LARGE SCALE EVIDENCE GENERATION FOR THE UTILISATION AND














- Slides: 24

LARGE SCALE EVIDENCE GENERATION FOR THE UTILISATION AND SAFETY OF BIOLOGIC MEDICINES ASSOCIATE PROFESSOR NICOLE PRATT QUALITY USE OF MEDICINES AND PHARMACY RESEARCH CENTRE UNIVERSITY OF SOUTH AUSTRALIA

OVERVIEW Biologic medicines, or immune based therapies, are a rapidly evolving group of pharmaceutical products whose active component is biologically derived rather than chemically synthesised. They have widespread clinical applications in cancer, multiple sclerosis, organ transplantation and autoimmune disorders such as rheumatoid arthritis, multiple sclerosis and inflammatory bowel disease Biologic medicines have transformed management of difficult-to-treat disease and provide more effective treatment alternatives or therapeutic options where previously none existed. The mechanism of action of biologics and their administration in difficult-to-treat patients can result in an atypical and clinically unpredictable safety profile, particularly through modification of the immune system. For example, all approved biologic medicines that are monoclonal antibodies (m. Abs) have been noted to exhibit some level of immunogenicity 1.

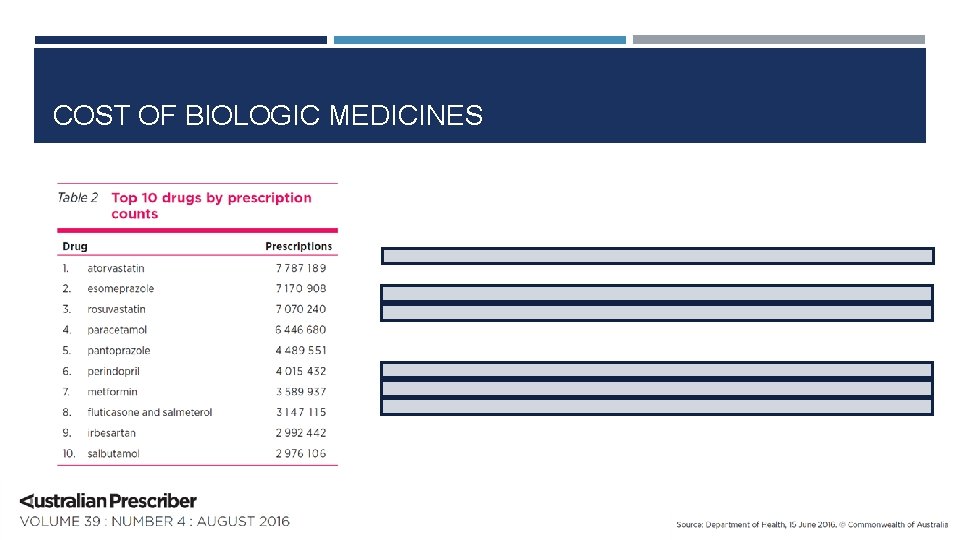
COST OF BIOLOGIC MEDICINES

SAFETY CONCERNS Biologics have a unique mechanisms of action that can result in unpredictable and serious adverse events Potential for Adverse events: Expanding indications, off-label use, use of multiple biologic medicines for multiple diseases, sequential use of biologics and interactions with other medications The move towards use of biologics for long-term treatment of chronic conditions An increasing proportion of the population will be exposed to biologics and for longer periods of time Due to the limitations of pre-market clinical trials, some serious safety concerns have only emerged as populations have become increasingly exposed to biologics in the post-market setting Of the 174 biologic medicines approved for use in the US and Europe, 25% have required some safety-related regulatory action in the post-market phase* *Giezen Tjet al. Safety-Related Regulatory Actions for Biologicals Approved in the United States and the European Union. JAMA. 2008;

POST MARKET SURVEILLANCE The current mechanism for monitoring post-market safety of biologics relies on passive surveillance of spontaneous reports limited due to under-reporting and inadequate for rare conditions or rare outcomes Delayed detection of safety issues has resulted in delayed action by regulators mean time to regulatory action estimated at 3. 7 years after market approval 2. Strategies to both monitor the use of biologics in the community and to assess the safety of biologics in the real-world are lacking but are critically important given the rapid population uptake, costs and potential for serious adverse effects of these treatments

ONCE UPON A TIME……. IPIL B A M IMU In South Australia, of 56 patients dispensed ipilimumab for malignant melanoma 1, eight were admitted to hospital for severe, steroid refractory colitis and two of these patients received a colectomy after another biologic, infliximab failed to resolve the colitis. In practice the rate of colitis was 3 times higher than in clinical trials and the estimated cost to manage the adverse events in these patients was over $400, 000.

ANOTHER DAY ANOTHER STORY…. . Efalizumab, for treatment of psoriasis, was withdrawn 6 years post approval after three cases of PML were detected 2 E ESSIV PML is rare, generally occurs in people with weakened R PROG CAL O F I T L MU CEPH N E O K LEU THY A P O L A (PML) immune systems, has no known treatment, and leads to irreversible decline in neurologic function usually resulting in death. PML has also been identified post-market with natalizumab 3, for treatment of multiple sclerosis, which resulted in a temporary removal from the market Risk of PML was shown to increase with duration of use of natalizumab and modified with prior use of immunosuppressants

These examples highlight the need for a rapid post-market surveillance system for biologics that not only identifies and quantifies harms, but identifies particular characteristics that make patients more vulnerable to harm

STUDY AIM To generate large-scale real-world evidence of the safety of biologics including identifying risk, quantifying risk and identifying factors that modify risk of adverse events Aim 1: To describe biologic medicine use and map treatment pathways over time Aim 2: To quantify incidence and risk of severe acute and long-term adverse events with biologics Aim 3: To investigate how risk of adverse events with biologic medicines varies dependent on dose, cumulative dose, concurrent use and sequential use within and across indications Aim 4: To identify factors that modify the risk of adverse events associated with biologic medicines using novel machine learning methods

RESEARCH PLAN & METHODOLOGY To develop a comprehensive post-market surveillance program for biologic medicines using existing collaborations with two large Data Research Networks (DRNs). Data Research Networks are collaborations between data custodians, in different countries, working together to generate evidence at scale while preserving data confidentiality.

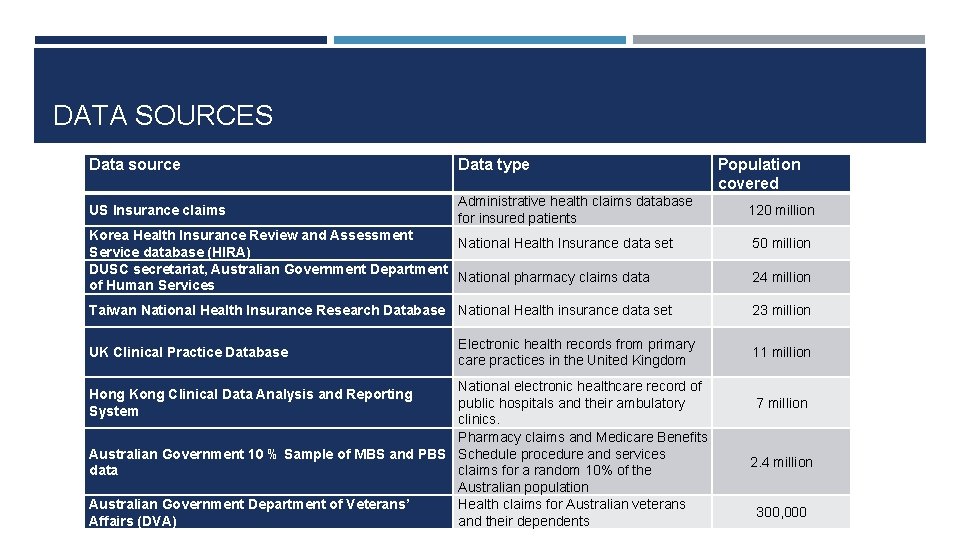
DATA SOURCES Data source Data type US Insurance claims Administrative health claims database for insured patients Korea Health Insurance Review and Assessment National Health Insurance data set Service database (HIRA) DUSC secretariat, Australian Government Department National pharmacy claims data of Human Services Taiwan National Health Insurance Research Database National Health insurance data set UK Clinical Practice Database Electronic health records from primary care practices in the United Kingdom National electronic healthcare record of public hospitals and their ambulatory clinics. Pharmacy claims and Medicare Benefits Australian Government 10 % Sample of MBS and PBS Schedule procedure and services data claims for a random 10% of the Australian population Australian Government Department of Veterans’ Health claims for Australian veterans Affairs (DVA) and their dependents Hong Kong Clinical Data Analysis and Reporting System Population covered 120 million 50 million 24 million 23 million 11 million 7 million 2. 4 million 300, 000

HOW ARE WE GOING TO DO IT? Chief Investigators Nicole Pratt, Libby Roughead, Michael Ward, Lisa Kalisch Ellett (Australia), Martijn Schuemie, Marc Suchard (OHDSI), Ian Wong (Hong-Kong & UK), Ju-Young Shin (Korea) Associate Investigators Edward Lai, Yea-Huei Yang, (Taiwan) Nam-Kyong Choi (Korea) Michal Abrahamowicz (Canada) 4 years funding for new Staff: • Post-doc • Statistician • 4 part-time post docs (Korea, Taiwan, Hong. Kong, US)

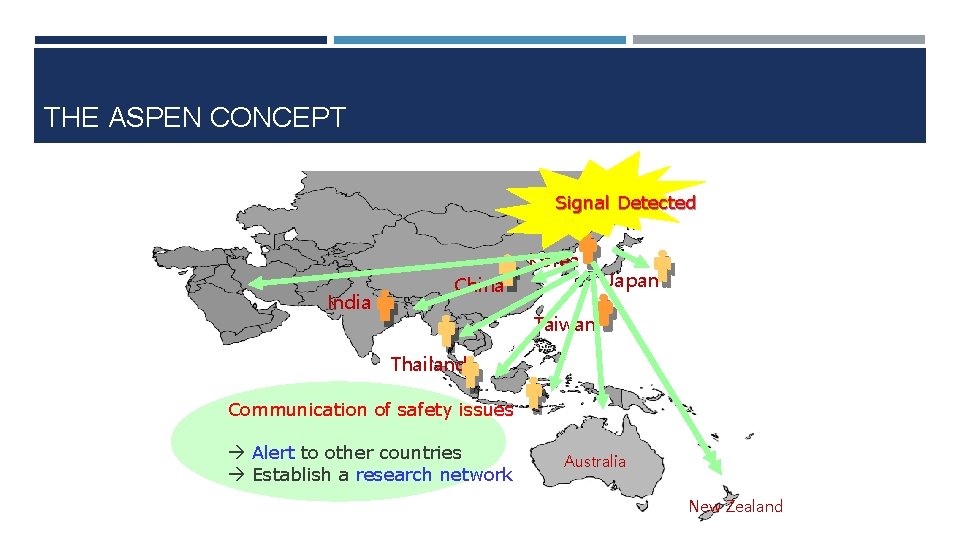
THE ASPEN CONCEPT Signal Detected India China Korea Japan Taiwan Thailand Communication of safety issues Alert to other countries Establish a research network Australia New Zealand

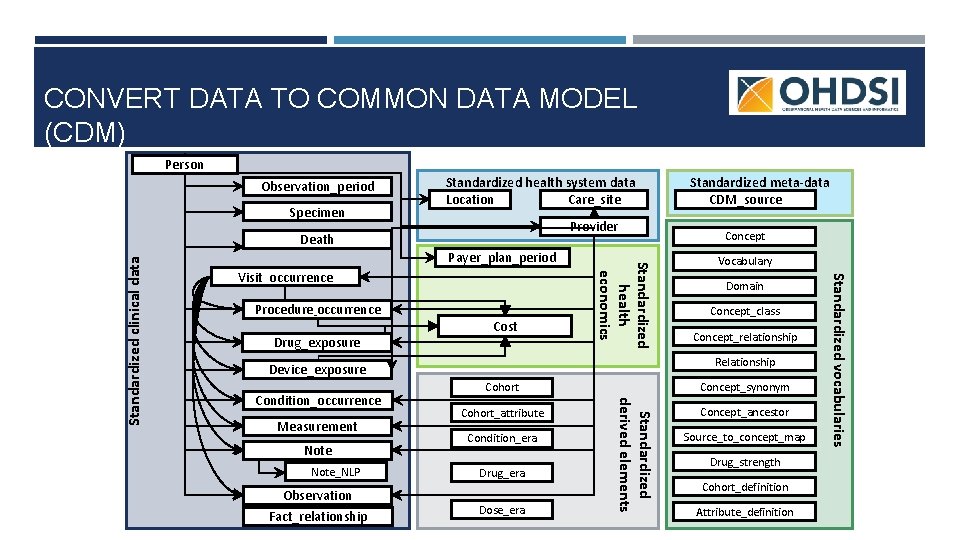
CONVERT DATA TO COMMON DATA MODEL (CDM) Person Observation_period Specimen Standardized health system data Location Care_site Provider Payer_plan_period Procedure_occurrence Drug_exposure Cost Note_NLP Observation Fact_relationship Domain Concept_class Concept_relationship Concept_synonym Cohort_attribute Condition_era Drug_era Dose_era Standardized derived elements Measurement Vocabulary Relationship Device_exposure Condition_occurrence Concept_ancestor Source_to_concept_map Drug_strength Cohort_definition Attribute_definition Standardized vocabularies Visit_occurrence Standardized health economics Standardized clinical data Death Standardized meta-data CDM_source

CONVERSION OF ASIAN DATABASES TO CDM Lai et al. Applying a common data model to Asian databases for multinational pharmacoepidemiologic studies: opportunities and challenges. Clin Epidemiol

ADVANTAGE OF COMMON DATA MODEL Research infrastructure for ongoing safety studies Take advantage of the analytic tools developed by the Observational Health Data Sciences and Informatics (OHDSI) community (www. ohdsi. org) Avoids each country creating their own analytic code Easy to make cross-country comparisons

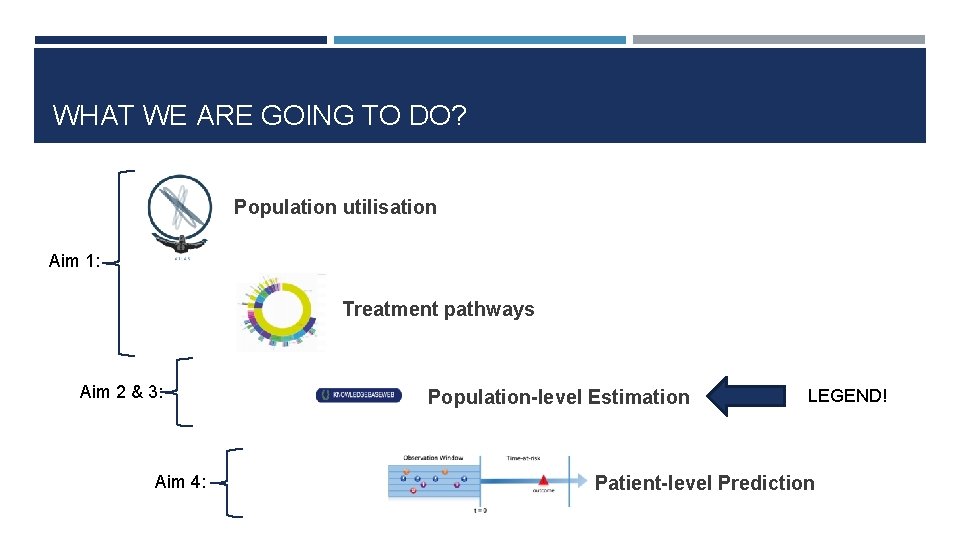
WHAT WE ARE GOING TO DO? Population utilisation Aim 1: Treatment pathways Aim 2 & 3: Aim 4: Population-level Estimation LEGEND! Patient-level Prediction

AIM 1: TO DESCRIBE BIOLOGIC USE AND MAP TREATMENT PATHWAYS OVER TIME Figure 1: Treatment Pathways Examine trends over time in (1) uptake of biologic medicines, (2) incident and prevalent use of biologic medicines, (3) use of different biologic medicines concurrently for the same condition or across multiple conditions, and (4) average doses used. Characterising treatment pathways will reveal how countries differ in their treatment approaches with biologic medicines and will reveal differences in the availability and potential appropriateness of treatment. Practice differences between regions will provide a mechanism to investigate potential modifying factors associated with risk. Outcomes of Aim 1: An international profile of (1) trends in biologics use by product, specific condition, use of multiple treatments (2) treatment pathways in the use of biologics for specific conditions.

AIM 2: TO QUANTIFY INCIDENCE AND RISK OF SEVERE ACUTE AND LONG-TERM ADVERSE EVENTS WITH BIOLOGIC MEDICINES Prioritise Biologic medicines to study? Collaborating with the TGA’s Signal Investigation Unit, Pharmacovigilance and Special Access Branch have indicated as high priority and which will be addressed include quantifying 1) risk of colitis associated with ipilimumab 2) risk of fracture associated with denosumab and 3) cardiotoxicity associated with etanercept and infliximab. Data-driven approach: Spontaneous reports , OHDSI signal detection tools Methods: Associations between biologic medicines and adverse events will be generated using observational study designs including prescription sequence symmetry analysis (PSSA), self-controlled case series (SCCS) and cohort studies. Outcomes of Aim 2: Clinical evidence of the risks and extent of harm associated with biologics in real world practice will be generated including estimates of numbers needed to harm suitable for clinical use and evidence of population harm to improve timeliness of potential regulatory action.

AIM 3: TO INVESTIGATE HOW RISK OF ADVERSE EVENTS WITH BIOLOGIC MEDICINES VARIES DEPENDENT ON DOSE, CUMULATIVE DOSE, CONCURRENT USE AND SEQUENTIAL USE WITHIN AND ACROSS INDICATIONS Biologics have the potential for immunogenicity and prior use of biologic medicines where immunogenicity has resulted may affect both harms and efficacy of current biologic use, particularly where the biologic targets are the same. For example, risk of PML with natalizumab was found to increase with duration and prior use of immunosuppressants Concurrent use of biologics may result in interactions For example, combination treatment of a fusion protein, etanercept with the receptor antagonist anakinra, resulted in a higher frequency of adverse events (14. 8%) compared with single biologic treatment (4. 5%) Methods: Weighted cumulative exposure (WCE) methods will be used to establish whether different patterns of medication use affect the occurrence of adverse events. WCE models the weighted sum of past doses with estimated weights to reflect the relative importance of doses taken at different times in relation to the adverse event where weights are estimated using flexible cubic splines. Exposureadjusted SCCS “adaptation we also compare the rates at various levels of accumulated exposure, regardless of whether the person is still exposed or not” Outcomes of Aim 3: Clinical evidence of how timing and dose in treatment patterns of biologics contribute to harm.

DEVELOPING NEW TOOLS? Estimation of the cumulative effects of treatment on risk of adverse events Abrahamowicz et al (2006) defined a weight function which assigned weights to past doses. 1. Weights were dependent on the drug half-life, with doses further in the past assigned lower weights. 2. Hauptmann et al (2003) and Sylvestre et al (2009) modelled cumulative exposure over aetiologically relevant time windows using flexible cubic splines, WCE models have been shown to have a better fit than conventional models (Abrahamowicz et al 2012). WCE models account for varying intensity, timing and duration of exposure, predictions for different clinically relevant patterns of medicine dose are possible (for example, Dixon et al 2012). Freely available statistical software by Sylvestre et al (Sylvestre 2018) R package ‘WCE’

AIM 4: TO IDENTIFY PATIENT RELATED FACTORS THAT MODIFY THE RISK OF ADVERSE EVENTS ASSOCIATED WITH BIOLOGIC MEDICINES USING NOVEL MACHINE LEARNING METHODS Further to determining whether biologics are associated with increased risk of harm at the population level, it is important to identify the particular characteristics of patients that may make them more vulnerable to these risks. Understanding how adverse effects of biologics are modified by particular patient characteristics will be of importance for clinicians and patients as, in the real world, biologics are often required to be used in patients who bear little resemblance to those in the pre-market clinical trials. Methods: data mining techniques will be used including deep learning with artificial neural networks, lasso regularised regression, random forests and gradient boosted machines. Models will be validated internally using cross-validation, while each of the datasets in the network will be used for external validation. Key factors consistent across datasets will help identify predictors of side effects at the global level. Outcomes of Aim 4: Globally relevant predictive models that highlight factors predictive of safety issues associated with biologic medicines. Application and validation of innovative machine learning techniques for patient level prediction of risk factors which can be used in applications of precision medicine to create tailored treatment pathways for patients.

PLAN FOR YEAR 1 Identify availability of biologic medicines; Indications: variability / availability / reimbursement policies Coding issues: Products/biosimilars Feasibility: sample size (particularly for rare or long term outcomes) Drug Utilisation Treatment pathways Which medicines to focus on Meeting with Australia’s TGA to discuss priorities Meeting with TGA today! • Vaccination of people on biologics (timing and efficacy) • Long-term risk of cancer • Immune related AES – infections, reactivation of viral infections • Stopping treatment Rebound disease, etc Spontaneous Reports: eg Minnema et al. Drug Safety 2019 m. Abs and depression and suicidal ideation and behaviour & Araujo GS et al. Safety of Biologics Approved for RA and other Austo-immune diseases: Disproportionality Analysis from FAERS Data driven signal detection: SSA, other OHDSI tools
