. Only 1 unacceptable variation 19