Laser LASER L ight A mplification C H


























- Slides: 56

Laser


LASER • L ight • A mplification • • • C. H. Towns, 1954 by S timulated E mission T. H. Maiman, 1960 Noble prize, 1964 of R adiation

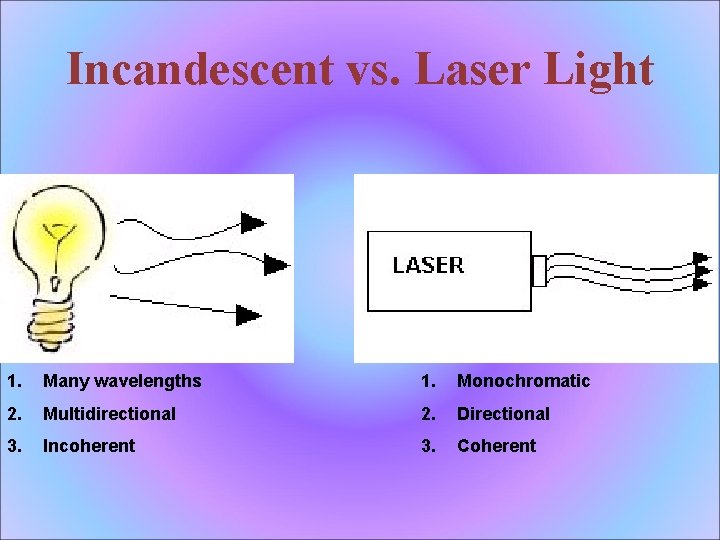
Incandescent vs. Laser Light 1. Many wavelengths 1. Monochromatic 2. Multidirectional 2. Directional 3. Incoherent 3. Coherent

Coherence

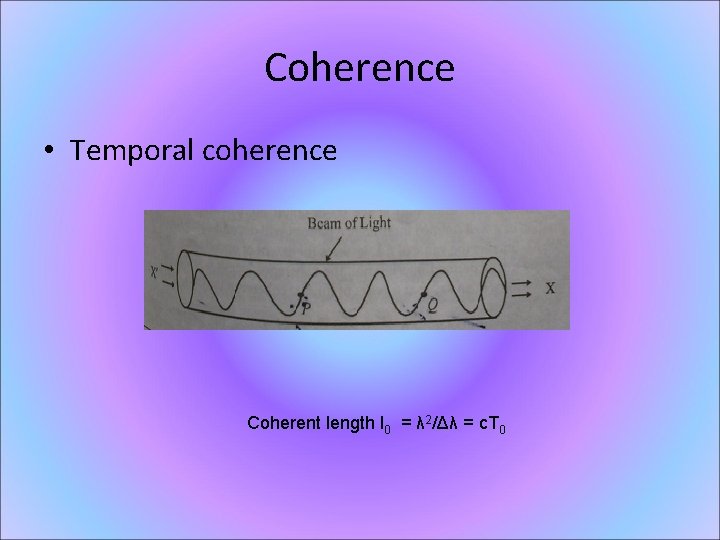
Coherence • Temporal coherence Coherent length l 0 = λ 2/Δλ = c. T 0

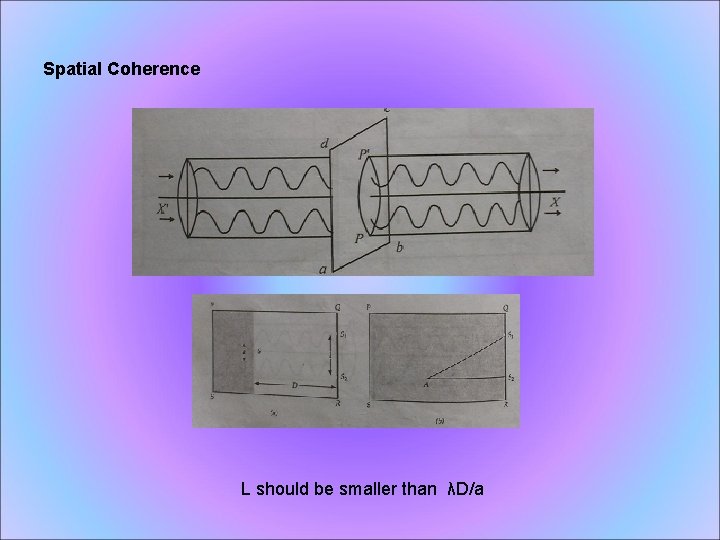
Spatial Coherence L should be smaller than λD/a

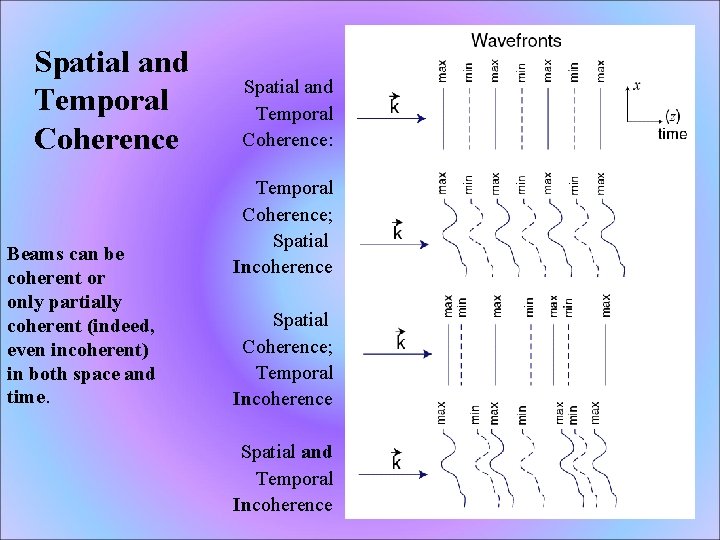
Spatial and Temporal Coherence Beams can be coherent or only partially coherent (indeed, even incoherent) in both space and time. Spatial and Temporal Coherence: Temporal Coherence; Spatial Incoherence Spatial Coherence; Temporal Incoherence Spatial and Temporal Incoherence

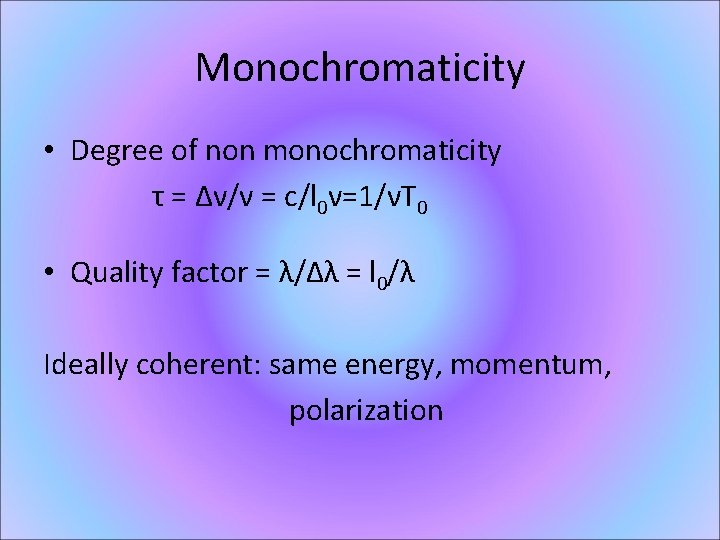
Monochromaticity • Degree of non monochromaticity τ = Δν/ν = c/l 0ν=1/νT 0 • Quality factor = λ/Δλ = l 0/λ Ideally coherent: same energy, momentum, polarization

Absorption and Emission • Excitation potential and critical potential

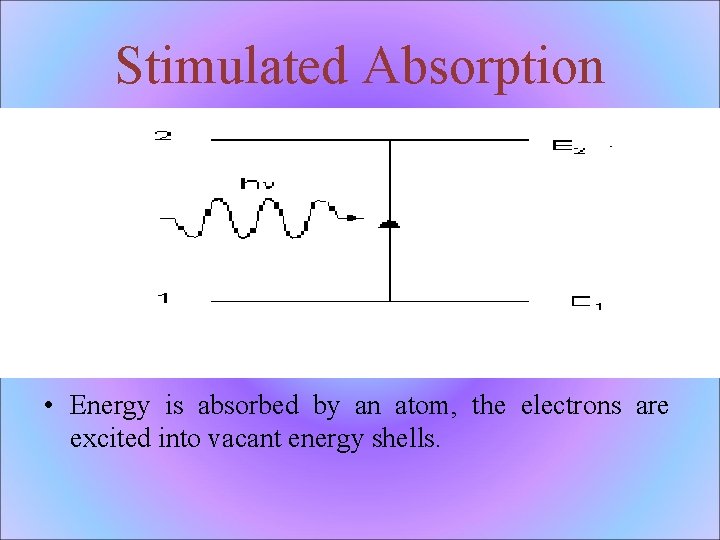
Stimulated Absorption • Energy is absorbed by an atom, the electrons are excited into vacant energy shells.

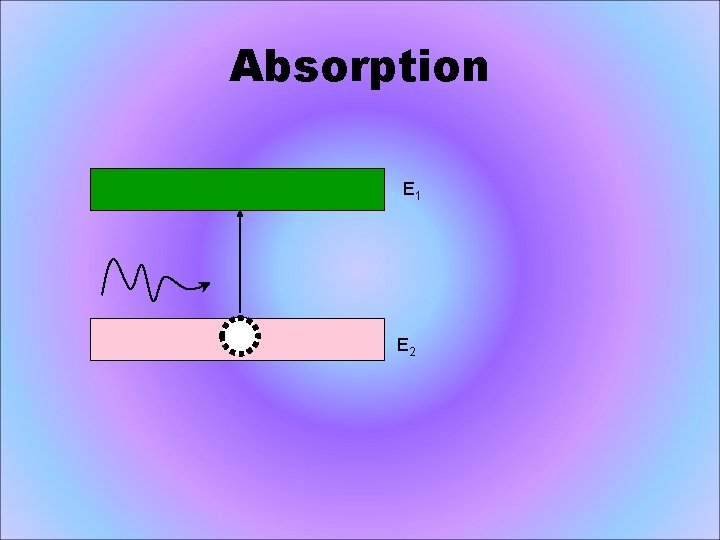
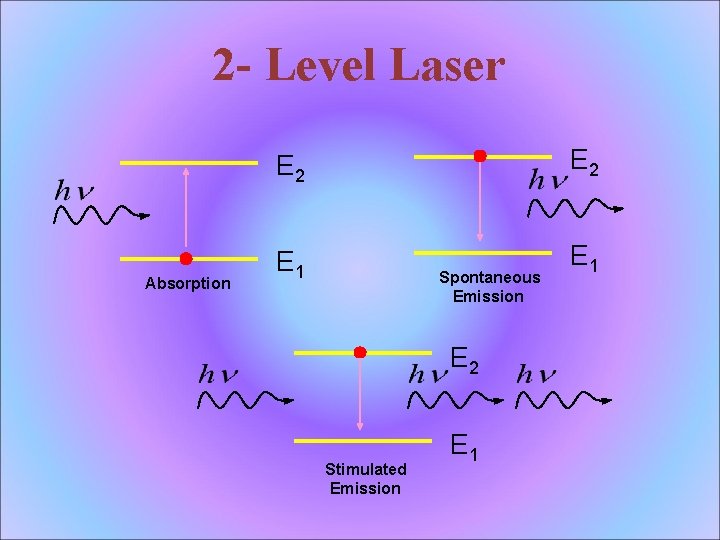
Absorption E 1 E 2

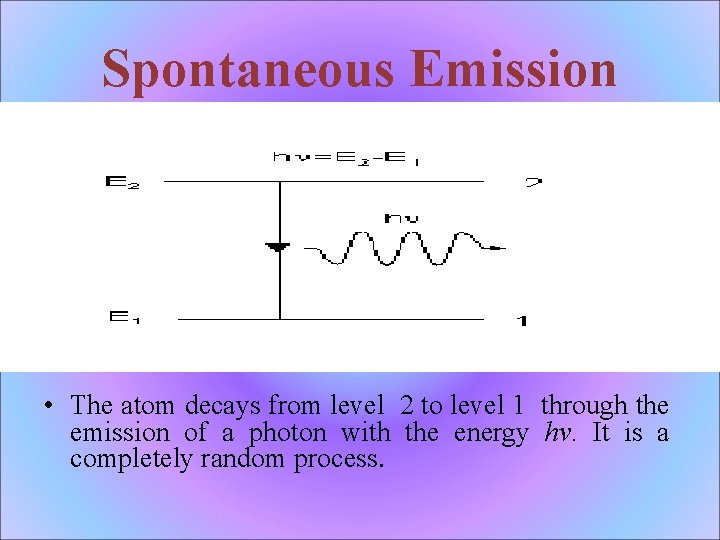
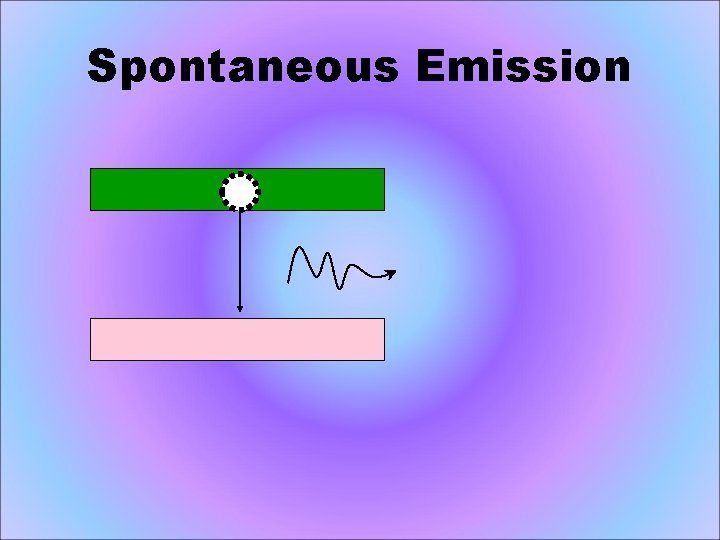
Spontaneous Emission • The atom decays from level 2 to level 1 through the emission of a photon with the energy hv. It is a completely random process.

Spontaneous Emission

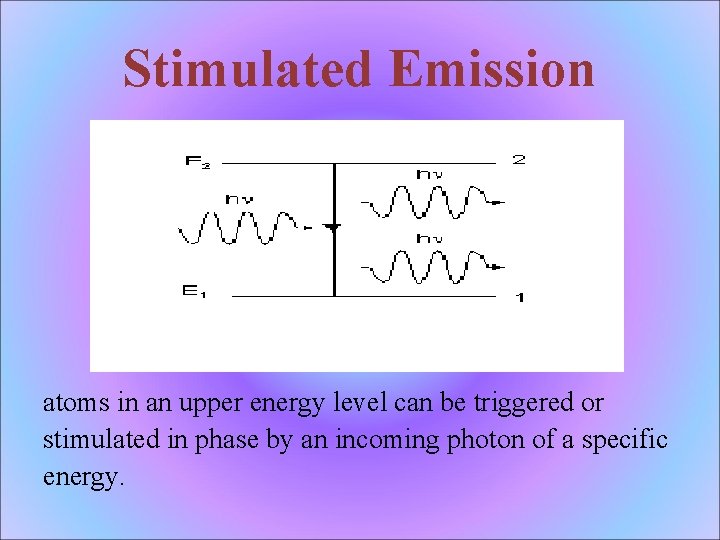
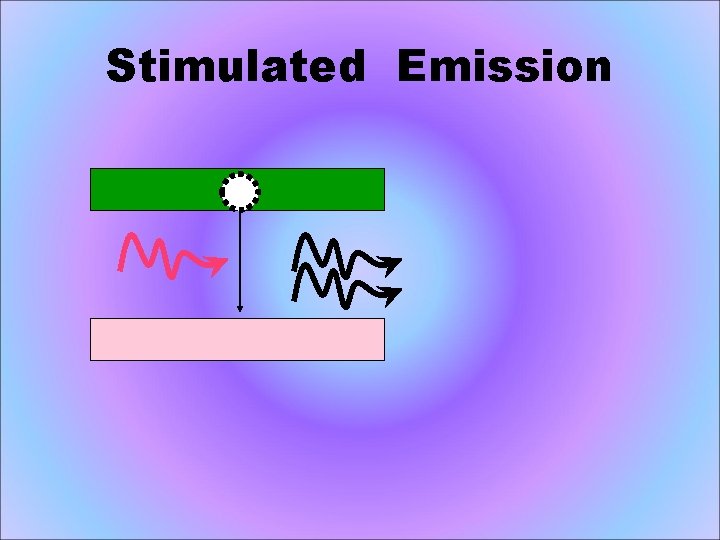
Stimulated Emission atoms in an upper energy level can be triggered or stimulated in phase by an incoming photon of a specific energy.

Stimulated Emission

Stimulated Emission The stimulated photons have unique properties: – In phase with the incident photon – Same wavelength as the incident photon – Travel in same direction as incident photon

Electron/Photon Interactions

WHY WE NEED META STABLE STATE? ANSWER IS With having the metastable state above the ground level. Atom reaches the meta stable state (after first stimulated emission) can remain there for longer time period. So the number of atom increases in the meta stable state. And when these atoms come back to the original ground level it emits laser beam.

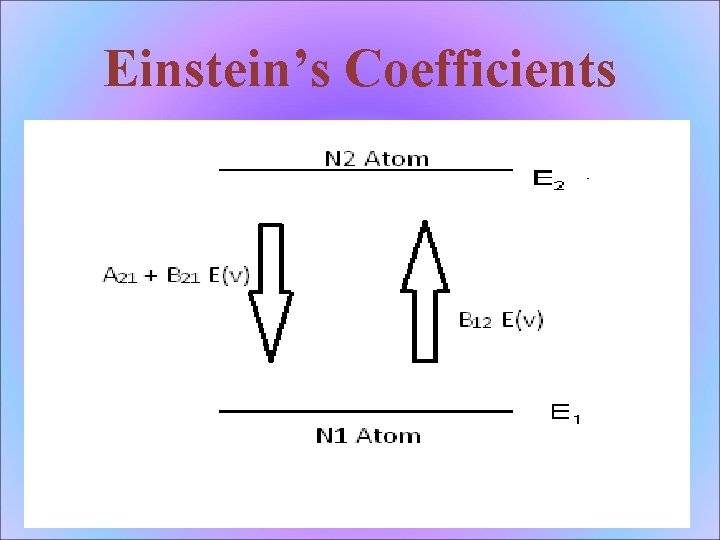
Einstein’s Coefficients

Spontaneous emission A 21 : - correspond to spontaneous emission probability per unit time This particular emission can occur without the presence of external field E(v)

Stimulated Absorption

Stimulated Emission B 21 : - correspond to stimulated emission probability per unit time This type of emission can occur in presence of external field E(v) only

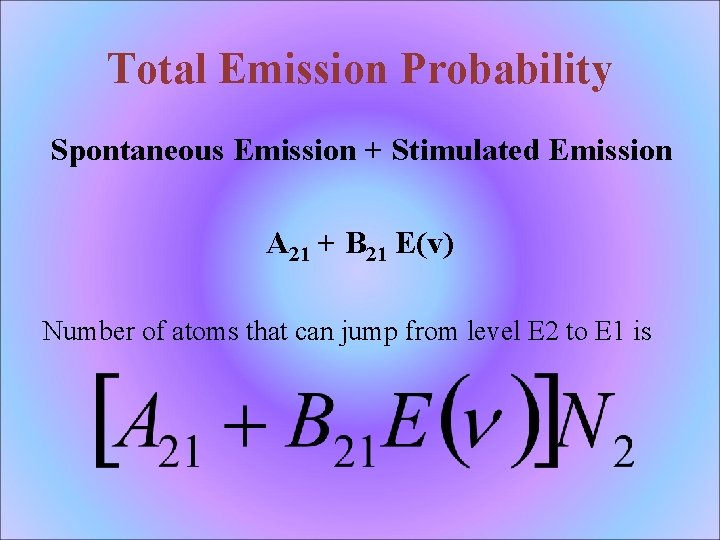
Total Emission Probability Spontaneous Emission + Stimulated Emission A 21 + B 21 E(v) Number of atoms that can jump from level E 2 to E 1 is

Total Absorption Probability

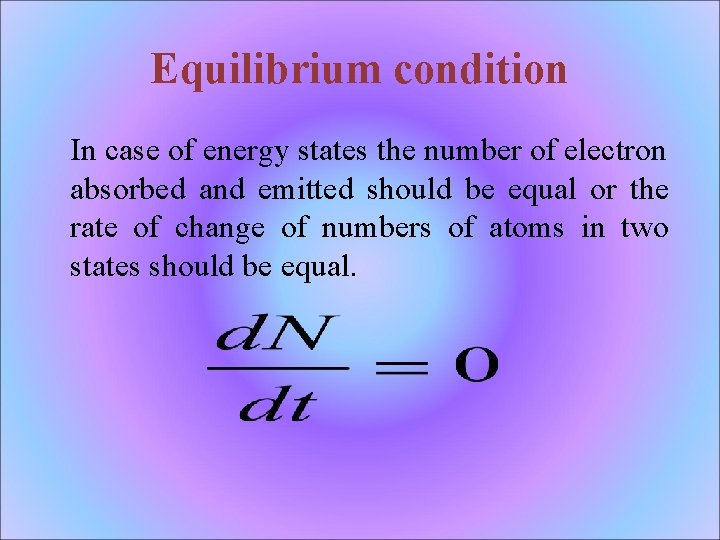
Equilibrium condition In case of energy states the number of electron absorbed and emitted should be equal or the rate of change of numbers of atoms in two states should be equal.

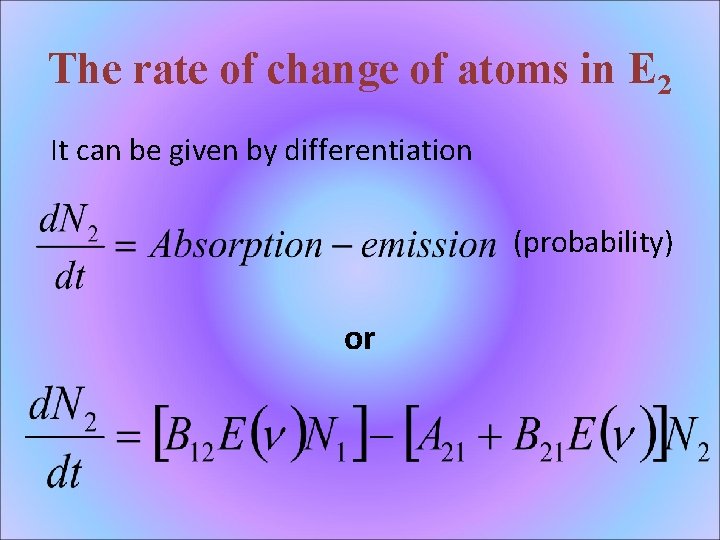
The rate of change of atoms in E 2 It can be given by differentiation (probability) or

At Equilibrium Then

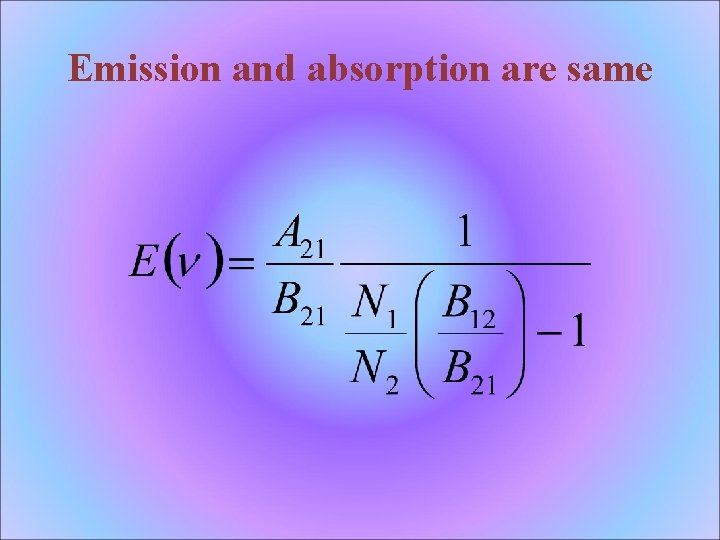
Emission and absorption are same

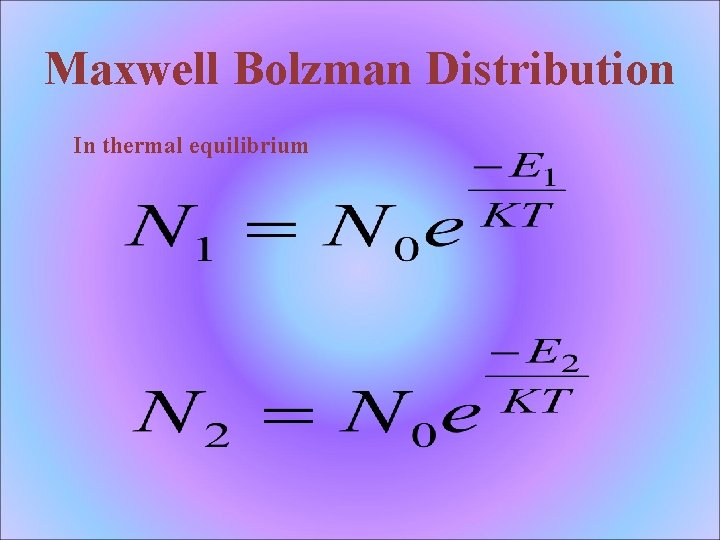
Maxwell Bolzman Distribution In thermal equilibrium

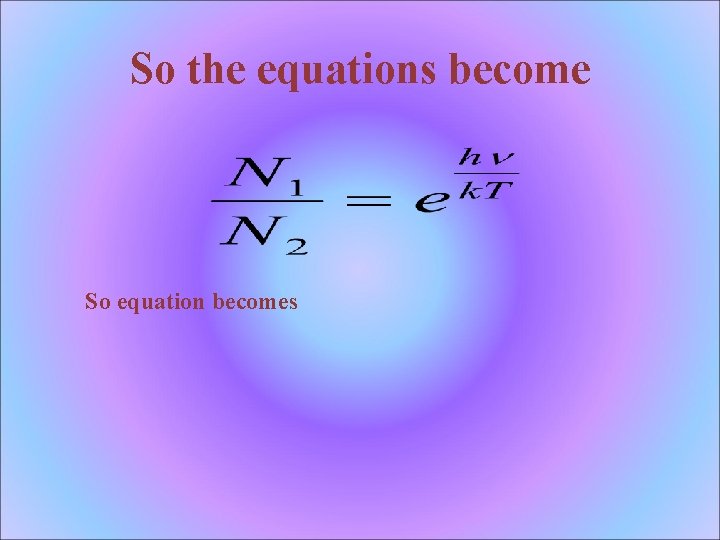
So the equations become So equation becomes

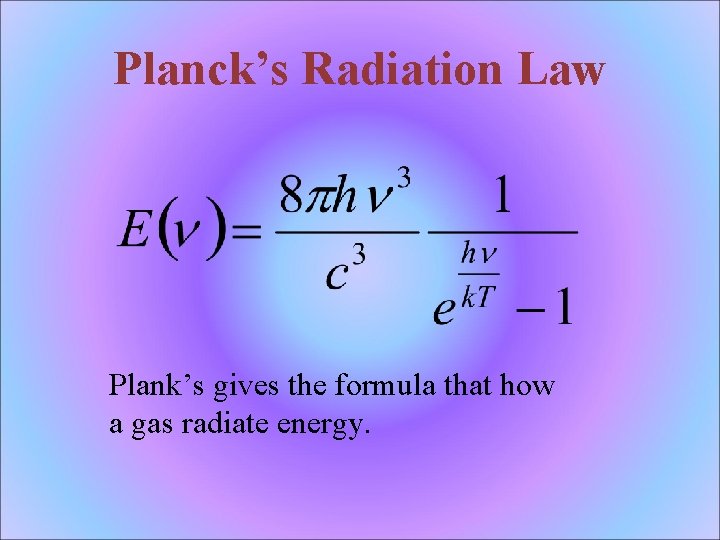
Planck’s Radiation Law Plank’s gives the formula that how a gas radiate energy.

Einstein’s Coefficients Einstein gives a probability that stimulated emission is same as absorption. Means that if a stimulated absorption can occur then there is same probability that stimulated emission can occur.

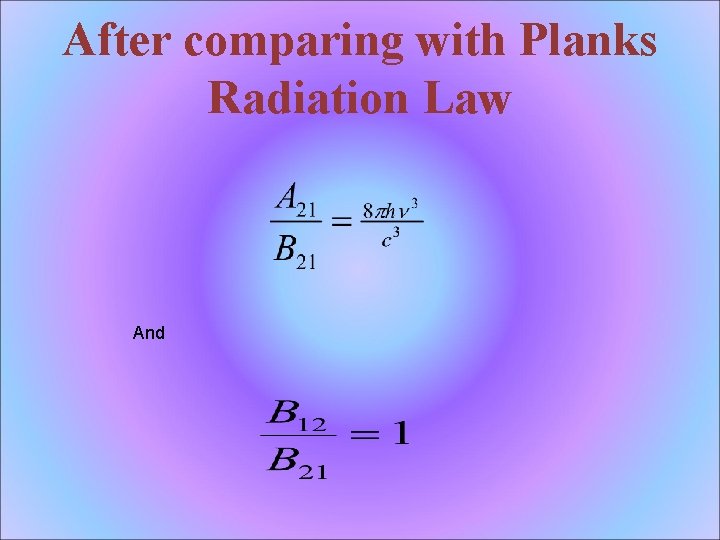
After comparing with Planks Radiation Law And

Conclusions • Stimulated emission have same probability as stimulated absorption • Ratio between spontaneous and stimulated emission varies with v 3 • All we need is to calculate one of the probability to find others.

BASIC PRINCIPLE NEEDED FOR LASER

POPULATION INVERSION • A state of a medium where a higher-lying electronic level has a higher population than a lower-lying level

PUMPING • The method particle of raising a particle from lower energy state to higher energy state is called pumping. • TYPES OF PUMPING : 1. Optical pumping 2. Electrical pumping 3. X-ray pumping 4. Chemical pumping

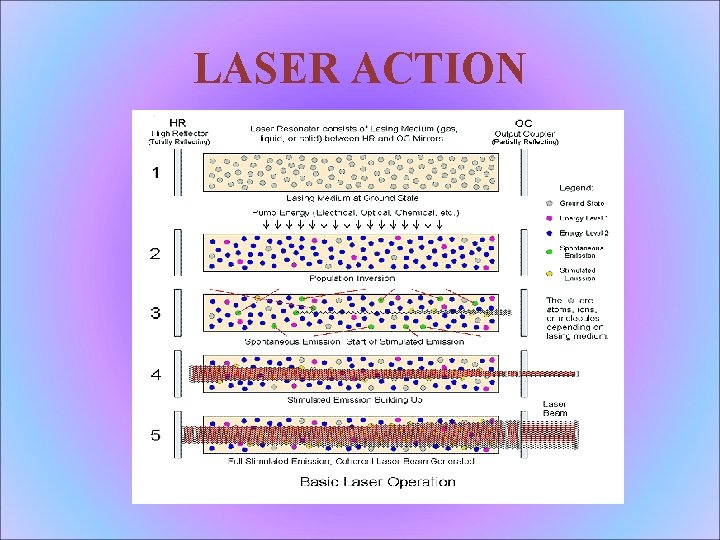
LASER COMPONENTS All lasers have 3 essential components: • A lasing or "gain" medium • A source of energy to excite electrons in the gain medium to high energy states, referred to as "pump" energy • An optical path which allows emitted photons to oscillate and interfere constructively as energy is added or "pumped" into the system, ie, a resonator


LASER ACTION

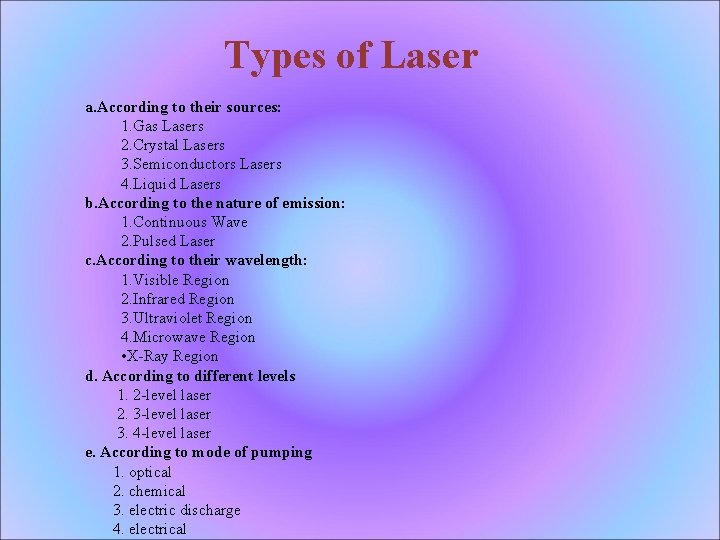
Types of Laser a. According to their sources: 1. Gas Lasers 2. Crystal Lasers 3. Semiconductors Lasers 4. Liquid Lasers b. According to the nature of emission: 1. Continuous Wave 2. Pulsed Laser c. According to their wavelength: 1. Visible Region 2. Infrared Region 3. Ultraviolet Region 4. Microwave Region • X-Ray Region d. According to different levels 1. 2 -level laser 2. 3 -level laser 3. 4 -level laser e. According to mode of pumping 1. optical 2. chemical 3. electric discharge 4. electrical

2 - Level Laser Absorption E 2 E 1 Spontaneous Emission E 2 Stimulated Emission E 1

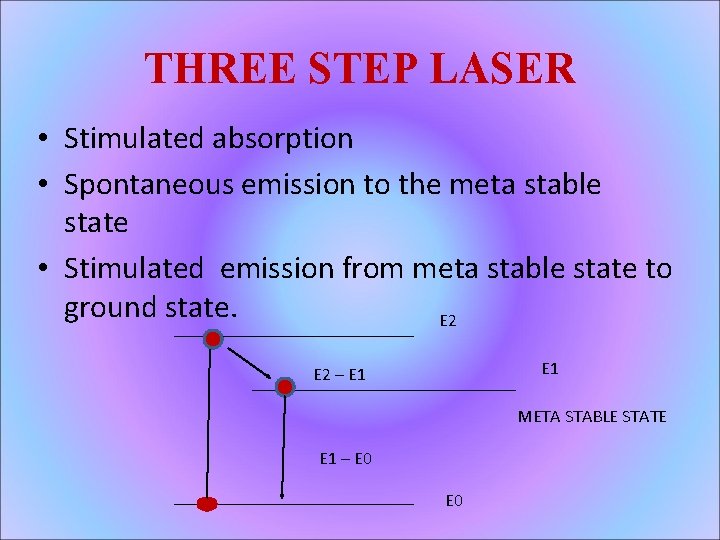
THREE STEP LASER • Stimulated absorption • Spontaneous emission to the meta stable state • Stimulated emission from meta stable state to ground state. E 2 E 1 E 2 – E 1 META STABLE STATE E 1 – E 0

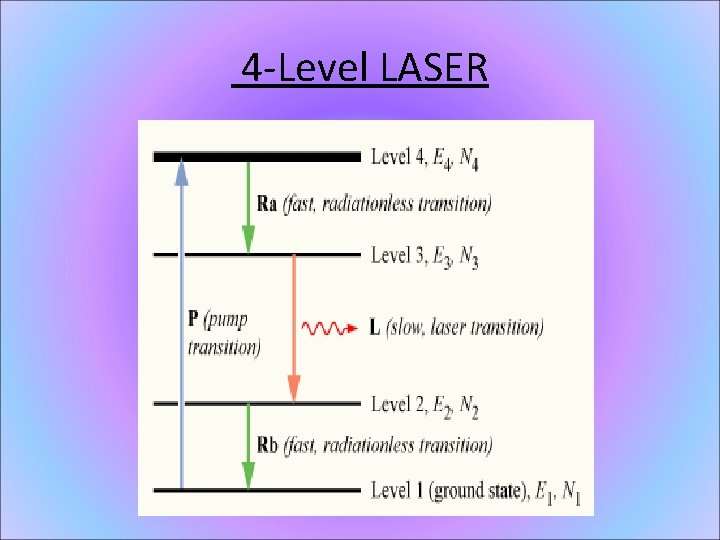
4 -Level LASER

PRACTICAL LASERS

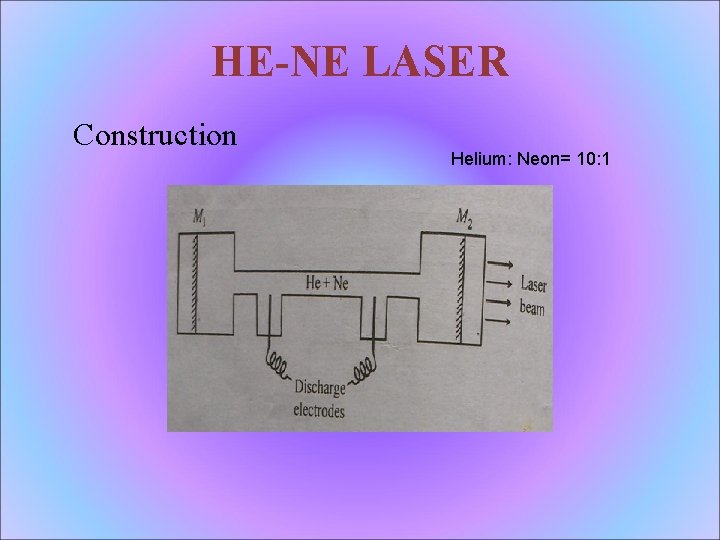
HE-NE LASER Construction Helium: Neon= 10: 1

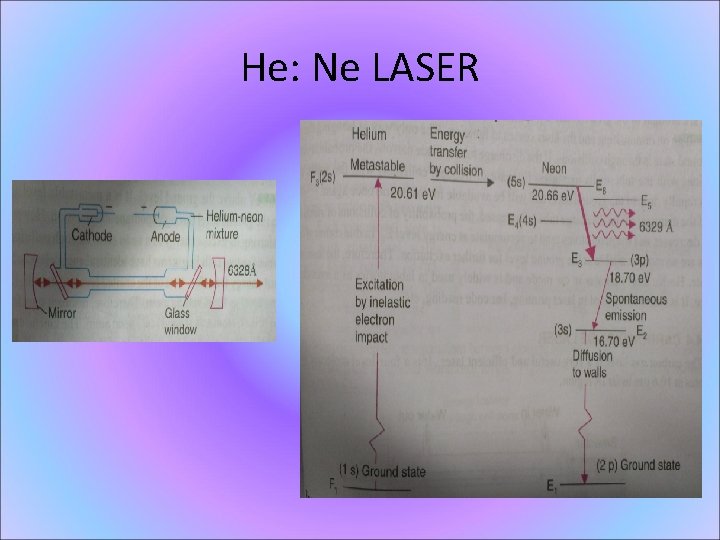
. Energy Level Diagram of He-Ne Power output: m. W

He: Ne LASER

Nd: YAG LASER

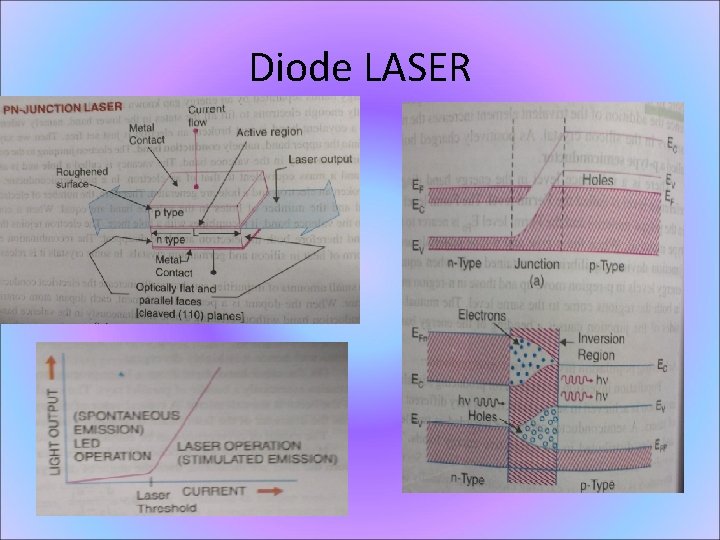
Diode LASER

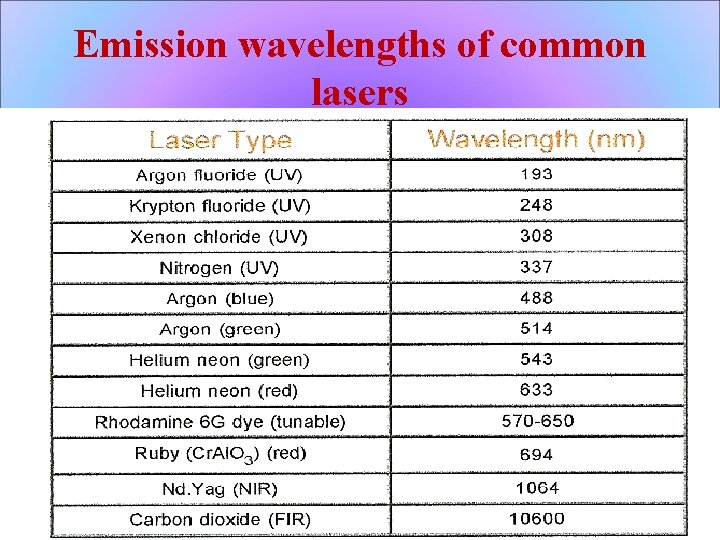
Emission wavelengths of common lasers

APPLICATION S OF LASER

Not to be Taken Lightly The Weighty Implications of Laser Technology Nuclear fusion Long distance measurement Holography Medical • Optical Surgery • General Surgery • Tattoo removal Entertainment Applications of Laser Technology • CD Players • DVD Players • Video Game Systems Telecommunications • Information tech. • Holograms • Satellites Military • Weapons • Radar 2020/11/25 Industry

Can You See the Light? Military and Space aircraft are equipped with laser guns Airplanes are equipped with laser radar Bad eyesight can be corrected by optical surgery using lasers Cd-Rom discs are read by lasers Dentists use laser drills Tattoo removal is done using lasers Laser tech. is used in printers, copiers, and scanners CD-Audio is read by a laser 2020/11/25 Laser pointers can enhance presentations DVD players read DVD’s using lasers Bar codes in grocery stores are scanned by lasers Video game systems such as Play. Station 2 utilize lasers

THANKS SEE YOU IN NEXT LECTURE