LASER CHARACTERISTICS OF LASER LIGHT MONOCHROMATICITY DIRECTIONALITY COHERENCE





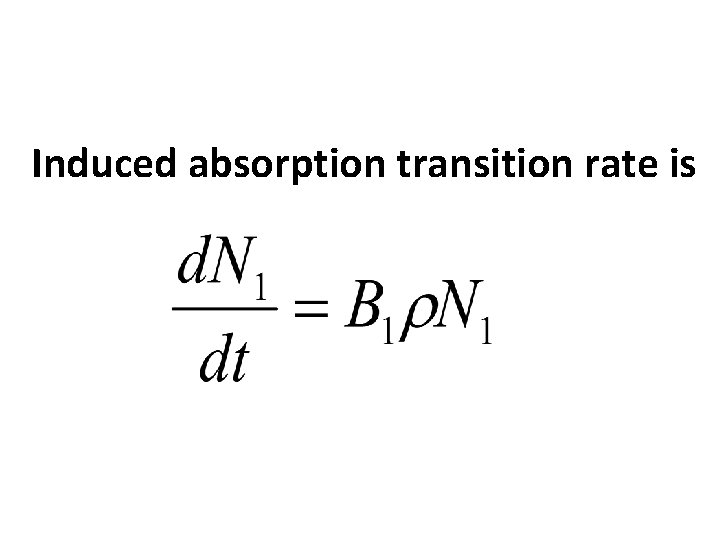

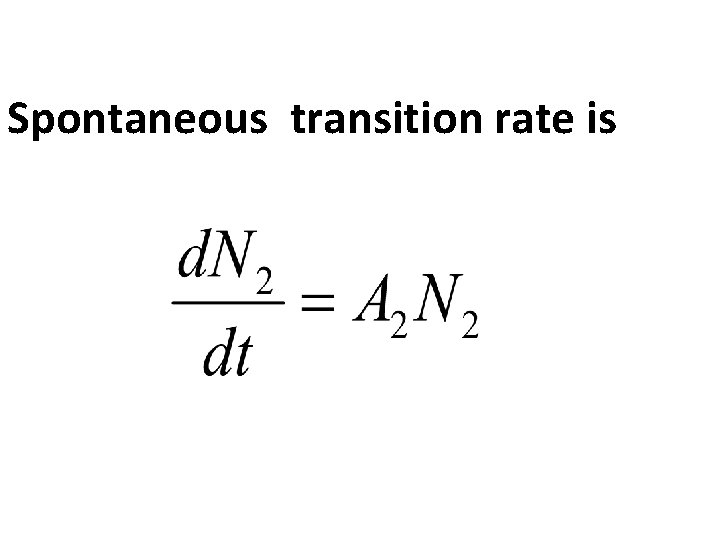

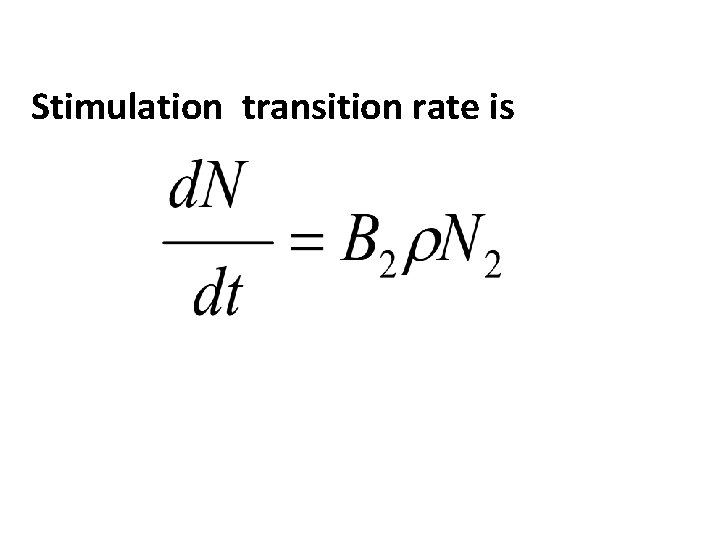
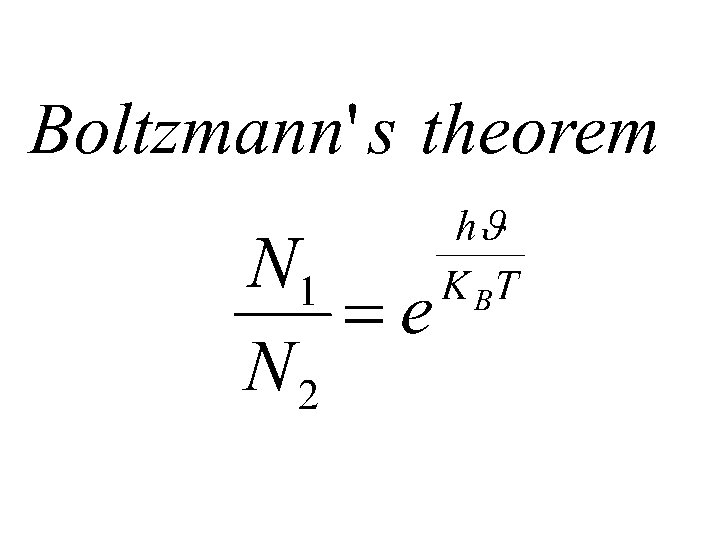

























- Slides: 86

LASER


CHARACTERISTICS OF LASER LIGHT MONOCHROMATICITY DIRECTIONALITY COHERENCE INTENCE BRIGHTNESS FOCUSSING &COLLIMATION PROPERTY The combination of these three properties makes laser light focus 100 times better than ordinary light Laser-Professionals. com

MONOCHROMATICITY: The light emitted from a laser is monochromatic, that is, it is of one color/wavelength. In contrast, ordinary white light is a combination of many colors (or wavelengths) of light.

DIRECTIONALITY: Lasers emit light that is highly directional, that is, laser light is emitted as a relatively narrow beam in a specific direction. Ordinary light, such as from a light bulb, is emitted in many directions away from the source

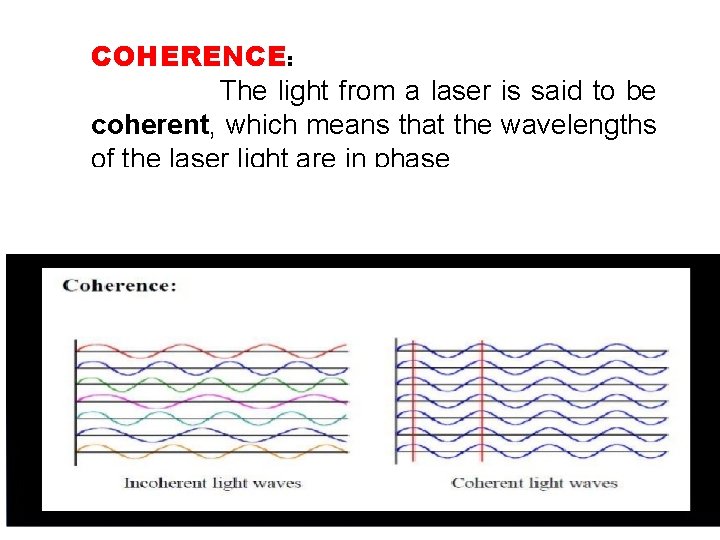
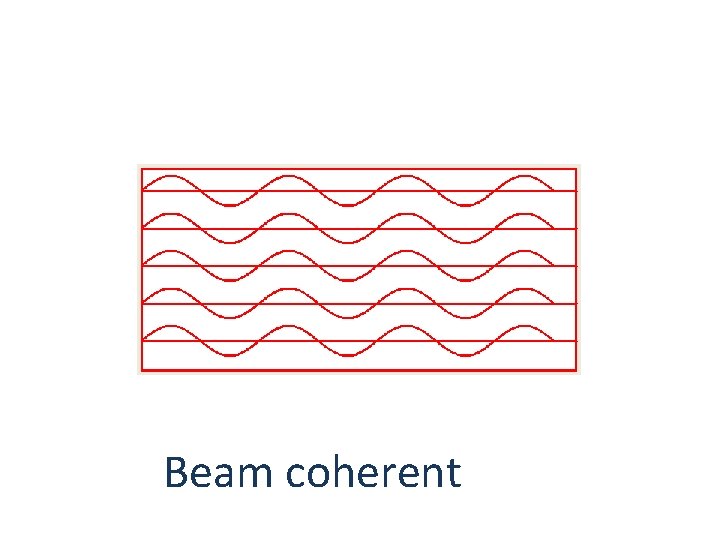
COHERENCE: The light from a laser is said to be coherent, which means that the wavelengths of the laser light are in phase

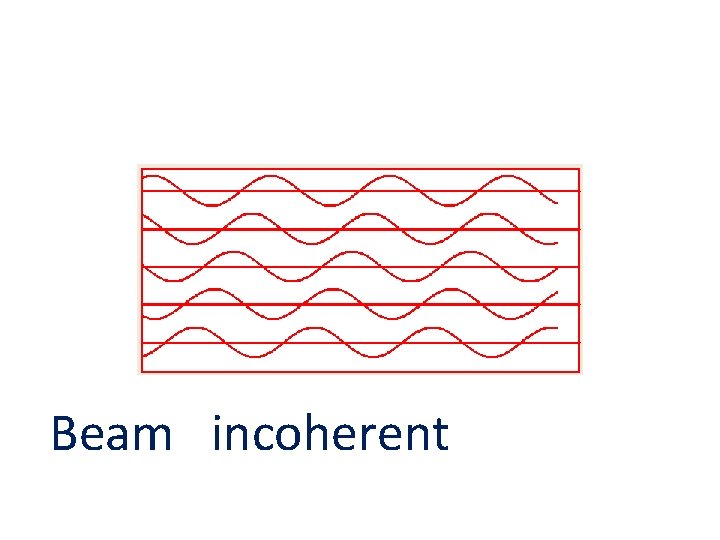
Beam incoherent

Beam coherent

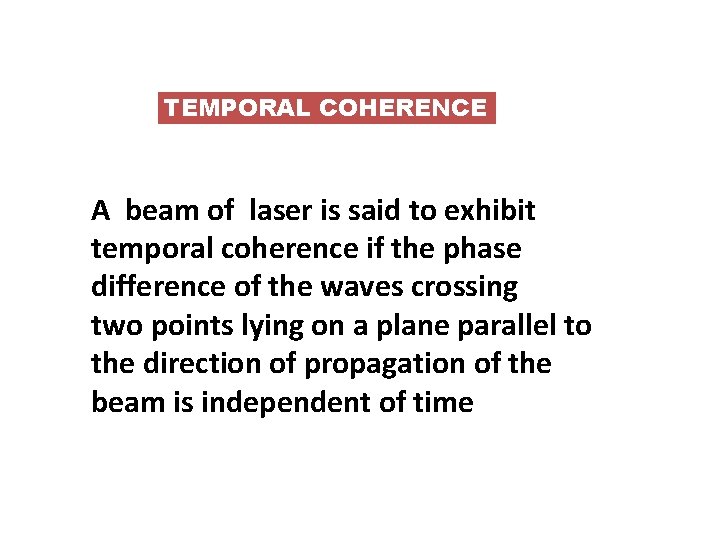
TEMPORAL COHERENCE A beam of laser is said to exhibit temporal coherence if the phase difference of the waves crossing two points lying on a plane parallel to the direction of propagation of the beam is independent of time

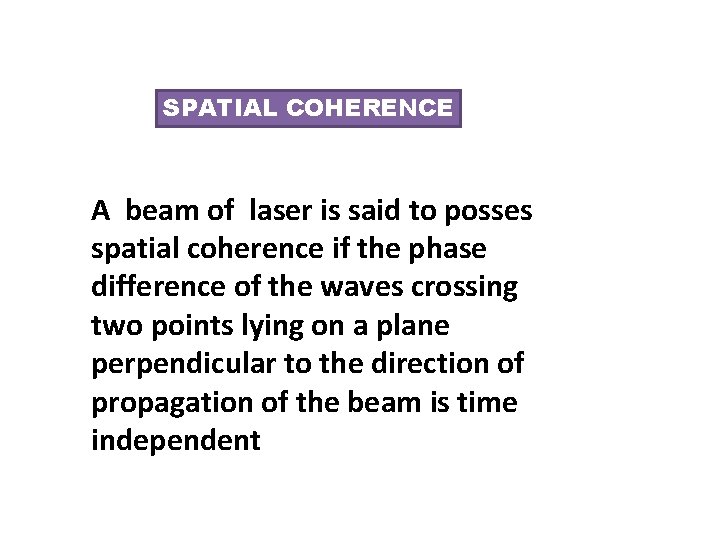
SPATIAL COHERENCE A beam of laser is said to posses spatial coherence if the phase difference of the waves crossing two points lying on a plane perpendicular to the direction of propagation of the beam is time independent

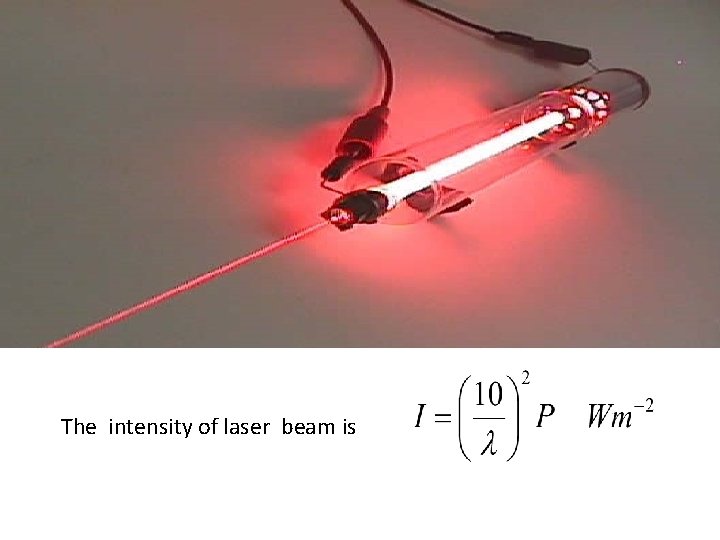
INTENSE BRIGHTNESS Laser beam is more intense than the light from any other source

The intensity of laser beam is

FOCUSSING & COLLIMATING PROPERTY Laser beam can be focussed & collimated to a very small area

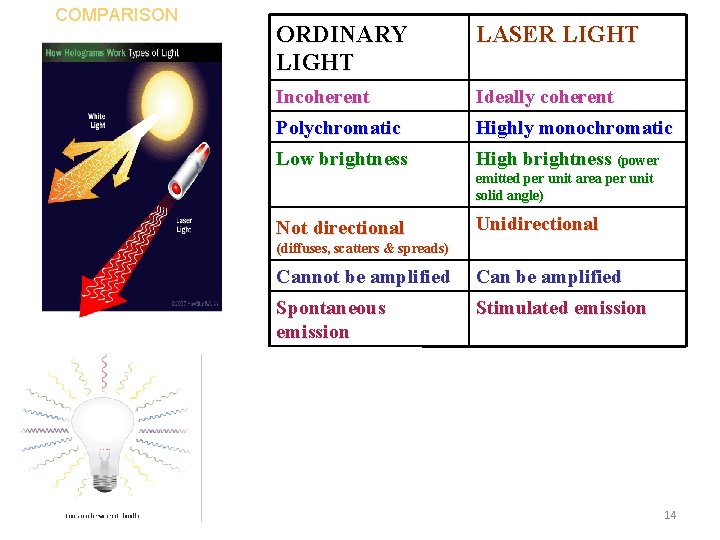
COMPARISON ORDINARY LIGHT LASER LIGHT Incoherent Ideally coherent Polychromatic Highly monochromatic Low brightness High brightness (power emitted per unit area per unit solid angle) Not directional Unidirectional (diffuses, scatters & spreads) 2/6/2022 Cannot be amplified Can be amplified Spontaneous emission Stimulated emission 14

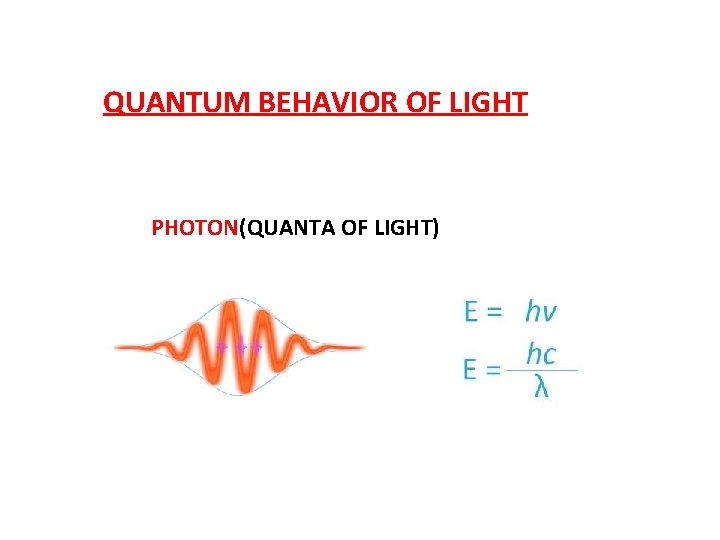
QUANTUM BEHAVIOR OF LIGHT PHOTON(QUANTA OF LIGHT)

BASIC CONCEPTS OF LASER

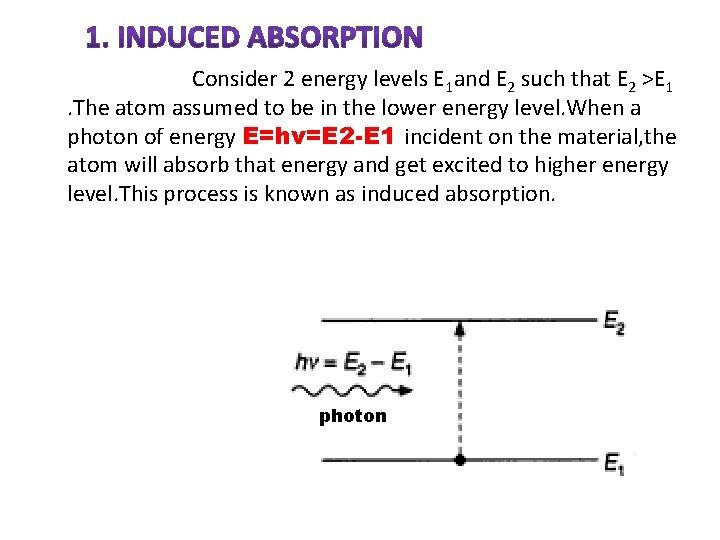
INDUCED ABSORPTION

Consider 2 energy levels E 1 and E 2 such that E 2 >E 1. The atom assumed to be in the lower energy level. When a photon of energy E=hν=E 2 -E 1 incident on the material, the atom will absorb that energy and get excited to higher energy level. This process is known as induced absorption. photon
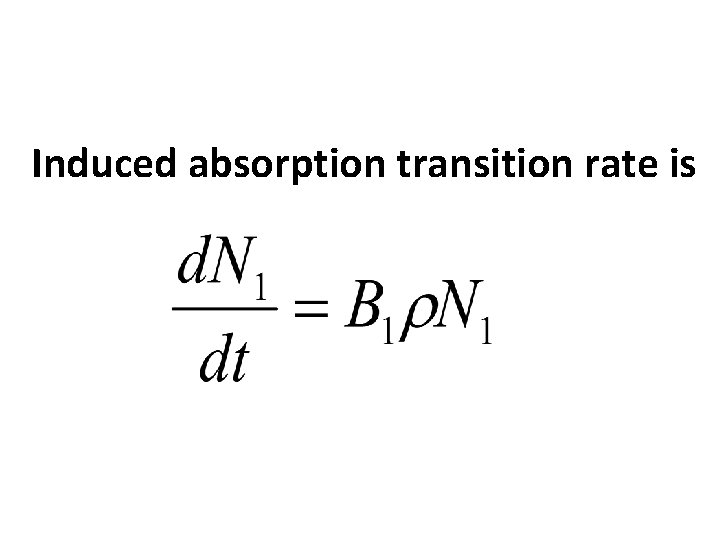
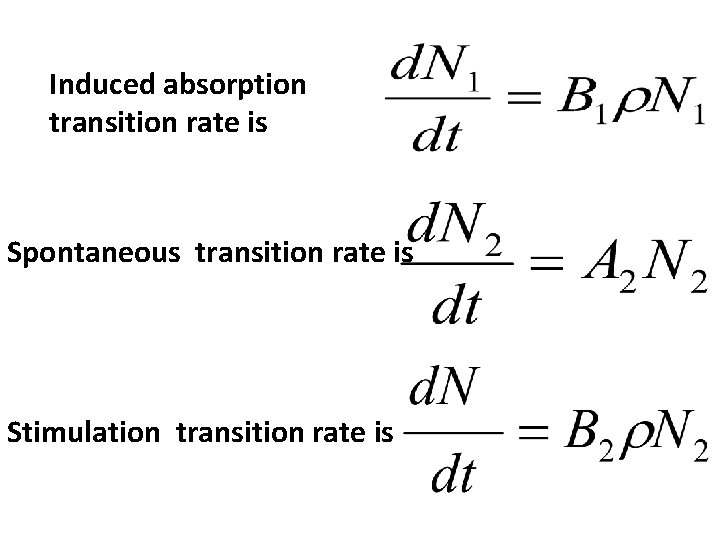
Induced absorption transition rate is

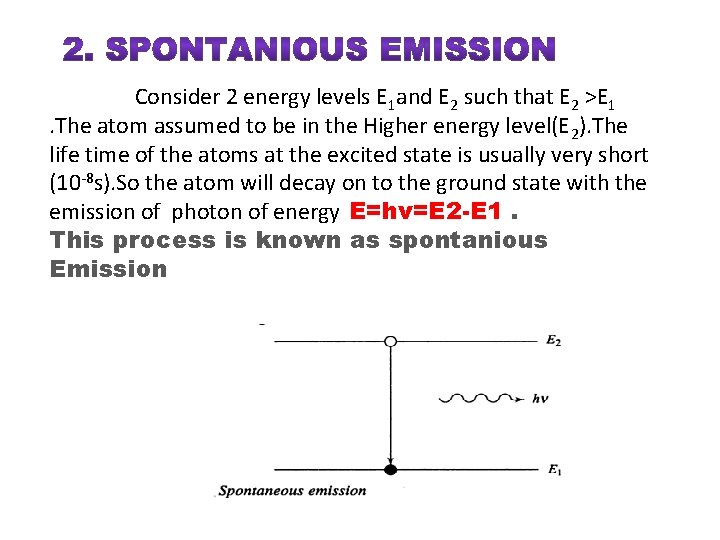
SPONTANIOUS EMISSION

Consider 2 energy levels E 1 and E 2 such that E 2 >E 1. The atom assumed to be in the Higher energy level(E 2). The life time of the atoms at the excited state is usually very short (10 -8 s). So the atom will decay on to the ground state with the emission of photon of energy E=hν=E 2 -E 1. This process is known as spontanious Emission
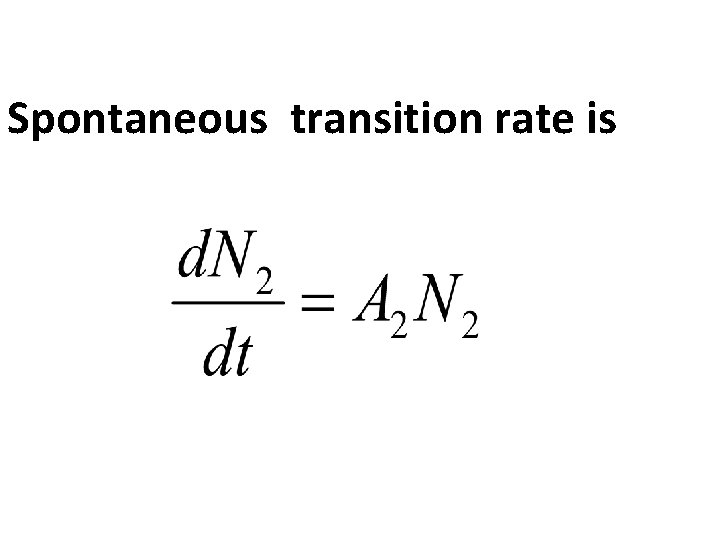
Spontaneous transition rate is

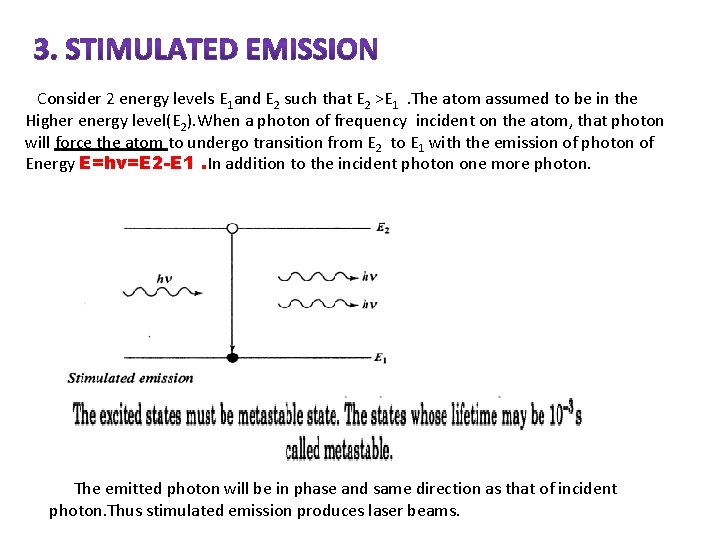
STIMULATED EMISSION

Consider 2 energy levels E 1 and E 2 such that E 2 >E 1. The atom assumed to be in the Higher energy level(E 2). When a photon of frequency incident on the atom, that photon will force the atom to undergo transition from E 2 to E 1 with the emission of photon of Energy E=hν=E 2 -E 1. In addition to the incident photon one more photon. The emitted photon will be in phase and same direction as that of incident photon. Thus stimulated emission produces laser beams.
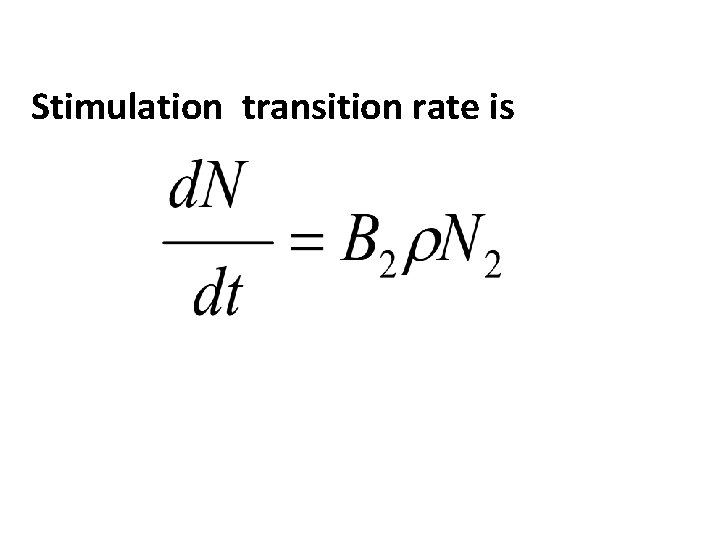
Stimulation transition rate is

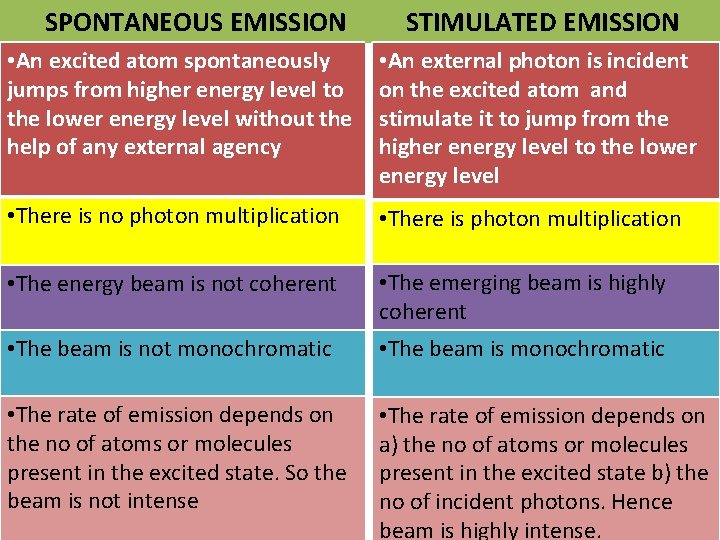
SPONTANEOUS EMISSION STIMULATED EMISSION • An excited atom spontaneously jumps from higher energy level to the lower energy level without the help of any external agency • An external photon is incident on the excited atom and stimulate it to jump from the higher energy level to the lower energy level • There is no photon multiplication • There is photon multiplication • The energy beam is not coherent • The emerging beam is highly coherent • The beam is not monochromatic • The beam is monochromatic • The rate of emission depends on the no of atoms or molecules present in the excited state. So the beam is not intense • The rate of emission depends on a) the no of atoms or molecules present in the excited state b) the no of incident photons. Hence beam is highly intense.

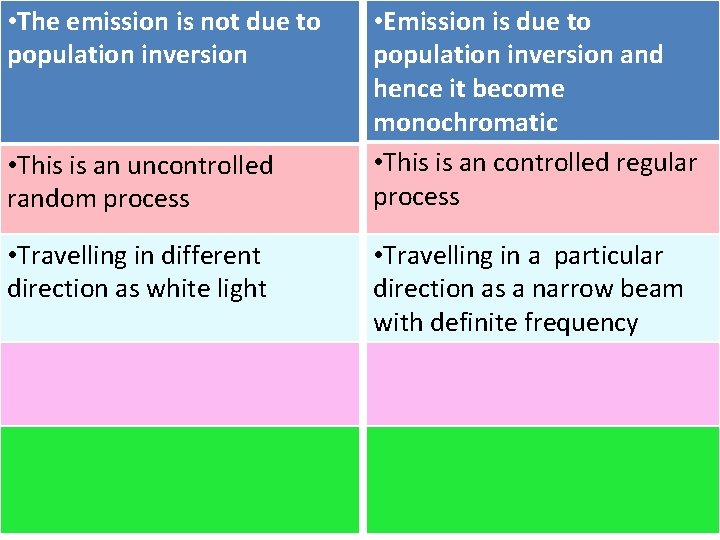
• The emission is not due to population inversion • This is an uncontrolled random process • Travelling in different direction as white light • Emission is due to population inversion and hence it become monochromatic • This is an controlled regular process • Travelling in a particular direction as a narrow beam with definite frequency

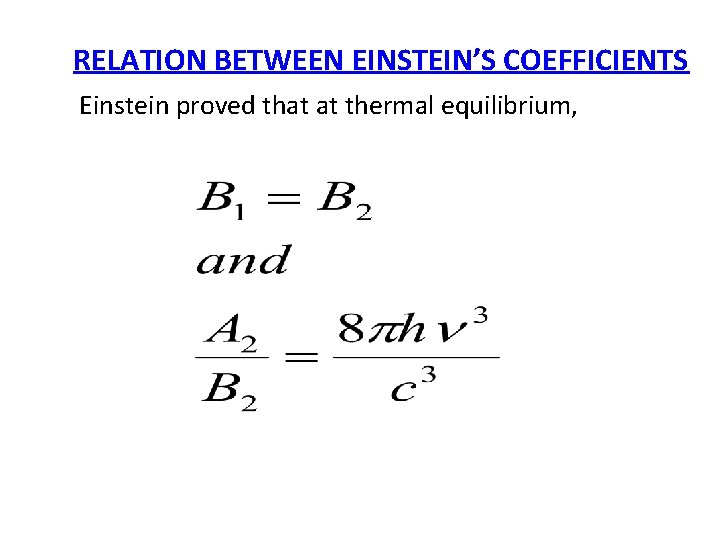
RELATION BETWEEN EINSTEIN’S COEFFICIENTS Einstein proved that at thermal equilibrium,

Induced absorption transition rate is Spontaneous transition rate is Stimulation transition rate is
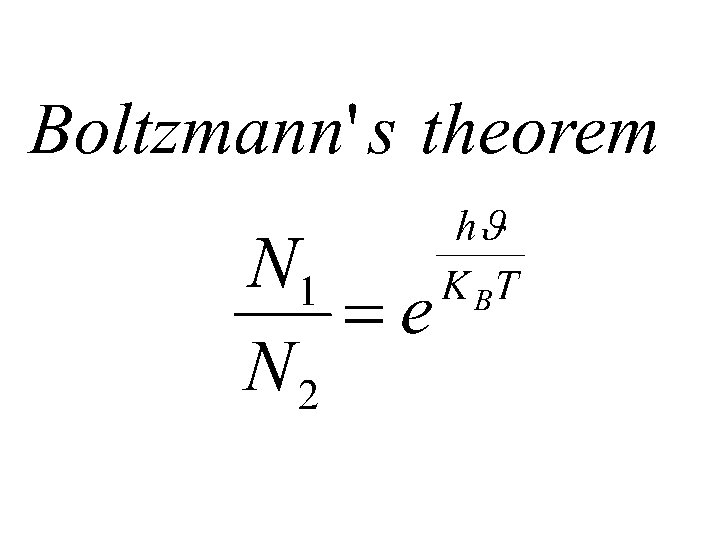

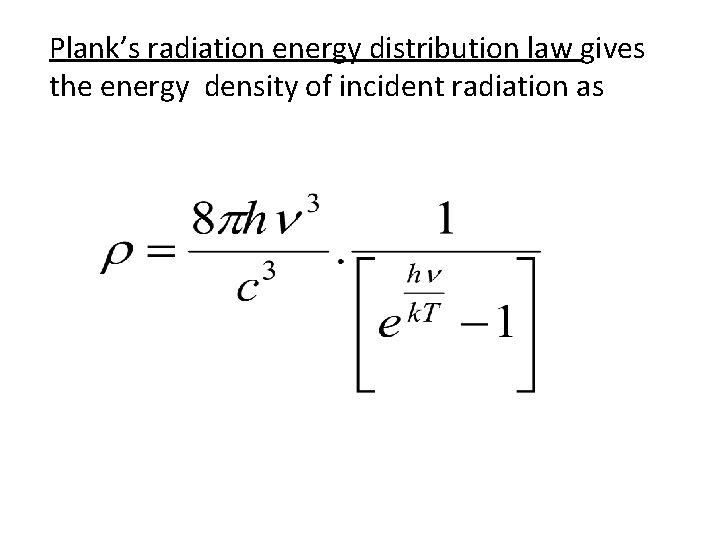
Plank’s radiation energy distribution law gives the energy density of incident radiation as

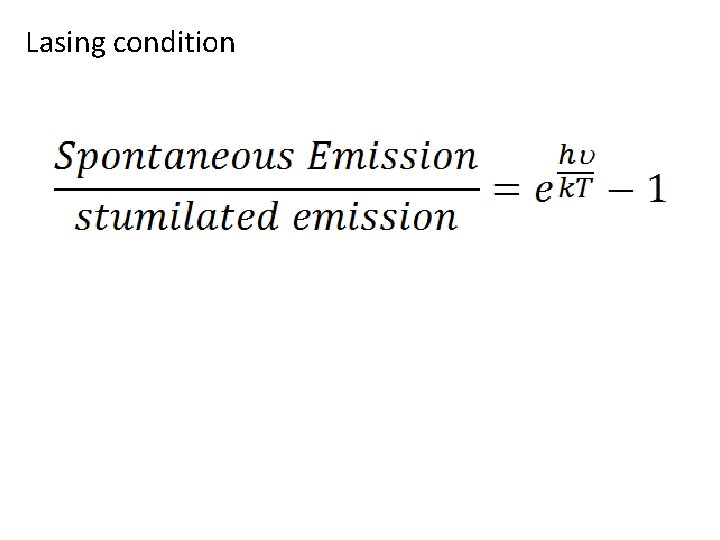
Lasing condition

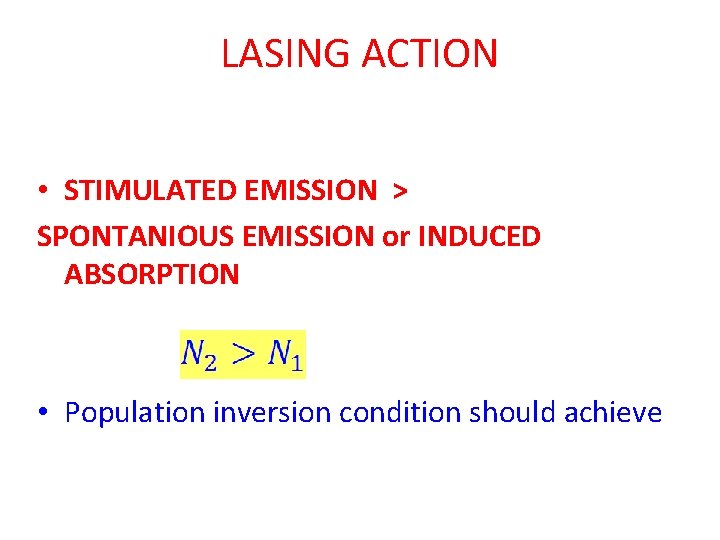
LASING ACTION • STIMULATED EMISSION > SPONTANIOUS EMISSION or INDUCED ABSORPTION • Population inversion condition should achieve

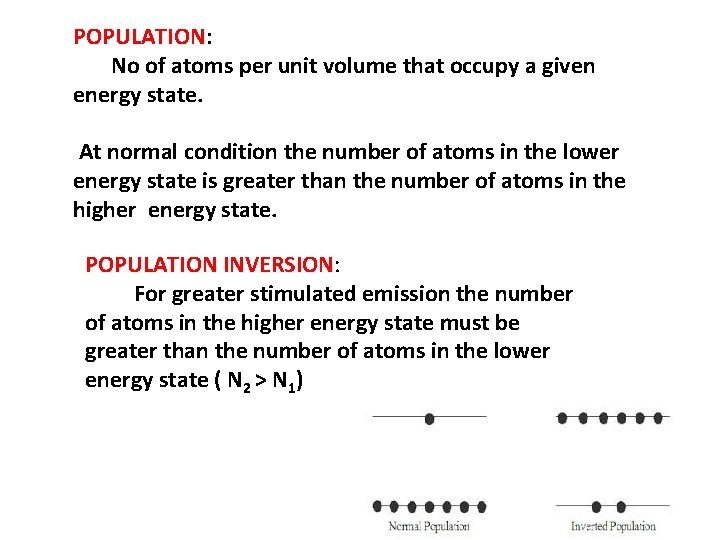
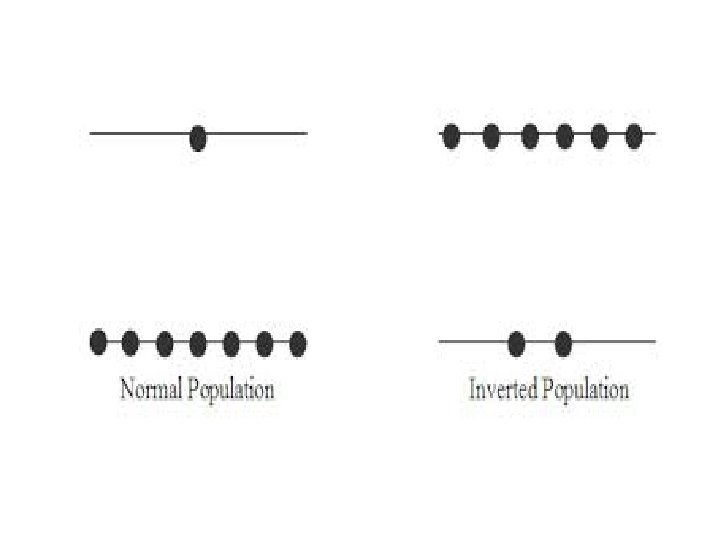
POPULATION: No of atoms per unit volume that occupy a given energy state. At normal condition the number of atoms in the lower energy state is greater than the number of atoms in the higher energy state. POPULATION INVERSION: For greater stimulated emission the number of atoms in the higher energy state must be greater than the number of atoms in the lower energy state ( N 2 > N 1)


PUMPING: The method by which population inversion is achieved It is the process for achieving population inversion by transferring atoms from the lower energy level to the higher energy level

PUMPING • Optical pumping • Electric discharge or electrical pumping • Direct conversion or Electron Beam pumping • Chemical pumping • Thermal pumping

Optical pumping A light source is used to supply energy. Atoms absorb energy from the light flashes and they are excited to higher energy level Electric discharge or electrical pumping The electrons are excited using electric discharges Direct conversion or Electron Beam pumping A beam of electrons is injected in to the semiconductor

Chemical pumping The energy required for pumping is directly obtained from chemical reaction Thermal pumping The active material is heated to a very high temperature

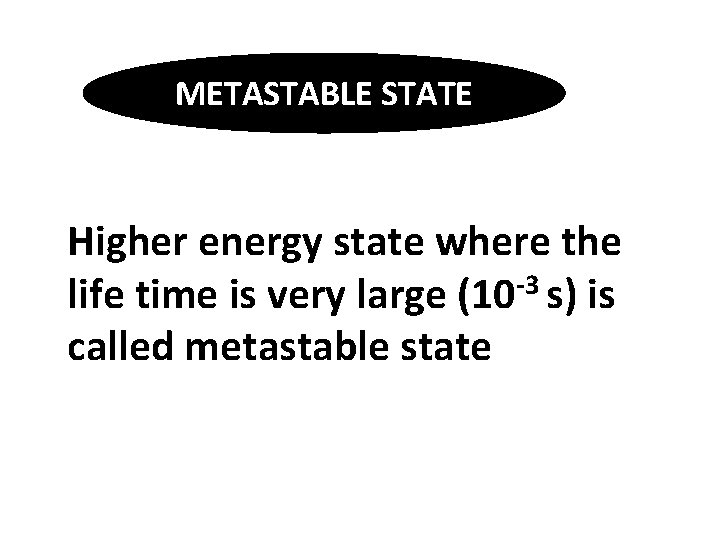
METASTABLE STATE Higher energy state where the life time is very large (10 -3 s) is called metastable state

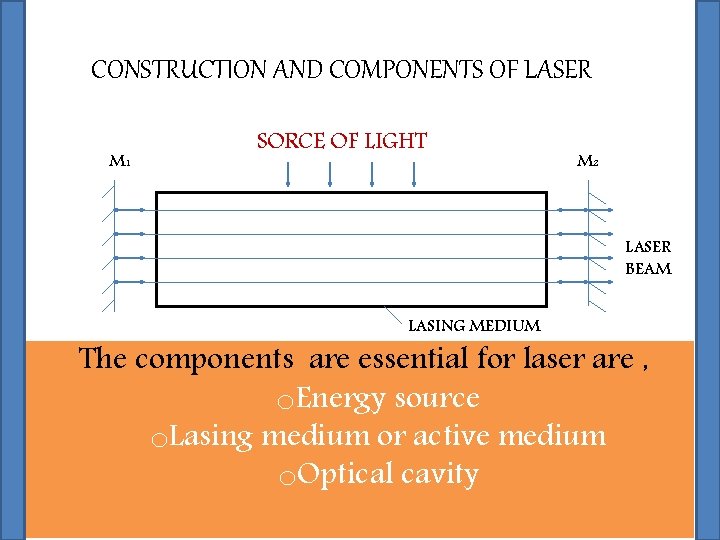
CONSTRUCTION AND COMPONENTS OF LASER


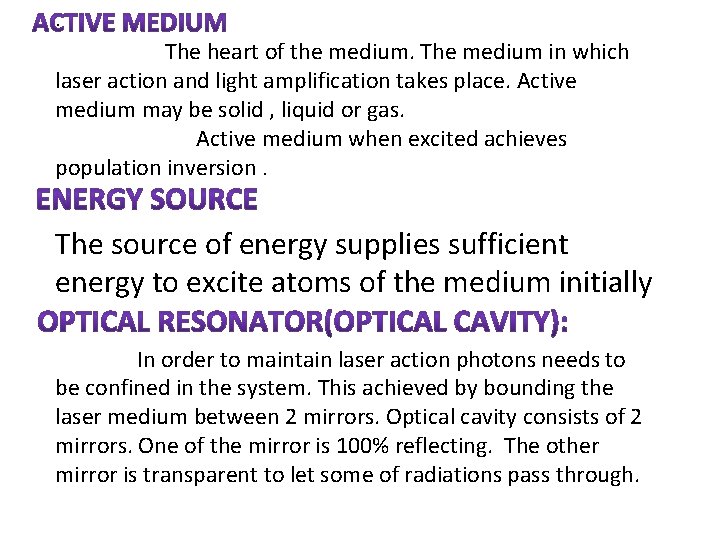
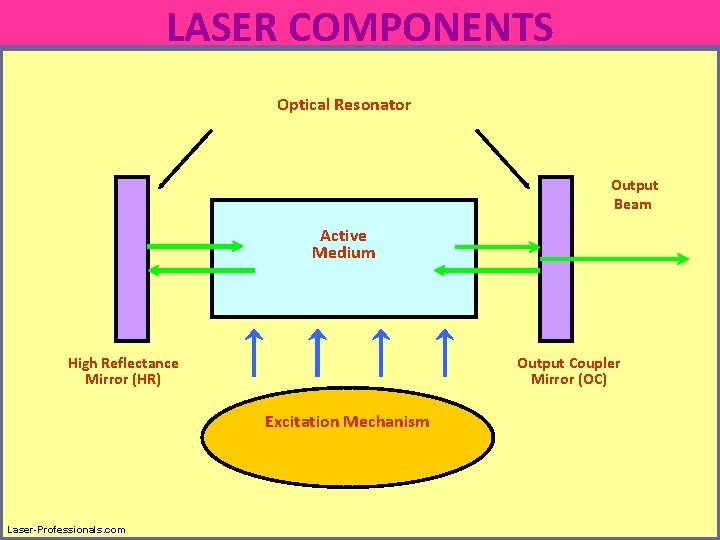
: The heart of the medium. The medium in which laser action and light amplification takes place. Active medium may be solid , liquid or gas. Active medium when excited achieves population inversion. The source of energy supplies sufficient energy to excite atoms of the medium initially In order to maintain laser action photons needs to be confined in the system. This achieved by bounding the laser medium between 2 mirrors. Optical cavity consists of 2 mirrors. One of the mirror is 100% reflecting. The other mirror is transparent to let some of radiations pass through.

LASER COMPONENTS Optical Resonator Output Beam Active Medium High Reflectance Mirror (HR) Output Coupler Mirror (OC) Excitation Mechanism Laser-Professionals. com

CONSTRUCTION AND COMPONENTS OF LASER M 1 SORCE OF LIGHT M 2 LASER BEAM LASING MEDIUM The components are essential for laser are , o. Energy source o. Lasing medium or active medium o. Optical cavity

DIFFERENT TYPES OF LASERS


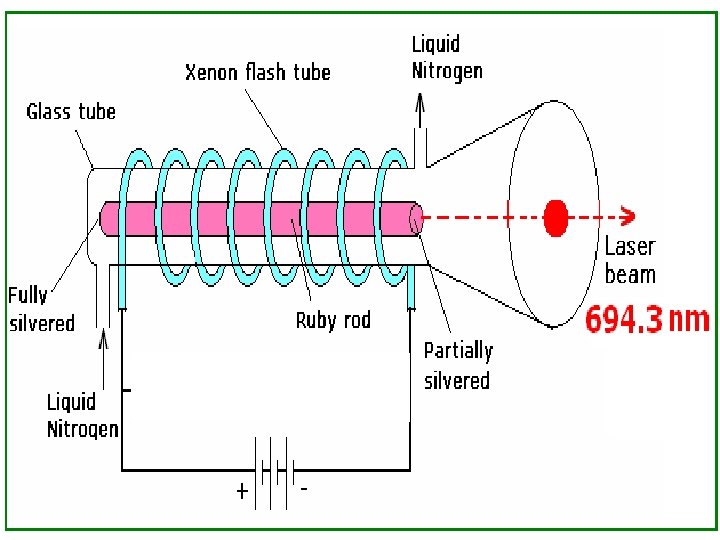
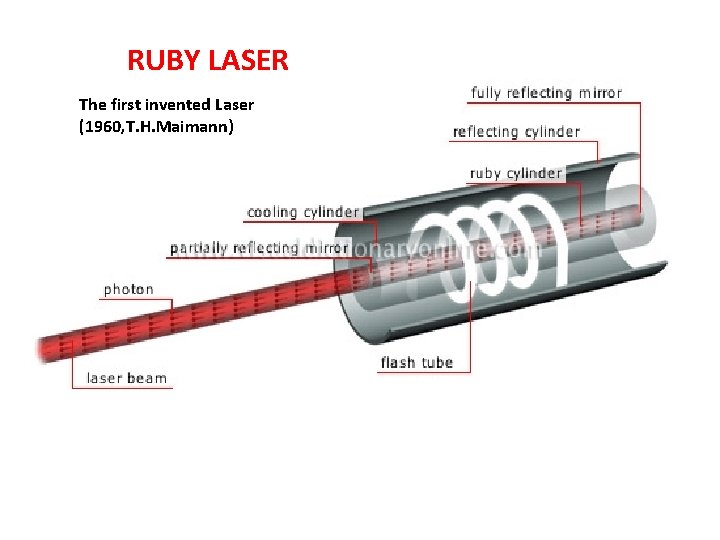
RUBY LASER


RUBY LASER The first invented Laser (1960, T. H. Maimann)

The first laser produced. Fabricated by Maiman. 1960.

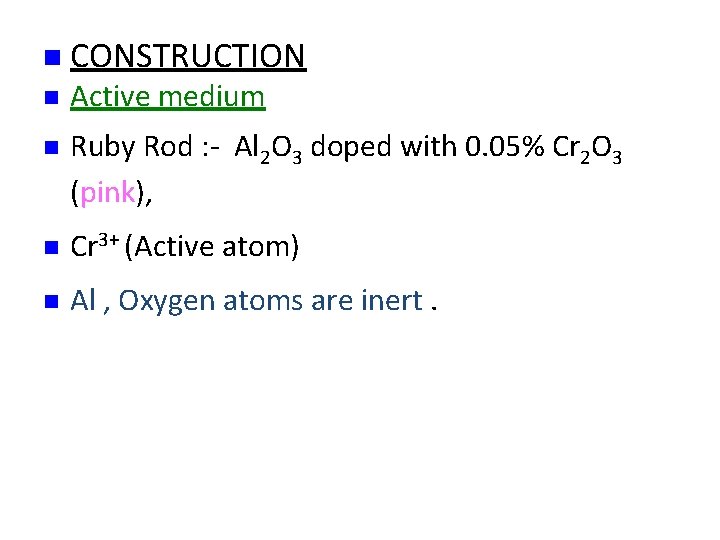
n CONSTRUCTION n Active medium n Ruby Rod : - Al 2 O 3 doped with 0. 05% Cr 2 O 3 (pink), n Cr 3+ (Active atom) n Al , Oxygen atoms are inert.

• It’s end faces are polished to make them optically flat and perfectly parallel to each other. The ruby rod is enclosed in a transparent glass jacket in which a coolant liquid nitrogen is circulating.

n n Pumping mechanism The ruby rod is surrounded by a helical xenon flash lamp. The flash lamp produces photons necessary for pumping. Optical resonators One end of the ruby rod is fully coated with silver so that it is 100 % reflecting while the other end is partially silvered so that it is partially reflecting and partially transparent. This forms the resonant cavity.

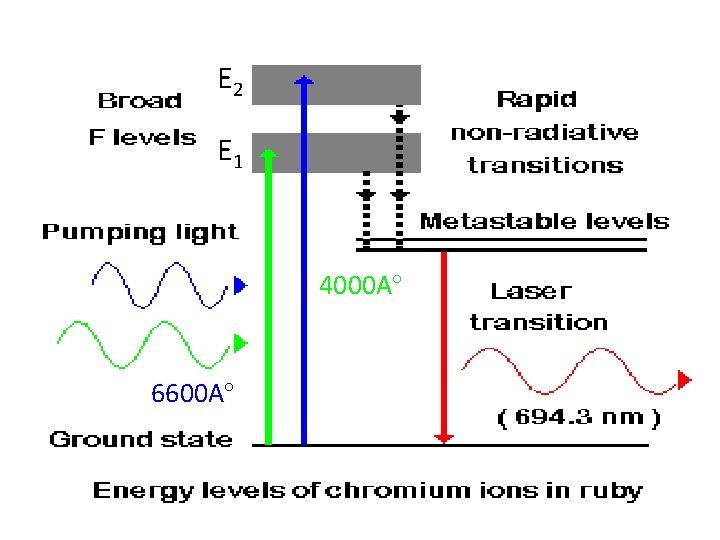
E 2 E 1 4000 A 6600 A

• When light radiations are incident , the chromium ions absorb this energy and get excited to higher energy state E 1 and E 2 which are broad levels. • Transitions to E 1 and E 2 are cause by 6600 A and 4000 A respectively. • E 1 and E 2 levels have very short life time=10 -9 secs • Hence Cr 3+ ions suddenly jump to the metastable state making non radiative transition. • The life time of metastable state M is 10 -3 secs. • Hence number of chromium atoms gets increased at metastable state.

• Finally population inversion is achieved. • At this stage the photons produced shuttles between the end faces. • This produces lasing action. • Stimulated emission takes from M to ground state G. • As a result intense laser beam of =6943 A is emerging through the partially silvered face. • The laser beam is in the form of pulses.

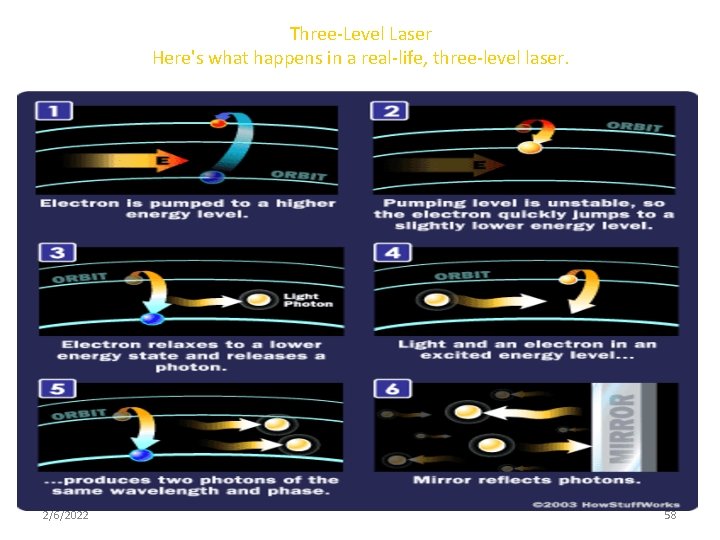
Three-Level Laser Here's what happens in a real-life, three-level laser. 2/6/2022 58

APPLICATIONS To study about matter and quality of material To study fusion, nuclear bombs etc As laser range finders and laser weapon In spectroscopy For surgery, ophthalmology and general diagnosis For laser entertainments For drilling, cutting, welding and trimming of very hard materials • For remote sensing • •

He-Ne LASER

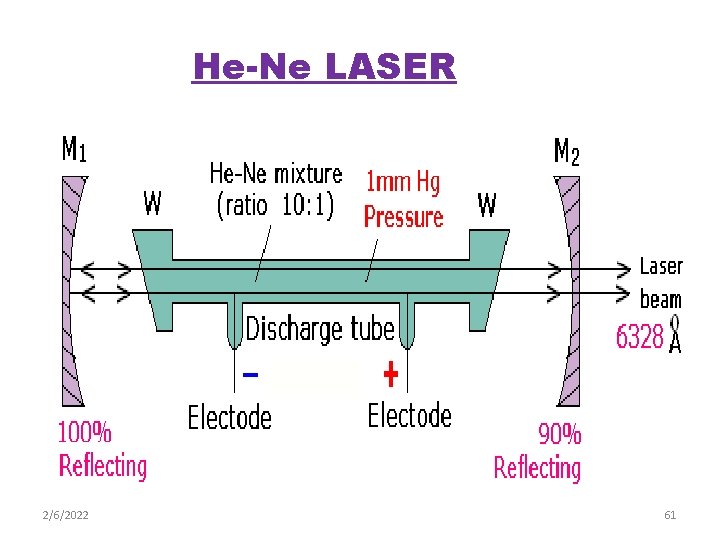
He-Ne LASER 2/6/2022 61


Lasing medium: A mixture of He and Ne in the ratio 10: 1 Energy source: RF electrical discharge Optical cavity: Two reflecting mirrors-one fully silvered and the other partially silvered.

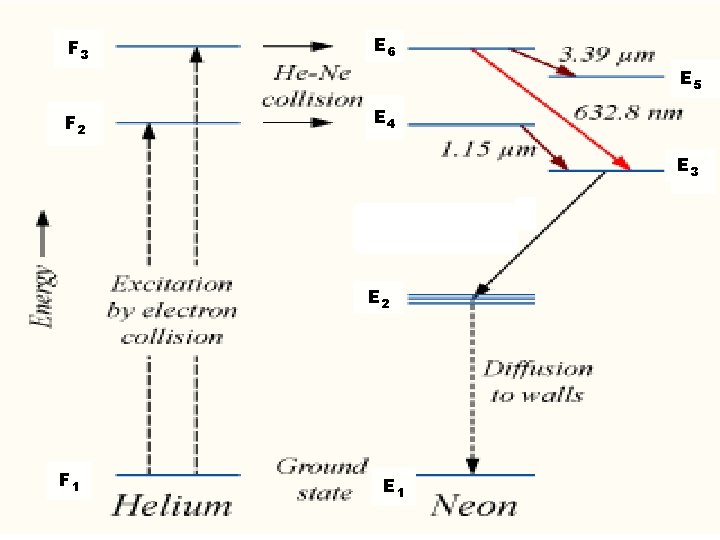
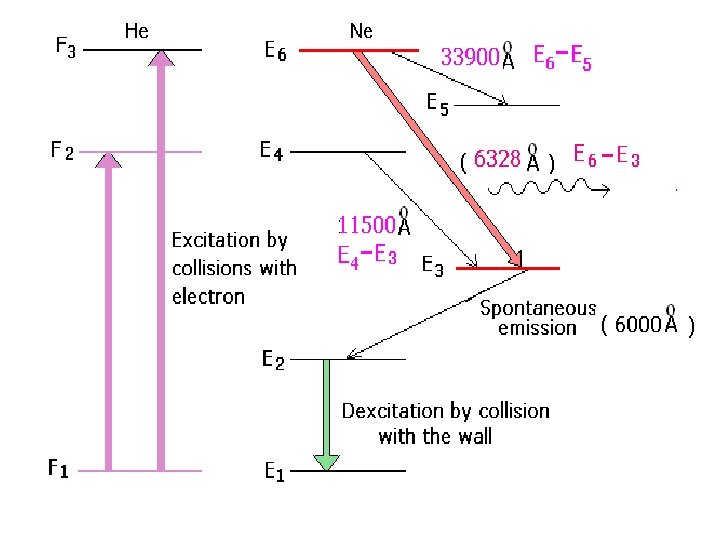
F 3 E 6 E 5 F 2 E 4 E 3 E 2 F 1 E 1


when RF discharge is passed through this gas mixture, ionization takes place. • Electrons are accelerated to anode and positive ions to cathode. • Accelerated electrons collide with He atoms which are lighter than Ne.

• As a result He atoms get excited to levels F 2 and F 3 which are metastable states • These excited He atoms are now colliding with Ne atoms and transferring their energy to them and reaching to the ground state. • As a result Ne atoms are excited to the energy levels E 4 and E 6 which are also metastable states in the same level of F 2 and F 3 • This process is continued. • Now population inversion takes place in E 4 and E 6.

Stimulated emission takes place from E 6 to E 5, E 6 to E 3 and E 4 to E 3 emitting radiations of 3 wavelengths. 1. E 6 to E 3 6328 Ao in the visible region. 2. E 6 to E 5 3. 39 m in IR region 3. E 4 to E 3 1. 15 m in IR region • Hence a plane polarized beam of laser is obtained.

ADVANTAGES • Continuous plane polarized laser beam is obtained • Highly monochromatic with stable frequency and more directional • Pure spectrum is obtained as there is no thermal distortion and scattering • Easily constructed and operated without any need of cooling

DISADVANTAGE • Efficiency is only 0. 1%.

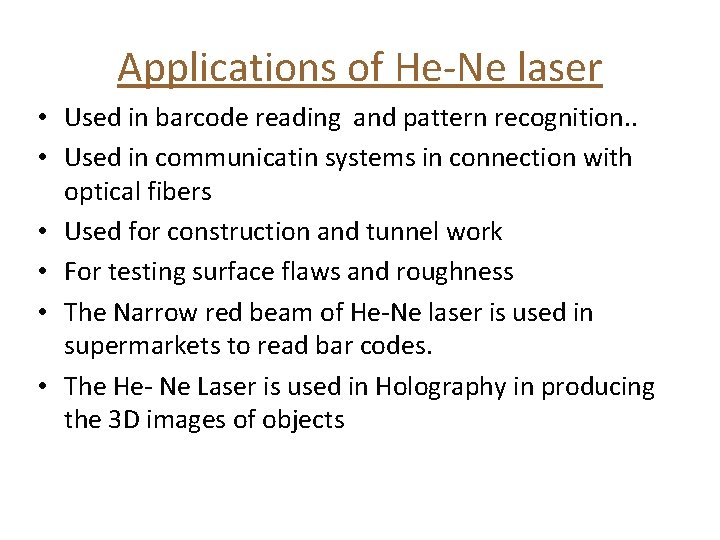
Applications of He-Ne laser • Used in barcode reading and pattern recognition. . • Used in communicatin systems in connection with optical fibers • Used for construction and tunnel work • For testing surface flaws and roughness • The Narrow red beam of He-Ne laser is used in supermarkets to read bar codes. • The He- Ne Laser is used in Holography in producing the 3 D images of objects

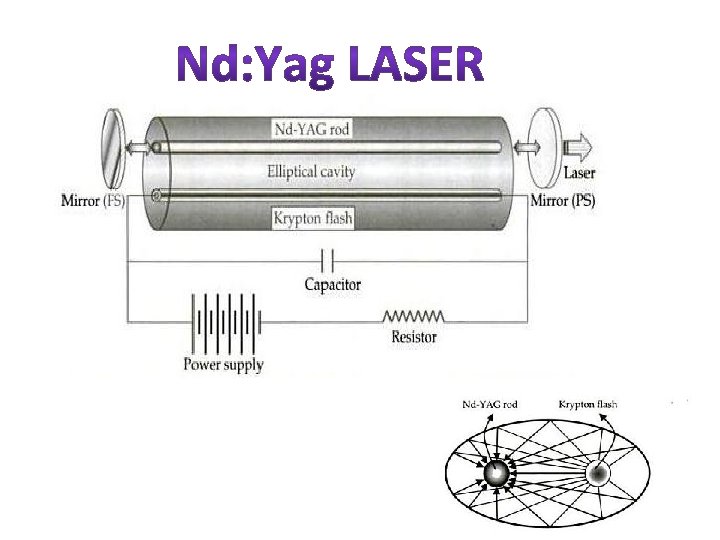
Nd: YAG LASER




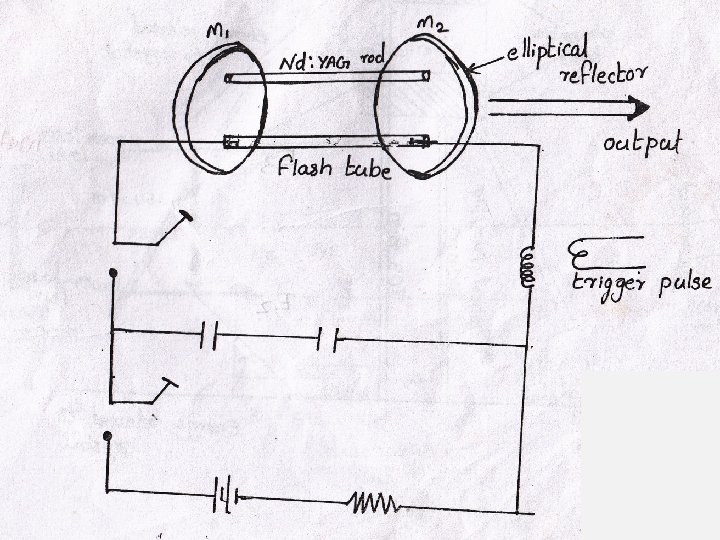
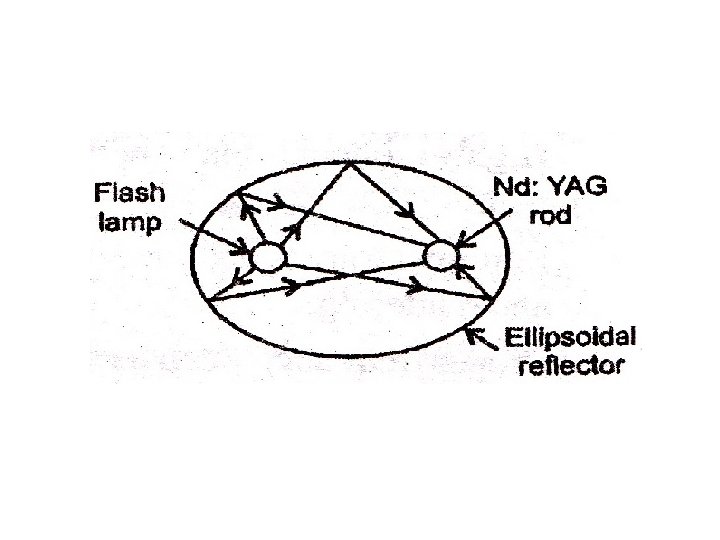
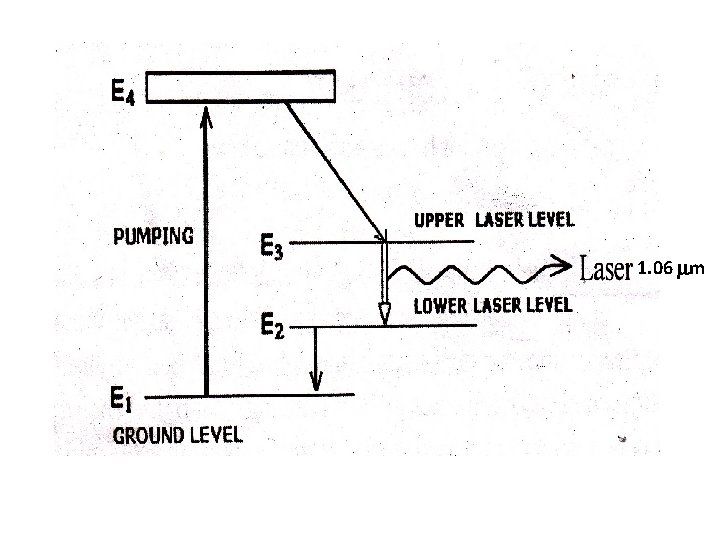
This is a four level solid state laser Active medium YAG (Yitrium Aluminium Garnet Y 3 Al 5 O 12)crystal is doped with 1% of neodymium ions. Nd-YAG rod of about 0. 1 m in length and 6 mm in dia. Is kept along one of the foci of an elliptical reflector. Energy source is Krypton arc lamp kept along the other focal line of the reflector. Optical resonators: These are two mirrors kept at each ends. One end mirror is fully silvered and the other partially silvered.

1. 06 m

Working Flash lamp is switched on. Nd ions are excited to higher energy level E 4. Within 10 -9 sec. these atoms make a quick nonradiative transition to level E 3 So atoms accumulate in this metastable state. Thus population inversion is achieved between E 3 and E 2. A powerful laser beam is emitted through the partially silvered mirror. It is s continuous laser with wavelength 1. 06 m

Advantages: At ordinary temperature the lower laser level is unpopulated. Hence population inversion can be easily achieved

USES • In military they are used as range finders • In ophthalmology for surgery to correct vision • In fluid mechanics to visualize the flow of liquids • In cosmetics to remove tattoos and hair on skin • For welding and drilling

Graphics used to make still, dynamic or animated graphics



Coherence Temporal Coherence Spatial Coherence

Temporal Coherence This refers to the Coherence of the wave with respect to time The phase of the wave change with time If wave will have the constant phase change in equal interval of time , then the wave is said to have the temporal coherence

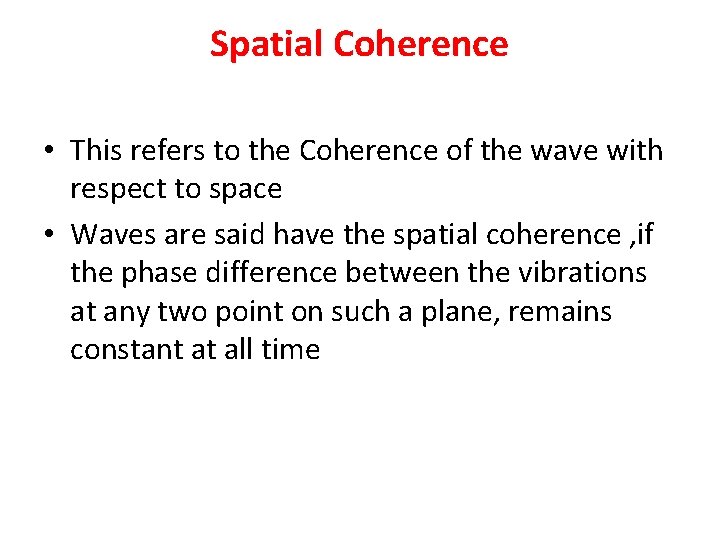
Spatial Coherence • This refers to the Coherence of the wave with respect to space • Waves are said have the spatial coherence , if the phase difference between the vibrations at any two point on such a plane, remains constant at all time